| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Anna-Jasmina Donaubauer | + 1934 word(s) | 1934 | 2020-09-03 08:37:38 | | | |
| 2 | Vicky Zhou | + 1 word(s) | 1935 | 2020-09-10 10:55:01 | | | | |
| 3 | Vicky Zhou | + 1 word(s) | 1935 | 2020-09-10 11:12:44 | | | | |
| 4 | Vicky Zhou | Meta information modification | 1935 | 2020-10-27 09:44:21 | | |
Video Upload Options
The bone is a complex organ that is dependent on a tight regulation between bone formation by osteoblasts (OBs) and bone resorption by osteoclasts (OCs). The OC is a multinucleated giant cell, arising from the fusion of many mononuclear OC precursors with a myeloid/monocyte origin. Differently from OCs, OBs arise from pluripotent mesenchymal stem cells (MSCs). The main function of OBs is the synthesis of new bone matrix. Bone metabolism is regulated by various hormones or cytokines and dysregulation in this complex system can lead to numerous diseases characterized either by enhanced bone resorption (osteoporotic phenotype) or enhanced bone formation (osteopetrotic phenotype).
1. Introduction
1.1 Osteoclast Differentiation and Function
Osteoclastogenesis from precursor cells is a strictly regulated differentiation process. Similar to all other hematopoietic lineage cells, OCs originate from hematopoietic stem cells (HSCs) in the bone marrow. More precisely, OC progenitors differentiate from the common myeloid progenitor lineage (CMP). Cells of this lineage give rise to monocytes and macrophages, dendritic cells and to osteoclasts[1]. In the bone, the stimulation of the progenitor cells with the cytokines receptor activator of nuclear factor kappa B ligand (RANK-L) and macrophage colony-stimulating factor (M-CSF), amongst other factors, triggers a further differentiation into pre-osteoclasts. Here, M-CSF serves as a key survival signal, whereas RANK-L mainly induces OC specific differentiation processes in progenitor cells[1][2]. In the bone, osteocytes, as well as OBs, but also immune cells, serve as a source of RANKL, creating a local microenvironment that enables osteoclastogenesis specifically in bone tissue[3]. In the differentiating cell, these cytokine signals lead to an activation of nuclear factor kappa-light-chain-enhancer of activated B-cells (NF-ƙB), an essential transcription factor for osteoclastogenesis. NF-ƙB activation in turn, leads to an activation of c-fos and nuclear factor of activated T cells 1 (NFATc1), the master transcription factor for osteoclastogenesis. Due to RANK-L stimulation, the expression of NFATc1 is strongly upregulated by autoamplification. Finally, OC-specific genes are induced, amongst them are dendritic cell-specific transmembrane protein (Dcstamp), v-type proton ATPase subunit d2 (Atp6v0d2), tartate-resistant acid phosphatase (Acp5, TRAP) or Cathepsin K (Ctsk) enabling common OC functions [1]. By now, a mononuclear OC has formed.
For efficient bone resorption, the formation of multinucleated giant cells from the mononuclear osteoclast precursor cell is essential. For these differentiation processes, excessive arrangements occur in the mononuclear osteoclasts. Similar to the previous described differentiation processes, also OC multinucleation is a tightly regulated process. Amongst others, DC-STAMP and OC-STAMP (osteoclast stimulatory transmembrane protein) are essential fusogens in OC multinucleation. In summary, the multinucleation can be divided into three main steps. First, the cells need to come into close proximity by chemotaxis, adhere to one another and finally become multinucleated cells by membrane fusion. The chemotactic approximation of the cells is mainly mediated through C-C motif chemokine ligand 2 (CCL2) signaling. Second, the approached cells subsequently adhere to one another by the expression of integrins and E-cadherin which initiates cytoskeletal rearrangements preparing the cellular fusion. Third, as the actin cytoskeleton reorganizes, membrane fusion is enabled leading to cell multinucleation. Finally, a fully functional, multinucleated OC has formed[4].
