| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yong Chool Boo | + 3579 word(s) | 3579 | 2020-09-09 05:34:04 | | | |
| 2 | Yong Chool Boo | + 2 word(s) | 3581 | 2020-09-09 14:23:18 | | | | |
| 3 | Yong Chool Boo | Meta information modification | 3581 | 2020-09-09 14:37:16 | | | | |
| 4 | Yong Chool Boo | Meta information modification | 3581 | 2020-09-09 15:26:06 | | | | |
| 5 | Yong Chool Boo | + 4 word(s) | 3585 | 2020-09-10 03:08:32 | | | | |
| 6 | Yong Chool Boo | -75 word(s) | 3506 | 2020-09-10 03:52:55 | | | | |
| 7 | Yong Chool Boo | Meta information modification | 3506 | 2020-09-10 06:45:17 | | | | |
| 8 | Yong Chool Boo | -1615 word(s) | 1891 | 2020-09-10 08:36:07 | | | | |
| 9 | Camila Xu | -4 word(s) | 1887 | 2020-09-10 10:29:01 | | | | |
| 10 | Yong Chool Boo | + 4 word(s) | 1891 | 2020-09-11 02:25:10 | | | | |
| 11 | Yong Chool Boo | Meta information modification | 1891 | 2020-09-12 10:19:50 | | | | |
| 12 | Yong Chool Boo | Meta information modification | 1891 | 2020-09-12 10:40:03 | | | | |
| 13 | Yong Chool Boo | Meta information modification | 1891 | 2020-09-16 11:35:14 | | | | |
| 14 | Yong Chool Boo | Meta information modification | 1891 | 2020-09-16 11:40:06 | | | | |
| 15 | Yong Chool Boo | Meta information modification | 1891 | 2020-09-16 11:46:54 | | | | |
| 16 | Camila Xu | -25 word(s) | 1866 | 2020-10-26 10:49:46 | | | | |
| 17 | Catherine Yang | Meta information modification | 1866 | 2021-09-28 11:24:22 | | |
Video Upload Options
Certain analogs of α-melanocyte stimulating hormone (MSH) and peptides with the sequences derived from the hormone were shown to promote or suppress melanin synthesis in cells and in vivo models. Various amino acids, peptides, their analogs, and their hybrid compounds with other chemical moieties were shown to inhibit tyrosinase (TYR) catalytic activity or downregulate TYR gene expression. Certain peptides were shown to inhibit melanosome biogenesis or induce autophagy, leading to decreased pigmentation. In vivo and clinical evidence are available for some compounds, including [Nle4-D-Phe7]-α-MSH, glutathione disulfide, and glycinamide hydrochloride.
1. Introduction
Melanin plays an important role in the appearance of skin color, protection against ultraviolet (UV) radiation, and maintenance of homeostasis in many organs [1][2]. Both over- and underproduction of melanin are a major research theme in cosmetology and dermatology, not only from the aesthetic viewpoint pursuing a harmonious skin tone, but also from a medical viewpoint preventing and treating various skin diseases [3][4][5][6][7].
As numerous amino acids and peptides directly and indirectly participate in the melanin synthesis process, it is reasonably assumed that the process could be artificially regulated by certain structurally related compounds. This review will introduce recent advances in the artificial regulation of skin pigmentation using amino acids, peptides, and their analogs.
