
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Izabela Mlynarczuk-Bialy | + 2039 word(s) | 2039 | 2020-09-07 04:19:01 | | | |
| 2 | Dean Liu | -615 word(s) | 1424 | 2020-09-08 05:50:49 | | | | |
| 3 | Dean Liu | Meta information modification | 1424 | 2020-09-08 05:59:13 | | |
Video Upload Options
Entosis is a phenomenon, in which one cell enters a second one. New clinico-histopathological studies of entosis prompted us to summarize its significance in cancer. It appears that entosis might be a novel, independent prognostic predictor factor in cancer histopathology. We briefly discuss the biological basis of entosis, followed by a summary of published clinico-histopathological studies on entosis significance in cancer prognosis. The correlation of entosis with cancer prognosis in head and neck squamous cell carcinoma, anal carcinoma, lung adenocarcinoma, pancreatic ductal carcinoma and breast ductal carcinoma, is shown. Numerous entotic figures are associated with a more malignant cancer phenotype and poor prognosis in many cancers. We also showed that some anticancer drugs could induce entosis in cell culture, even as an escape mechanism. Thus, entosis is likely beneficial for survival of malignant cells, i.e., an entotic cell can hide from unfavourable factors in another cell and subsequently leave the host cell remaining intact, leading to failure in therapy or cancer recurrence. Finally, we highlight the potential relationship of cell adhesion with entosis in vitro, based on the model of the BxPc3 cells cultured in full adhesive conditions, comparing them to a commonly used MCF7 semiadhesive model of entosis.
1. Introduction
Entosis refers to the invasion of one living cell into another of the same type with involvement of adhesion molecules, actin cytoskeleton and expenditure of energy [1][2],. The term entosis was first defined by Overholtzer in 2007, as a new type of cell death[2]. Entosis is derived from the Greek word that means “inside” or “within”, based on the observation that one viable cell invades the other viable cell. To simplify the nomenclature in this article, we refer to the internalized or engulfed cell as “inner”, while the internalizing or engulfing cell is referred to as the “outer” cell. Entosis is characteristic for epithelial cells and epithelial cancers, and is triggered by detachment of cells from the basement membrane. As a result of entosis, characteristic cell-in-cell (CIC) structures are formed[3]. Once engulfed, entotic cells can be eliminated through regulated cell death within the entotic vacuole (entosome), via a specific autophagy-related process, commonly known as LC3-associated phagocytosis (LAP)[4]. This process is independent of the apoptotic pathway[2]. However, various fates of entosis are possible (see Figure 1). The inner entotic cell can undergo division within the host cell or can escape from the host cell, without any signs of degeneration[1][2]. Therefore, the European Cell Death Organization (ECDO) questioned whether entosis is a form of cell death, since one cell can exist for an extended time within the other one[5].
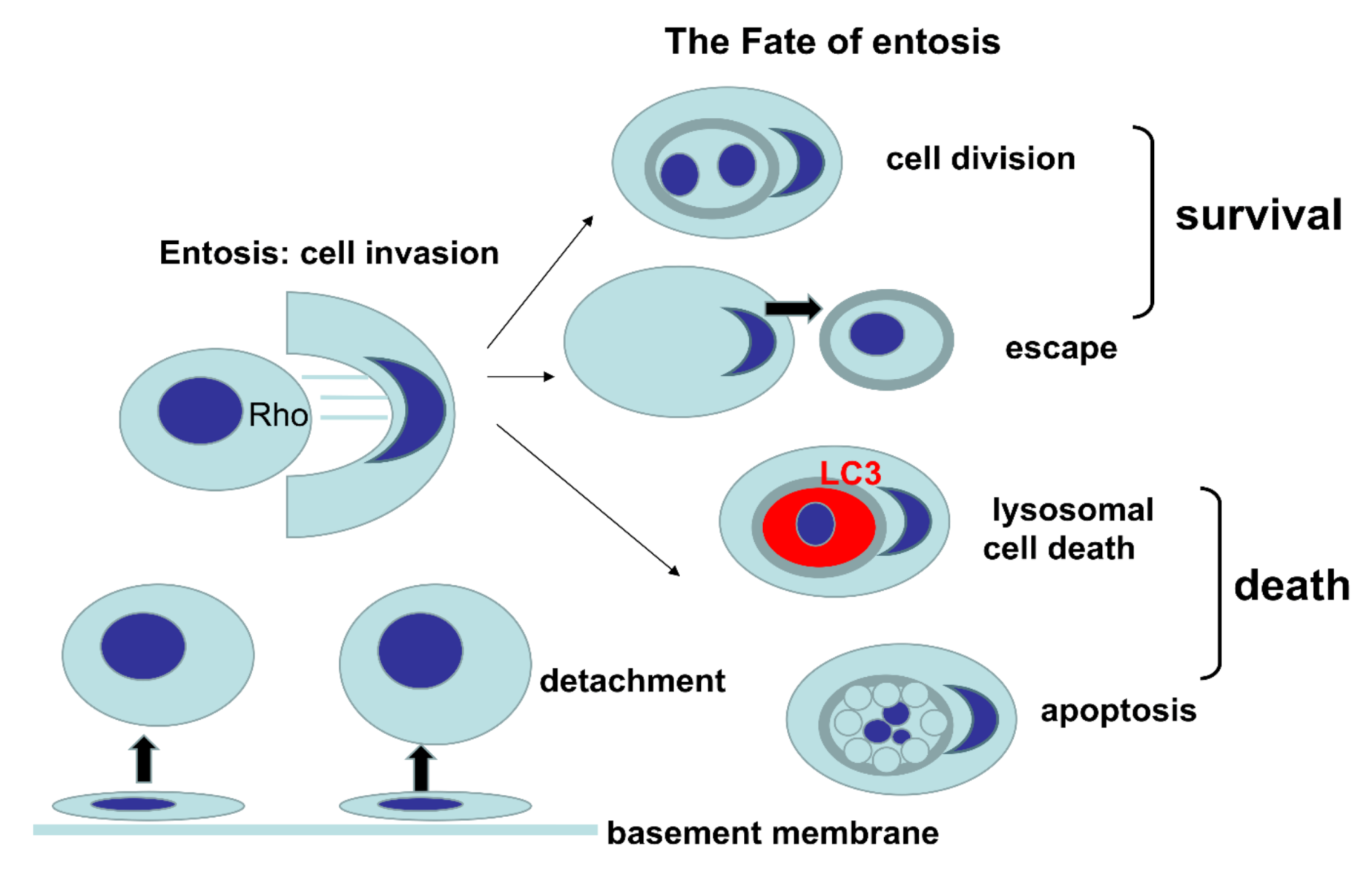 Figure 1. Graphical presentation of various fates of entosis.
Figure 1. Graphical presentation of various fates of entosis.