Size, as well as the number of nuclei correlates positively with the bone resorbing activities of OCs[5][6]. These highly specialized cells cannot only degrade the inorganic, mineralized components of the bone, which consists mainly of hydroxyapartite, but also the organic components of the matrix, which are composed mainly of collagen[7][8]. By adhering tightly to the bone surface via integrins, the OC generates a sealed resorption pit. This sealed zone is further supported by the reorganization of the actin cytoskeleton, in order to form an actin ring for an even stronger adhesion. In addition, the OC gets polarized and forms a ruffled border structure at the site of bone resorption. The fully differentiated OC secretes H+ through vacuolar ATPases into the resorption pit to resolve the inorganic components. Moreover, a variety of proteinases are secreted to dissociate extracellular matrix proteins. The enzymes for degradation are secreted via vesicle trafficking into the resorption pit, while degradation products of the bone matrix are finally endocytosed by the OC. Cathepsins (Cts) as well as matrixmetalloproteinases (MMPs) are the best-characterized enzymes involved [8][9].
1.2. Osteoblast Differentiation and Function
For the proper functionality of OCs, their tightly regulated interaction with bone forming OBs is necessary. Differently from OCs, OBs arise from pluripotent mesenchymal stem cells (MSCs). The main function of OBs is the synthesis of new bone matrix. In detail, OBs secrete organic matrix components, such as collagens and proteoglycans to form the osteoid. Moreover, OBs secrete hydroxyapatite crystals via vesicles into the extracellular space, in order to build the inorganic component of the bone matrix. Amongst those functions, OBs also have a key role in the regulation of osteoclastogenesis. Here, they stimulate the differentiation of pre-OCs via the secretion of RANK-L, and MCS-F[10]. Bone, as a complex organ contains numerous cell types, additionally to the bone forming and resorbing cells. Next to OCs and OBs, osteocytes serve as major component of bone. In this organ, osteocytes function as mechanosensory cells that pass their signals down to OBs and OCs in order to impact on bone homeostasis. Osteocytes develop from mature OBs that become embedded in the secreted bone matrix. These cells orchestrate bone resorption and formation, as the mechano-sensation leads to an adaption in the rate of bone formation and secretion. High mechanical loads trigger bone formation and reduce bone resorption, whereas mechanical unloading, e.g. in space travel, leads to the opposite effects. Appropriately, osteocytes can impact directly on OC, as well as OB differentiation and activity[10][11].
2. The Influence of Radiation on Bone Remodeling Cells
2.1 The Influence of High Doses of Ionizing Radiation
Bone homeostasis, especially OC and OB differentiation and functionality are tightly regulated processes. Consequently, those processes are prone to numerous disruptive factors. Internal factors such as hormonal imbalances during menopause or rheumatic diseases can cause severe skeletal transformation[12][13]. On the other hand, also external influences can affect the bone in general, as well as OCs and OBs. Those factors include medical drugs, mechanical stress but also environmental or therapeutically delivered ionizing radiation (IR)[14][15][16][17].
As IR can induce cell death, radiation therapy (RT) is employed in cancer treatment[18][19]. By irradiating tumors with fractionated high doses of IR (single dose: ≥ 2 Gy; total dose: 50–70 Gy), IR accumulates in the tumor tissue, leading to the death or senescence of fast dividing, malignant cells. Even though IR is delivered with a high precision to the target tissue, the damage of adjacent healthy cells cannot be prevented completely[20]. As well, the bone is often affected by radiation damage. Consequently, many cancer patients undergoing RT suffer from side effects ranging from radiation osteitis to more severe complications such as fractures. Such complications have been described in numerous entities such as breast or brain cancer or in pelvic tumor diseases[17][21][22][23]. Moreover, osteopenia or even osteoporosis are side effects that have been unavoidable after RT in distinct constellations[24].
Numerous studies suggest that bone damage is mainly due to IR-induced defects in bone-forming OBs. Since OBs are essential for a proper OC differentiation and thereby for a functional bone homeostasis, radiation effects on OBs will affect OC biology at least indirectly. IR influences on OB functionality, such as on collagen production, but also on OB proliferation. Furthermore, IR is reported to induce cell cycle arrest in OBs[25][26][27]. Likewise, numerous studies on animal models reported a reduced deposition of bone matrix after RT, coming along with either stable or decreasing OB cell numbers.
The studies on IR effects on OCs reveal primarily conflicting results. Several published studies report either decreasing or stable OC numbers after RT[28][29][30][31][32]. Surprisingly, numerous reports even described increasing OC numbers following RT, suggesting that OCs may be key players in radiation-induced bone loss. In accordance with this, exposure to IR leads to an upregulation of the expression of OC marker genes in marrow tissue[33]. These findings can be supported from rodent studies. As high doses of IR induce a damage response and inflammatory processes in affected tissues such as bone, the secretion of pro-inflammatory cytokines e.g. tumor necrosis factor (TNF) α, interleukin (IL) -6 and IL-1 may be stimulated. The differentiation of OCs in turn, can be increased in such a rather pro-inflammatory milieu, as TNF-α and IL-1 directly stimulate the expression of RANK-L[34].