2. Targets for the control of skin pigmentation
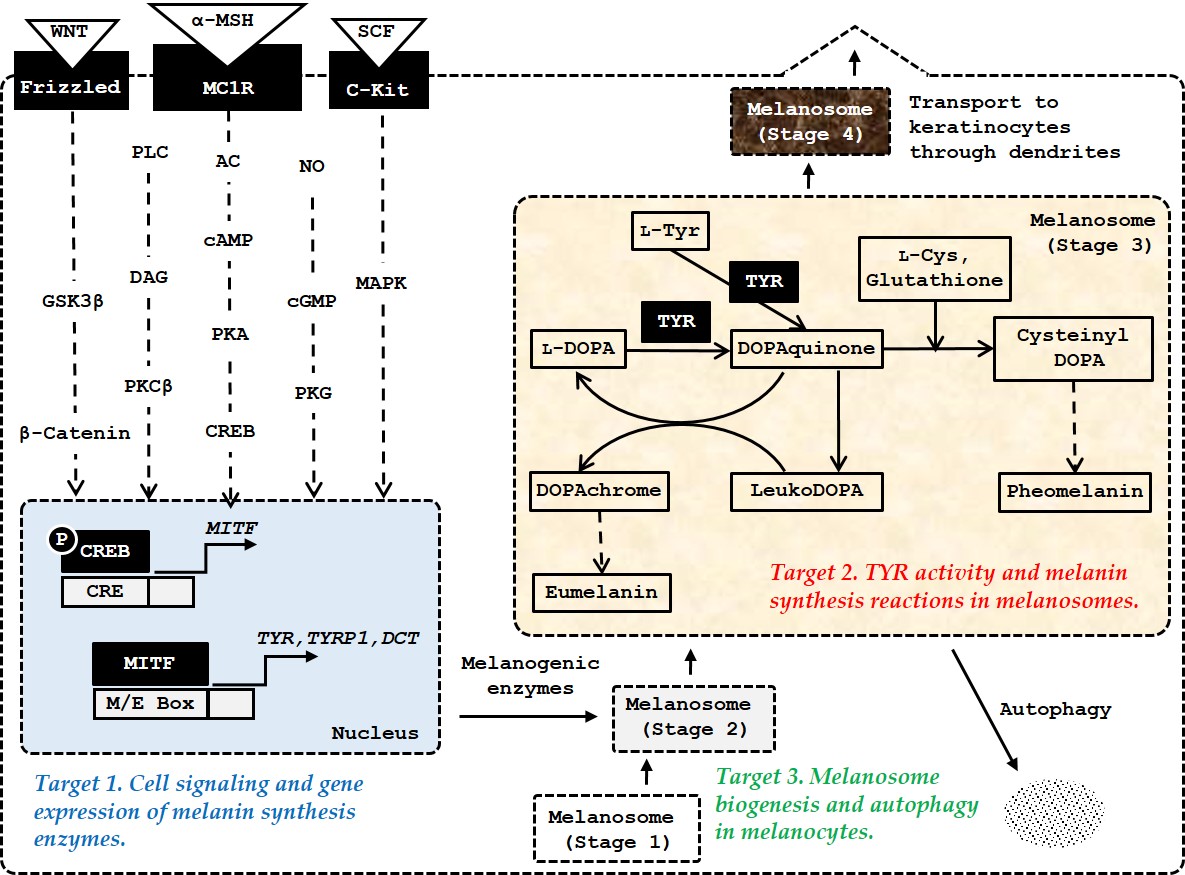
The most-studied molecular targets are the receptors on the surface of melanocytes which transmit intracellular signals, and the enzymes and proteins within melanocytes involved in melanin synthesis, and melanosome biogenesis and autophagy in melanocytes (Figure 1).
Figure 1. The major targets of amino acids, peptides, and their analogs for the control of skin pigmentation. Microphthalmia-associated transcription factor (MITF) plays a primary role in inducing gene expression of melanogenic enzymes, such as tyrosinase (TYR), tyrosinase-related protein 1 (TYRP1), and dopachrome tautomerase (DCT) in response to various internal and external stimuli. In addition to the α-melanocyte stimulating hormone (MSH)/ melanocortin 1 receptor (MC1R) /adenyl cyclase (AC)/ cyclic adenosine monophosphate (cAMP)/protein kinase A (PKA)/cAMP-responsive element-binding protein (CREB) pathway, the stem cell factor (SCF)/receptor tyrosine kinase protein, c-Kit/mitogen-activated protein kinases (MAPK) pathway, and WNT/frizzled/glycogen synthase kinase (GSK) 3β/β-catenin pathway can activate MITF. Other signaling pathways, such as phospholipase C (PLC)/diacylglycerol (DAG)/protein kinase C (PKC) β cascade, and nitric oxide (NO)/cGMP/protein kinase G (PKG) cascade are also involved in the activation of MITF. Melanosome biogenesis occurs through morphologically distinct stages 1, −2, −3, and −4. Melanogenic enzymes matured through post-translational modifications in endoplasmic reticulum and metal-loading in Golgi apparatus are sorted and transported to stage 2 melanosomes. Melanin is synthesized thereafter and the mature stage 4 melanosomes with accumulated melanin are transferred through dendrites to keratinocytes.
3. Artificial Upregulation of Melanin Synthesis
In this chapter, we discuss promotion of melanin synthesis by α-MSH analogs and oligopeptides derived from the hormone sequence, L-Tyr and L-DOPA, and other peptide hormones. Selected studies are listed in Table 1.
Table 1. Amino acids, peptides, and their analogs that stimulate melanin synthesis.
|
Compounds |
Key Points |
Literature |
|
[Nle4-D-Phe7]-α-MSH |
This α-MSH analog was more resistant to enzymatic degradation and more potent in biological activity compared with α-MSH or [Nle4]-α-MSH. |
[8] |
|
Ac-Phe-Arg-Trp-Gly-NH2 |
This peptide enhanced the α-MSH-induced increase in TYR activity in S-91 murine melanoma cells. |
[9] |
|
Ac-His-D-Phe-Arg-Trp-NH2 n-Pentadecanoyl-His-D-Phe-Arg-Trp-NH2 4-Phenylbutyryl-His-D-Phe-Arg-Trp-NH2 |
These tetrapeptides increased melanin synthesis and viability of human melanocytes under UV-irradiated conditions. |
[10] |
|
Bz-Gly-His-D-Phe-D-Arg-D-Trp-N(CH2CH2CH3)2 |
This pentapeptide induced protein expression of MITF, TYR, and TYRP1, and enhanced the activation of NRF2 after UVA-irradiation. |
[11] |
|
L-Tyr L-DOPA |
L-Tyr and L-DOPA enhanced expression of TYR and stimulated melanin synthesis. |
[12] |
|
Vasoactive intestinal peptide (HSDAVFXDNYXRLRKQMAVKKYLNSXLN) |
Vasoactive intestinal peptide increased melanin production by increasing TYR activity and gene expression in a PKA, CREB, and MITF–dependent mechanism. |
[13] |
|
Angiotensin II (DRVYIHPF) |
Angiotensin II upregulated TYR activity and melanin content in melanocytes through an AT1-dependent mechanism. |
[14] |
4. Artificial Downregulation of Melanin Synthesis
In this chapter, we discuss basic amino acids and peptides (Section 4.1), peptides isolated from plants or derived from natural protein sequences (Section 4.2), and hybrid peptides with other chemical moieties (Section 4.3) that inhibit TYR catalytic activity in vitro. We additionally discuss certain peptides that downregulate TYR gene expression or its protein level in melanocytes (Section 4.4). Finally, we discuss the peptides that inhibit melanosome biogenesis or induce autophagy in melanocytes (Section 4.5). IC50 is defined as the 50% inhibitory concentration.
4.1. TYR Inhibitory Amino Acids, Peptides, and Their Analogs
Various amino acids and peptides are known to inhibit TYR activity and/or cellular melanin synthesis, and some of them show depigmenting effects in human skin (Table 2).
Table 2. Amino acids, peptides, and their analogs that inhibit TYR activity and melanin synthesis.
|
Compounds |
Key Points |
Literature |
|
L-Cys |
L-Cys extended an initial delay in DOPAchrome formation by avocado and mushroom TYRs. |
[15] |
|
Ergothioneine |
Ergothioneine inhibited mushroom TYR activity in a competitive manner, whereas L-His exhibited no inhibitory effect. |
[16] |
|
GD; GK; GH; GG; GF; GY |
Glycyl-dipeptides such as GD, GK, and GH inhibited TYR activity, and reduced the browning of apples and potatoes. |
[17] |
|
CA; YC; PD; DY; CE; CS; CY; CW |
Estimated TYR inhibitory activity of 20 × 20 dipeptides. N-terminal Cys-containing dipeptides were highly active. |
[18] |
|
CRY RCY |
These antimelanogenic peptides were identified in a pharmacophore modeling method. |
[19] |
|
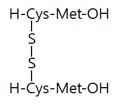
L-Cys L-Cystine H-Glu(Cys-Gly-OH)-OH H-Glo(Cys-Gly-OH)-OH Ergothioneine Taurine |
L-Cys, L-cystine, H-Glo(Cys-Gly-OH)-OH, and ergothioneine inhibited TYR activity more strongly than glutathione (H-Glu(Cys-Gly-OH)-OH) and taurine. |
[20] |
|
YRSRKYSSWY RADSRADC KFEKKFEK SFLLRN |
These oligopeptides were identified from an internal library and they inhibited TYR activity and reduced the melanin content of cells. |
[21] |
|
RRWWRRYY RRRYWYYR RRYWYWRR |
These peptides were identified from a docking study against mushroom TYR and they were also inhibitory against the human TYR. |
[22] |
|
D-Tyr |
D-Tyr inhibited TYR activity by a competitive mechanism and reduced melanin content in cells and a three-dimensional human skin model. |
[23] |
|
D-Tyr-D-Ala-Gly-Phe-Leu D-Ala-Gly-Phe-Leu-D-Tyr Gly-His-Lys-D-Tyr |
The addition of D-Tyr to functional peptides endowed antimelanogenic activity without altering other bioactivities. |
[24] |
|
Glutathione |
Oral administration of glutathione induced skin lightening of human volunteers. |
[25] |
|
Glutathione disulfide |
Topical application of glutathione disulfide lowered melanin index in human skin. |
[26] |
4.2. TYR Inhibitory Peptides Derived from Natural Protein Sequences
Various peptides derived from natural protein sequences inhibit TYR activity and display antimelanogenic effects in cells (Table 3).