In summary, the deleterious effects of high doses of IR in cancer therapy are well described, whereas the underlying cellular mechanisms remain mostly elusive, as the effects on bone forming cells are not well understood.
2.2 The Influence of Low Doses of Ionizing Radiation
The therapeutic application of IR is not limited to cancer therapy. If delivered in low doses, IR does not induce cell death, senescence or inflammatory processes, but ameliorates preexisting inflammation[35]. Today, low doses of X-rays or α radiation are commonly applied for the therapy of chronic degenerative or inflammatory diseases, such as arthrosis or rheumatoid arthritis (single dose: ≤ 1 Gy; total dose: 3 – 6 Gy). Even though exposure to low doses of IR can have analgesic and immunomodulatory effects, the underlying osteoimmunological mechanisms are not understood in detail. As bone destruction and remodeling are a hallmark of chronic degenerative and inflammatory diseases, the effects of low doses of IR on bone and bone forming cells are subject of current research. Low doses of IR are modulating the immune system by various molecular mechanisms and impact thereby also on bone homeostasis.
As OBs produce bone matrix, they can potentially counteract the destructive processes in degenerative and inflammatory diseases. Therefore, OBs are a promising target in the therapy of chronic degenerative and inflammatory diseases. Appropriately, exposure to low doses of IR can have stimulatory effects on OBs. In detail, low dose IR is reported to induce OB proliferation, as well as OB differentiation and functionality. These findings are supported by in vitro, as well as animal studies, especially when doses between 0.5 and 1.0 Gy were applied. Even fracture healing can be promoted after exposure to low doses of X-rays in rats[36].
For OCs on the other hand, it is hypothesized that low doses of IR have a positive impact on bone homeostasis, as OCs are inhibited in their viability and their function. In accordance with this, numerous publications confirm the inhibitive effect of low doses of IR on OC differentiation and functionality. A possible mechanism of action might be the downregulation of the production of cellular reactive oxygen species that are known to stimulate osteoclastogenesis.
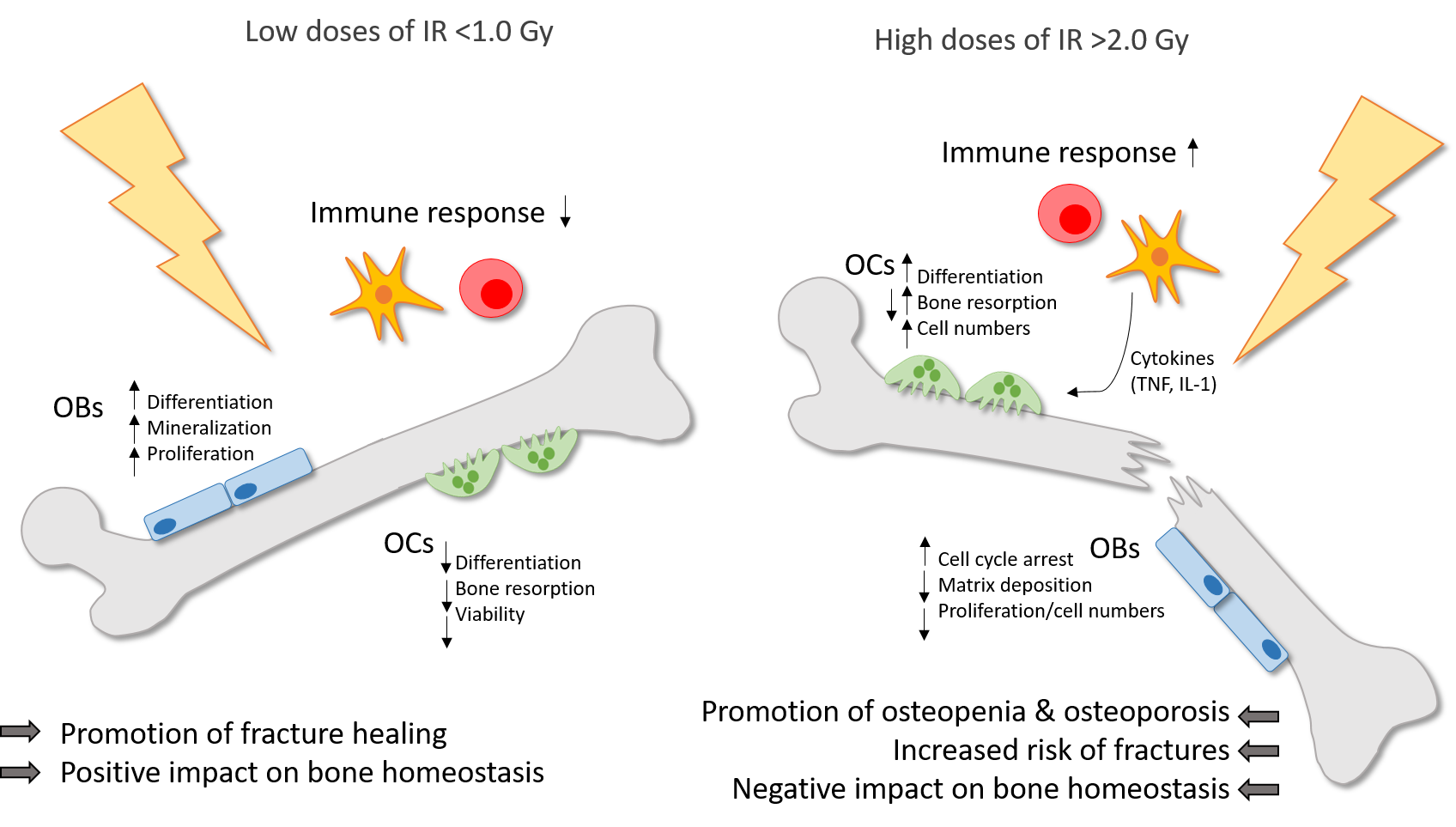
In summary, low doses of IR have positive effects on the bone homeostasis, especially in inflammatory settings. OBs, as well as OCs are modulated leading to promoted bone formation and reduced bone resorption. The figure below summarizes these key findings.