Table 3. TYR inhibitory peptides derived from natural protein sequences.
|
Compounds |
Key Points |
Literature |
|
Cyclo[GGYLPPLS] Cyclo[GTLPSPFL] Cyclo[PFSFGPLA] |
These cyclic peptides from Pseudostellaria heterophylla inhibited TYR activity. |
|
|
MMSFVSLL VSLLLVGI LILVLLAI |
These antimelanogenic peptides were selected from octameric peptides with sequences of industrial proteins. |
[29] |
|
LQPSHY HGGEGGRPY HPTSEVY |
LQPSHY derived from rice bran protein hydrolysates inhibited TYR activity and reduced melanin content in B16 cells. |
[30] |
|
SSEYYGGEGSSSEQGYYGEG |
Of the peptides from the rice bran albumin hydrolysates, this peptide showed the highest TYR inhibition activity. |
[31] |
|
ECGYF |
The peptide with a sequence of the protein midasin inhibited TYR activity and reduced melanin content in A375 melanoma cells. |
[32] |
|
NGVQPKY NGVQPKC CNGVQPK |
These antimicrobial peptides inhibited TYR activity and reduced melanin content in B16F1 melanoma cells. |
[33] |
4.3. TYR Inhibitory Peptides Conjugated with Other Chemical Moieties
Some amino acids and peptides have been hybridized with other antimelanogenic compounds, such as kojic acid, protocatechuic acid, α-resocylic acid, gentisic acid, gallic acid, caffeic acid, para-coumaric acid, and ascorbic acid to improve their activity, stability, or bioavailability (Table 4).
Table 4. TYR inhibitory peptides conjugated with other chemical moieties.
|
Compounds |
Key Points |
Literature |
|
Kojic acid-FWY Kojic acid-FHY Kojic acid-FRY Kojic acid-FWY-NH2 Kojic acid-FHY-NH2 Kojic acid-FRY-NH2 |
These kojic acid-tripeptide amides showed enhanced stability and potent inhibition against TYR activity. |
[34] |
|
Kojic acid-F-NH2 Kojic acid-C-NH2 |
Of the kojic acid-amino acid amides, kojic acid-F-NH2 and kojic acid-C-NH2 showed the highest and lowest TYR inhibition, respectively. |
[35] |
|
Kojic acid-PS Kojic acid-CDPGYIGSR |
These kojic acid-peptides inhibited TYR activity and reduced melanin synthesis in B16F10 cells. |
[36] |
|
Protocatechuic acid-F-NH2 Protocatechuic acid-W-NH2 Protocatechuic acid-Y-NH2 |
These hybrid compounds inhibited TYR activity and protocatechuic acid-F-NH2 reduced melanin synthesis in B16 cells most effectively. |
[37] |
|
Caffeic acid-MHIR |
β-Lactoglobulin fragment peptides were conjugated with caffeic acid. |
[38] |
|
para-Coumaric acid-GGG-ARP |
The compound inhibited TYR activity and decreased melanin content in cells. |
[39] |
|
Ascorbic acid-KTTKS |
Ascorbic acid-KTTKS hybrid inhibited TYR activity and decreased melanin content in cells. |
[40] |
4.4. Peptides That Inhibit TYR Gene Expression
Some peptides are known to downregulate TYR expression by acting as a MC1R antagonist or by other mechanisms (Table 5).
Table 5. Peptides that reduce TYR gene expression or its protein level in melanocytes.
|
Compounds |
Key Points |
Literature |
|
H-His-D-Arg-Ala-Trp-D-Phe-Lys-NH2 |
This hybrid peptide analog derived from growth hormone-releasing peptide and α-MSH sequences demonstrated the antagonistic efficacy, attenuating the response to α-MSH or [Nle4,D-Phe7]-α-MSH in the lizards. |
|
|
|
The tetrapeptide reduced melanin synthesis in cells by a receptor-mediated, ERK-dependent suppression of MITF and TYR expression. |
[42] |
|
SFKLRY-NH2 |
The peptide decreased TYR protein level in cells and showed antimelanogenic effects in B16 cells. |
[43] |
|
INHHLG-NH2 ISHHLG-NH2 INHNLG-NH2 ISHNLG-NH2 FNHHLG-NH2 FNHNLG-NH2 FSHNLG-NH2 |
These antimelanogenic hexapeptides were identified using PS-SCL. FNHHLG-NH2 reduced TYR expression and melanin synthesis in cells stimulated by α-MSH. |
[44] |
|
RFWG-NH2 RLWG-NH2 FRWG-NH2 FWG-NH2 LWG-NH2 RWG-NH2 WG-NH2 G-NH2 |
These low molecular antimelanogenic peptides with sequences overlapping with α-MSH inhibited melanin synthesis in cells stimulated by α-MSH. G-NH2 (glycinamide) attenuated phosphorylation of CREB and expression of MITF and TYR. Neither Ac-G-NH2 nor G showed antimelanogenic activity. |
[45] |
|
Gly-NH2•HCl |
Glycinamide hydrochloride exhibited depigmenting effects without noted adverse effects in the human skin. |
[46] |
4.5. Peptides That Inhibit Melanosome Biogenesis or Induce Autophagy in Melanocytes
A few peptides are known to display antimelanogenic effects in melanocytes through modulation of melanosome biogenesis and autophagy (Table 6).
Table 6. Peptides and peptidic compounds that inhibit melanosome biogenesis or induce autophagy in melanocytes.
|
Compounds |
Key Points |
Literature |
|
EPLNNLQVAVK QTVEISLPLST QVAVK QVA |
Peptides derived from β1-adaptin inhibited the binding of AP-1 subunit to KIF13A, thereby inhibiting the maturation of melanosomes and melanin synthesis in cells. |
[47] |
|
|
Pentasodium tetracarboxymethyl palmitoyl 21 dipeptide-12 induced autophagy in melanocytes and decreased pigmentation. |
[48] |
5. MC1R-targeting peptides
The sequences of endogenous melanocortin hormones derived from the POMC gene product and numerous synthetic oligopeptides that showed melanogenic or antimelanogenic activity are shown in Figure 2.

Figure 2. Sequences of proopiomelanocortin (POMC)-derived peptide hormones and synthetic peptides with melanogenic or antimelanogenic effects. (a) The entire amino acid sequence of the human POMC protein is shown. Sequences for different POMC-derived hormones are indicated with different colors: adrenocorticotrophic hormone (ACTH) in blue; α-melanocyte stimulating hormone (MSH) in underlined blue; β-MSH in green; γ3-MSH in red; and γ1-MSH in underlined red. (b) Amino acid sequences of ACTH, α-MSH, β-MSH, γ3-MSH, and γ1-MSH including posttranslational modifications are shown. A conserved sequence, His-Phe-Arg-Trp, is highlighted. (c) Tetrapeptides that stimulate melanin synthesis [10]. (d) A pentapeptide that stimulates melanin synthesis [11]. (e) Tetra-, tri-, di-, and mono-peptides that inhibit melanin synthesis [45]. (f) Molecules with no or unclear effects on melanin synthesis [9][45].
6. Discussion
A variety of peptides and amino acid analogs were described to modulate melanin synthesis in cells, although their therapeutic utility remains to be further verified. In vivo and clinical results have been provided for MC1R targeting molecules, such as [Nle4-D-Phe7]-α-MSH [49][50], and glycinamide hydrochloride [46], and an inhibitor of melanin synthetic reaction, such as oxidized glutathione [26]. These studies suggest that certain amino acids, peptides, and their analogs may be a promising drug candidate for up- and downregulating skin pigmentation. Melanin increasing molecules can be used to alleviate photosensitive skin, to prevent photocarcinogenesis, and to treat vitiligo vulgaris [49][50][51]. Conversely, melanin decreasing molecules can be used to treat various types of hyperpigmentation for medical and aesthetic purposes [26][46][52][53].