Figure 1. Ionizing radiation (IR) induces differential effects on bone in dependence of the dose. Low doses of IR (<1.0 Gy) are commonly applied for the therapy of chronic degenerative and inflammatory diseases and can counteract the destructive processes on bone with a positive impact on bone homeostasis and a promotion of fracture healing. Preexisting inflammatory processes are downregulated by low doses of IR. Also, the differentiation, mineralization and proliferation of OBs is stimulated. On the contrary, the differentiation, viability and bone resorbing capacity of OCs is reduced. In high doses (>2.0 Gy), IR is applied usually in cancer therapy and has a negative impact on bone homeostasis with a higher risk of osteopenia and osteoporosis and finally an increased fracture risk. In OCs, the differentiation, bone resorption and cell numbers are enhanced, whereas in OBs, the matrix deposition and proliferation rate in reduced and cell cycle arrest is induced. Also, high doses of IR induce the secretion of pro-inflammatory cytokines by immune cells, which in turn can stimulate OCs.
References
- Ono, T.; Nakashima, T. Recent advances in osteoclast biology. Histochem. Cell Biol. 2018, 149, 325–341, doi:10.1007/s00418-018-1636-2.
- Kim, N.; Takami, M.; Rho, J.; Josien, R.; Choi, Y. A novel member of the leukocyte receptor complex regulates osteoclast differentiation. J. Exp. Med. 2002, 195, 201–209, doi:10.1084/jem.20011681.
- Nakashima, T.; Hayashi, M.; Fukunaga, T.; Kurata, K.; Oh-Hora, M.; Feng, J.Q.; Bonewald, L.F.; Kodama, T.; Wutz, A.; Wagner, E.F.; et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat. Med. 2011, 17, 1231–1234, doi:10.1038/nm.2452.
- Pereira, M.; Petretto, E.; Gordon, S.; Bassett, J.H.D.; Williams, G.R.; Behmoaras, J. Common signalling pathways in macrophage and osteoclast multinucleation. J. Cell Sci. 2018, 131, doi:10.1242/jcs.216267.
- Lees, R.L.; Sabharwal, V.K.; Heersche, J.N. Resorptive state and cell size influence intracellular pH regulation in rabbit osteoclasts cultured on collagen-hydroxyapatite films. Bone 2001, 28, 187–194, doi:10.1016/s8756-3282(00)00433-6.
- Yagi, M.; Miyamoto, T.; Sawatani, Y.; Iwamoto, K.; Hosogane, N.; Fujita, N.; Morita, K.; Ninomiya, K.; Suzuki, T.; Miyamoto, K.; et al. DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J. Exp. Med. 2005, 202, 345–351, doi:10.1084/jem.20050645.