References
- Slominski, A.; Kim, T.K.; Brozyna, A.A.; Janjetovic, Z.; Brooks, D.L.; Schwab, L.P.; Skobowiat, C.; Jozwicki, W.; Seagroves, T.N. The role of melanogenesis in regulation of melanoma behavior: melanogenesis leads to stimulation of HIF-1alpha expression and HIF-dependent attendant pathways. Arch Biochem Biophys 2014, 563, 79-93
- Slominski, R.M.; Zmijewski, M.A.; Slominski, A.T. The role of melanin pigment in melanoma. Exp Dermatol 2015, 24, 258-259
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev 2004, 84, 1155-1228
- Yamaguchi, Y.; Beer, J.Z.; Hearing, V.J. Melanin mediated apoptosis of epidermal cells damaged by ultraviolet radiation: factors influencing the incidence of skin cancer. Arch Dermatol Res 2008, 300 Suppl 1, S43-50
- Fistarol, S.K.; Itin, P.H. Disorders of pigmentation. J Dtsch Dermatol Ges 2010, 8, 187-201
- Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Makrantonaki, E.; Zouboulis, C.C. Skin anti-aging strategies. Dermatoendocrinol 2012, 4, 308-319
- Ramos-e-Silva, M.; Celem, L.R.; Ramos-e-Silva, S.; Fucci-da-Costa, A.P. Anti-aging cosmetics: facts and controversies. Clin Dermatol 2013, 31, 750-758
- Sawyer, T.K.; Sanfilippo, P.J.; Hruby, V.J.; Engel, M.H.; Heward, C.B.; Burnett, J.B.; Hadley, M.E. 4-Norleucine, 7-D-phenylalanine-alpha-melanocyte-stimulating hormone: a highly potent alpha-melanotropin with ultralong biological activity. Proc Natl Acad Sci U S A 1980, 77, 5754-5758
- Castrucci, A.M.; Almeida, A.L.; al-Obeidi, F.A.; Hadley, M.E.; Hruby, V.J.; Staples, D.J.; Sawyer, T.K. Comparative biological activities of alpha-MSH antagonists in vertebrate pigment cells. Gen Comp Endocrinol 1997, 105, 410-416
- Abdel-Malek, Z.A.; Kadekaro, A.L.; Kavanagh, R.J.; Todorovic, A.; Koikov, L.N.; McNulty, J.C.; Jackson, P.J.; Millhauser, G.L.; Schwemberger, S.; Babcock, G.; Haskell-Luevano, C.; Knittel, J.J. Melanoma prevention strategy based on using tetrapeptide alpha-MSH analogs that protect human melanocytes from UV-induced DNA damage and cytotoxicity. Faseb Journal 2006, 20, 1561-1563
- Jackson, E.; Heidl, M.; Imfeld, D.; Meeus, L.; Schuetz, R.; Campiche, R. Discovery of a Highly Selective MC1R Agonists Pentapeptide to Be Used as a Skin Pigmentation Enhancer and with Potential Anti-Aging Properties. International Journal of Molecular Sciences 2019, 20, 6143
- Slominski, A.; Zmijewski, M.A.; Pawelek, J. L-tyrosine and L-dihydroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment Cell Melanoma Res 2012, 25, 14-27
- Yuan, X.H.; Yao, C.; Oh, J.H.; Park, C.H.; Tian, Y.D.; Han, M.; Kim, J.E.; Chung, J.H.; Jin, Z.H.; Lee, D.H. Vasoactive intestinal peptide stimulates melanogenesis in B16F10 mouse melanoma cells via CREB/MITF/tyrosinase signaling. Biochemical and Biophysical Research Communications 2016, 477, 336-342
- Liu, L.H.; Fan, X.; Li, H.T.; An, X.X.; Yang, R.Y. Angiotensin II promotes melanogenesis via angiotensin II type 1 receptors in human melanocytes. Mol Med Rep 2015, 12, 651-656
- Kahn, V. Effect of Proteins, Protein Hydrolyzates and Amino Acids on o-Dihydroxyphenolase Activity of Polyphenol Oxidase of Mushroom, Avocado, and Banana. Journal of Food Science 1985, 50, 111-115
- Liao, W.C.; Wu, W.H.; Tsai, P.C.; Wang, H.F.; Liu, Y.H.; Chan, C.F. Kinetics of Ergothioneine Inhibition of Mushroom Tyrosinase. Applied Biochemistry and Biotechnology 2012, 166, 259-267
- Girelli, A.M.; Mattei, E.; Messina, A.; Tarola, A.M. Inhibition of polyphenol oxidases activity by various dipeptides. Journal of Agricultural and Food Chemistry 2004, 52, 2741-2745
- Tseng, T.S.; Tsai, K.C.; Chen, W.C.; Wang, Y.T.; Lee, Y.C.; Lu, C.K.; Don, M.J.; Chang, C.Y.; Lee, C.H.; Lin, H.H.; Hsu, H.J.; Hsiao, N.W. Discovery of Potent Cysteine-Containing Dipeptide Inhibitors against Tyrosinase: A Comprehensive Investigation of 20 x 20 Dipeptides in Inhibiting Dopachrome Formation. J Agric Food Chem 2015, 63, 6181-6188
- Hsiao, N.W.; Tseng, T.S.; Lee, Y.C.; Chen, W.C.; Lin, H.H.; Chen, Y.R.; Wang, Y.T.; Hsu, H.J.; Tsai, K.C. Serendipitous Discovery of Short Peptides from Natural Products as Tyrosinase Inhibitors. Journal of Chemical Information and Modeling 2014, 54, 3099-3111
- Luisi, G.; Stefanucci, A.; Zengin, G.; Dimmito, M.P.; Mollica, A. Anti-Oxidant and Tyrosinase Inhibitory In Vitro Activity of Amino Acids and Small Peptides: New Hints for the Multifaceted Treatment of Neurologic and Metabolic Disfunctions. Antioxidants (Basel) 2018, 8, 7
- Abu Ubeid, A.; Zhao, L.; Wang, Y.; Hantash, B.M. Short-sequence oligopeptides with inhibitory activity against mushroom and human tyrosinase. J Invest Dermatol 2009, 129, 2242-2249
- Abu Ubeid, A.; Do, S.; Nye, C.; Hantash, B.M. Potent low toxicity inhibition of human melanogenesis by novel indole-containing octapeptides. Biochimica Et Biophysica Acta-General Subjects 2012, 1820, 1481-1489
- Park, J.; Jung, H.; Kim, K.; Lim, K.M.; Kim, J.Y.; Jho, E.H.; Oh, E.S. D-tyrosine negatively regulates melanin synthesis by competitively inhibiting tyrosinase activity. Pigment Cell Melanoma Res 2018, 31, 374-383
- Park, J.; Jung, H.; Jang, B.; Song, H.K.; Han, I.O.; Oh, E.S. D-tyrosine adds an anti-melanogenic effect to cosmetic peptides. Sci Rep 2020, 10, 262
- Arjinpathana, N.; Asawanonda, P. Glutathione as an oral whitening agent: A randomized, double-blind, placebo-controlled study. Journal of Dermatological Treatment 2012, 23, 97-102
- Watanabe, F.; Hashizume, E.; Chan, G.P.; Kamimura, A. Skin-whitening and skin-condition-improving effects of topical oxidized glutathione: a double-blind and placebo-controlled clinical trial in healthy women. Clin Cosmet Investig Dermatol 2014, 7, 267-274
- Morita, H.; Kayashita, T.; Kobata, H.; Gonda, A.; Takeya, K.; Itokawa, H. Pseudostellarins A-C, New Tyrosinase Inhibitory Cyclic-Peptides from Pseudostellaria-Heterophylla. Tetrahedron 1994, 50, 6797-6804
- Morita, H.; Kayashita, T.; Kobata, H.; Gonda, A.; Takeya, K.; Itokawa, H. Pseudostellarins D-F, New Tyrosinase Inhibitory Cyclic-Peptides from Pseudostellaria-Heterophylla. Tetrahedron 1994, 50, 9975-9982
- Schurink, M.; van Berkel, W.J.; Wichers, H.J.; Boeriu, C.G. Novel peptides with tyrosinase inhibitory activity. Peptides 2007, 28, 485-495
- Ochiai, A.; Tanaka, S.; Tanaka, T.; Taniguchi, M. Rice Bran Protein as a Potent Source of Antimelanogenic Peptides with Tyrosinase Inhibitory Activity. Journal of Natural Products 2016, 79, 2545-2551
- Kubglomsong, S.; Theerakulkait, C.; Reed, R.L.; Yang, L.; Maier, C.S.; Stevens, J.F. Isolation and Identification of Tyrosinase-Inhibitory and Copper-Chelating Peptides from Hydrolyzed Rice-Bran-Derived Albumin. J Agric Food Chem 2018, 66, 8346-8354
- Shen, Z.; Wang, Y.; Guo, Z.; Tan, T.; Zhang, Y. Novel tyrosinase inhibitory peptide with free radical scavenging ability. J Enzyme Inhib Med Chem 2019, 34, 1633-1640
- Joompang, A.; Jangpromma, N.; Choowongkomon, K.; Payoungkiattikun, W.; Tankrathok, A.; Viyoch, J.; Luangpraditkun, K.; Klaynongsruang, S. Evaluation of tyrosinase inhibitory activity and mechanism of Leucrocin I and its modified peptides. J Biosci Bioeng 2020,
- Noh, J.M.; Kwak, S.Y.; Kim, D.H.; Lee, Y.S. Kojic acid-tripeptide amide as a new tyrosinase inhibitor. Biopolymers 2007, 88, 300-307
- Noh, J.M.; Kwak, S.Y.; Seo, H.S.; Seo, J.H.; Kim, B.G.; Lee, Y.S. Kojic acid-amino acid conjugates as tyrosinase inhibitors. Bioorganic & Medicinal Chemistry Letters 2009, 19, 5586-5589