- Clarke, B. Normal bone anatomy and physiology. Clin. J. Am. Soc. Nephrol. 2008, 3 (Suppl. 3), S131–S139, doi:10.2215/CJN.04151206.
- Vaananen, H.K.; Zhao, H.; Mulari, M.; Halleen, J.M. The cell biology of osteoclast function. J. Cell Sci. 2000, 113, 377–381.
- Henriksen, K.; Bollerslev, J.; Everts, V.; Karsdal, M.A. Osteoclast activity and subtypes as a function of physiology and pathology—Implications for future treatments of osteoporosis. Endocr. Rev. 2011, 32, 31–63, doi:10.1210/er.2010-0006.
- Capulli, M.; Paone, R.; Rucci, N. Osteoblast and osteocyte: Games without frontiers. Arch. Biochem. Biophys. 2014, 561, 3–12, doi:10.1016/j.abb.2014.05.003.
- Uda, Y.; Azab, E.; Sun, N.; Shi, C.; Pajevic, P.D. Osteocyte Mechanobiology. Curr. Osteoporos. Rep. 2017, 15, 318–325, doi:10.1007/s11914-017-0373-0.
- Weitzmann, M.N. The Role of Inflammatory Cytokines, the RANKL/OPG Axis, and the Immunoskeletal Interface in Physiological Bone Turnover and Osteoporosis. Scientifica 2013, 2013, 125705, doi:10.1155/2013/125705.
- Almeida, M.; Laurent, M.R.; Dubois, V.; Claessens, F.; O’Brien, C.A.; Bouillon, R.; Vanderschueren, D.; Manolagas, S.C. Estrogens and Androgens in Skeletal Physiology and Pathophysiology. Physiol. Rev. 2017, 97, 135–187, doi:10.1152/physrev.00033.2015.
- Rachner, T.D.; Khosla, S.; Hofbauer, L.C. Osteoporosis: Now and the future. Lancet 2011, 377, 1276–1287, doi:10.1016/S0140-6736(10)62349-5.
- Prisby, R.D. Mechanical, hormonal and metabolic influences on blood vessels, blood flow and bone. J. Endocrinol. 2017, 235, R77–R100, doi:10.1530/JOE-16-0666.
- Shanmugarajan, S.; Zhang, Y.; Moreno-Villanueva, M.; Clanton, R.; Rohde, L.H.; Ramesh, G.T.; Sibonga, J.D.; Wu, H. Combined Effects of Simulated Microgravity and Radiation Exposure on Osteoclast Cell Fusion. Int. J. Mol. Sci. 2017, 18, 2443, doi:10.3390/ijms18112443.
- Baxter, N.N.; Habermann, E.B.; Tepper, J.E.; Durham, S.B.; Virnig, B.A. Risk of pelvic fractures in older women following pelvic irradiation. JAMA 2005, 294, 2587–2593, doi:10.1001/jama.294.20.2587.
- Delaney, G.; Jacob, S.; Featherstone, C.; Barton, M. The role of radiotherapy in cancer treatment: Estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer 2005, 104, 1129–1137, doi:10.1002/cncr.21324.
- Haussmann, J.; Tamaskovics, B.; Bolke, E.; Djiepmo-Njanang, F.J.; Kammers, K.; Corradini, S.; Hautmann, M.; Ghadjar, P.; Maas, K.; Schuler, P.J.; et al. Addition of chemotherapy to hyperfractionated radiotherapy in advanced head and neck cancer-a meta-analysis. Strahlenther. Onkol. 2019, 195, 1041–1049, doi:10.1007/s00066-019-01511-z.
- Thompson, M.K.; Poortmans, P.; Chalmers, A.J.; Faivre-Finn, C.; Hall, E.; Huddart, R.A.; Lievens, Y.; Sebag-Montefiore, D.; Coles, C.E. Practice-changing radiation therapy trials for the treatment of cancer: Where are we 150 years after the birth of Marie Curie? Br. J. Cancer 2018, 119, 389–407, doi:10.1038/s41416-018-0201-z.
- Darzy, K.H.; Shalet, S.M. Hypopituitarism after cranial irradiation. J. Endocrinol. Investig. 2005, 28, 78–87.
- Langlands, A.O.; Souter, W.A.; Samuel, E.; Redpath, A.T. Radiation osteitis following irradiation for breast cancer. Clin. Radiol. 1977, 28, 93–96, doi:10.1016/s0009-9260(77)80134-7.