- Singh, B.K.; Park, S.H.; Lee, H.B.; Goo, Y.A.; Kim, H.S.; Cho, S.H.; Lee, J.H.; Ahn, G.W.; Kim, J.P.; Kang, S.M.; Kim, E.K. Kojic Acid Peptide: A New Compound with Anti-Tyrosinase Potential. Ann Dermatol 2016, 28, 555-561
- Noh, J.M.; Lee, Y.S. Inhibitory activities of hydroxyphenolic acid-amino acid conjugates on tyrosinase. Food Chemistry 2011, 125, 953-957
- Yang, J.K.; Lee, E.; Hwang, I.J.; Yim, D.; Han, J.; Lee, Y.S.; Kim, J.H. beta-Lactoglobulin Peptide Fragments Conjugated with Caffeic Acid Displaying Dual Activities for Tyrosinase Inhibition and Antioxidant Effect. Bioconjugate Chemistry 2018, 29, 1000-1005
- Park, K.-Y.; Kim, J. Synthesis and Biological Evaluation of the Anti-Melanogenesis Effect of Coumaric and Caffeic Acid-Conjugated Peptides in Human Melanocytes. Frontiers in Pharmacology 2020, 11, 922
- Choi, H.I.; Park, J.I.; Kim, H.J.; Kim, D.W.; Kim, S.S. A novel L-ascorbic acid and peptide conjugate with increased stability and collagen biosynthesis. Bmb Reports 2009, 42, 743-746
- Castrucci, A.M.; Sherbrooke, W.C.; Sawyer, T.K.; Staples, D.J.; Tuma, M.C.; Hadley, M.E. Discovery of an alpha-melanotropin antagonist effective in vivo. Peptides 1994, 15, 627-632
- Choi, H.R.; Kang, Y.A.; Lee, H.S.; Park, K.C. Disulfanyl peptide decreases melanin synthesis via receptor-mediated ERK activation and the subsequent downregulation of MITF and tyrosinase. Int J Cosmet Sci 2015, 38, 279-285
- Lee, S.J.; Park, S.G.; Chung, H.M.; Choi, J.S.; Kim, D.D.; Sung, J.H. Antioxidant and Anti-Melanogenic Effect of the Novel Synthetic Hexapeptide (SFKLRY-NH2). International Journal of Peptide Research and Therapeutics 2009, 15, 281-286
- Seok, J.K.; Lee, S.W.; Choi, J.; Kim, Y.M.; Boo, Y.C. Identification of novel antimelanogenic hexapeptides via positional scanning of a synthetic peptide combinatorial library. Exp Dermatol 2017, 26, 742-744
- Kim, J.H.; Seok, J.K.; Kim, Y.M.; Boo, Y.C. Identification of small peptides and glycinamide that inhibit melanin synthesis using a positional scanning synthetic peptide combinatorial library. Br J Dermatol 2019, 181, 128-137
- Boo, Y.C.; Jo, D.J.; Oh, C.M.; Lee, S.Y.; Kim, Y.M. The First Human Clinical Trial on the Skin Depigmentation Efficacy of Glycinamide Hydrochloride. Biomedicines 2020, 8, 257
- Campagne, C.; Ripoll, L.; Gilles-Marsens, F.; Raposo, G.; Delevoye, C. AP-1/KIF13A Blocking Peptides Impair Melanosome Maturation and Melanin Synthesis. International Journal of Molecular Sciences 2018, 19, 568
- Kim, J.Y.; Kim, J.; Ahn, Y.; Lee, E.J.; Hwang, S.; Almurayshid, A.; Park, K.; Chung, H.J.; Kim, H.J.; Lee, S.H.; Lee, M.S.; Oh, S.H. Autophagy induction can regulate skin pigmentation by causing melanosome degradation in keratinocytes and melanocytes. Pigment Cell Melanoma Res 2020, 33, 403-415
- Dorr, R.T.; Ertl, G.; Levine, N.; Brooks, C.; Bangert, J.L.; Powell, M.B.; Humphrey, S.; Alberts, D.S. Effects of a superpotent melanotropic peptide in combination with solar UV radiation on tanning of the skin in human volunteers. Archives of Dermatology 2004, 140, 827-835
- Kreim, S.; Lautenschlager, S.; Minder, E. Safety and efficacy of an agonistic alpha-melanocyte stimulating hormone analogue, afamelanotide (Scenesse (R)), in treating patients with erythropoietic protoporphyria for 2.5 consecutive years. British Journal of Dermatology 2011, 164, 1149-1149
- Boo, Y.C. Emerging Strategies to Protect the Skin from Ultraviolet Rays Using Plant-Derived Materials. Antioxidants (Basel) 2020, 9, 637
- Boo, Y.C. p-Coumaric Acid as An Active Ingredient in Cosmetics: A Review Focusing on its Antimelanogenic Effects. Antioxidants (Basel) 2019, 8, 275
- Boo, Y.C. Human Skin Lightening Efficacy of Resveratrol and Its Analogs: From in Vitro Studies to Cosmetic Applications. Antioxidants (Basel) 2019, 8, 332