- Banfi, A.; Bianchi, G.; Galotto, M.; Cancedda, R.; Quarto, R. Bone marrow stromal damage after chemo/radiotherapy: Occurrence, consequences and possibilities of treatment. Leuk. Lymphoma 2001, 42, 863–870, doi:10.3109/10428190109097705.
- Ergun, H.; Howland, W.J. Postradiation atrophy of mature bone. CRC Crit. Rev. Diagn. Imaging 1980, 12, 225–243.
- Sakurai, T.; Sawada, Y.; Yoshimoto, M.; Kawai, M.; Miyakoshi, J. Radiation-induced reduction of osteoblast differentiation in C2C12 cells. J. Radiat. Res. 2007, 48, 515–521, doi:10.1269/jrr.07012.
- Gal, T.J.; Munoz-Antonia, T.; Muro-Cacho, C.A.; Klotch, D.W. Radiation effects on osteoblasts in vitro: A potential role in osteoradionecrosis. Arch. Otolaryngol. Head Neck Surg. 2000, 126, 1124–1128, doi:10.1001/archotol.126.9.1124.
- Szymczyk, K.H.; Shapiro, I.M.; Adams, C.S. Ionizing radiation sensitizes bone cells to apoptosis. Bone 2004, 34, 148–156, doi:10.1016/j.bone.2003.09.003.
- Sawajiri, M.; Mizoe, J.; Tanimoto, K. Changes in osteoclasts after irradiation with carbon ion particles. Radiat. Environ. Biophys. 2003, 42, 219–223, doi:10.1007/s00411-003-0204-9.
- Goblirsch, M.; Lynch, C.; Mathews, W.; Manivel, J.C.; Mantyh, P.W.; Clohisy, D.R. Radiation treatment decreases bone cancer pain through direct effect on tumor cells. Radiat. Res. 2005, 164, 400–408, doi:10.1667/rr3439.1.
- Vit, J.P.; Ohara, P.T.; Tien, D.A.; Fike, J.R.; Eikmeier, L.; Beitz, A.; Wilcox, G.L.; Jasmin, L. The analgesic effect of low dose focal irradiation in a mouse model of bone cancer is associated with spinal changes in neuro-mediators of nociception. Pain 2006, 120, 188–201, doi:10.1016/j.pain.2005.10.033.
- Anderson, N.D.; Colyer, R.A.; Riley, L.H., Jr. Skeletal changes during prolonged external irradiation: Alterations in marrow, growth plate and osteoclast populations. Johns Hopkins Med. J. 1979, 145, 73–83.
- Scheven, B.A.; Burger, E.H.; Kawilarang-de Haas, E.W.; Wassenaar, A.M.; Nijweide, P.J. Effects of ionizing irradiation on formation and resorbing activity of osteoclasts in vitro. Lab. Investig. 1985, 53, 72–79.
- Alwood, J.S.; Shahnazari, M.; Chicana, B.; Schreurs, A.S.; Kumar, A.; Bartolini, A.; Shirazi-Fard, Y.; Globus, R.K. Ionizing Radiation Stimulates Expression of Pro-Osteoclastogenic Genes in Marrow and Skeletal Tissue. J. Interferon Cytokine Res. 2015, 35, 480–487, doi:10.1089/jir.2014.0152.
- Willey, J.S.; Lloyd, S.A.; Nelson, G.A.; Bateman, T.A. Ionizing Radiation and Bone Loss: Space Exploration and Clinical Therapy Applications. Clin. Rev. Bone Miner. Metab. 2011, 9, 54–62, doi:10.1007/s12018-011-9092-8.
- Rodel, F.; Frey, B.; Gaipl, U.; Keilholz, L.; Fournier, C.; Manda, K.; Schollnberger, H.; Hildebrandt, G.; Rodel, C. Modulation of inflammatory immune reactions by low-dose ionizing radiation: Molecular mechanisms and clinical application. Curr. Med. Chem. 2012, 19, 1741–1750, doi:10.2174/092986712800099866.
- Xu, W.; Xu, L.; Chen, M.; Mao, Y.T.; Xie, Z.G.; Wu, S.L.; Dong, Q.R. The effects of low dose X-irradiation on osteoblastic MC3T3-E1 cells in vitro. BMC Musculoskelet. Disord. 2012, 13, 94, doi:10.1186/1471-2474-13-94.