Entosis is neither a type of phagocytosis nor cell cannibalism. During entosis, the inner entotic cell actively enters into the host cell through activation of Rho proteins, followed by formation of adhesion bonds and actomyosin filaments[6]. After cell invasion into the host cell, the inner cell is surrounded by a double membrane of the entotic vacuole, with an extensive space between membranes. The presence of the entotic vacuole is a feature distinguishing entosis from cell cannibalism, in which the phagocytosed cell is surrounded by a thin double-membrane space, with subsequent digestion of the inner membrane[2]. The fate of the two cells undergoing entosis can involve death of the inner cell, death of the outer cell, death of both cells, and survival of both cells. New clinico-histopathological studies prompted us to summarize the significance of entosis in cancer, as entotic figures can nowadays be considered a new predictor in routine histopathological evaluation[7].
Therefore, in this review we summarised published clinico-histopathological studies on the significance of entosis and its relationship to cancer prognosis. We also showed that some anticancer drugs could induce entosis in cell culture, either as a cell death or as a pro-survival escape mechanism. At the end, we highlight the potential relationship of cell adhesion with the fate of entotic cells in vitro, based on the model of BxPc3 cells.
2. Entosis in Cancer
Entotic cell death is postulated to act as a tumour suppressor mechanism by facilitating the death of entotic cancer cells[8][9][10][11][12]. However, entosis can lead to aneuploidy and polyploidy, which promote tumour progression. Moreover, multinucleation is a common feature observed in internalizing host cells, following matrix deadhesion[13], mitosis[14], and glucose starvation[14]. Furthermore, entosis does not always lead to the death of entotic cell. Thus, in certain situations, entotic inner cells remain viable, proliferate within the outer host cell, and can eventually escape from the outer cell. However, the reasons determining the fate of each inner entotic cell remain unknown.
An increased number of entotic structures correlates with tumour promotion and progression[15]. Thus, environmental conditions like lack of glucose are linked to an increase in the grade of genetic alteration. Interestingly, entosis can also indicate tumour suppression with the elimination of aberrant constituents[14]. Activated KRAS, one of the most prominent oncogenes, can stimulate entosis[9]. It is known that expression of cadherin proteins, E-cadherin or P-cadherin, induces entosis in human cancers[9].
Cancer tissues with numerous entotic figures represent a more malignant phenotype than the same variant without such structures[16][17]. Thus, entosis is likely beneficial for malignant cells. For instance, an internalizing cell can hide into the other, from environmental factors like chemotherapy, antibodies, cytotoxic T cells or malnutrition. Indeed, some anticancer drugs and substances induce entosis[14][18][19]. Moreover, in the case of prostate cancer cells, entosis was shown to be an escape mechanism from the anti-cancerous effects of the tyrosine kinase inhibitor nintedanib[19].
There was only one case in which CIC structures were associated with reduced metastasis in pancreatic cancer, which is the opposite to other tumour types[20][21]. However, one should keep in mind that the fate of entosis can be affected by the interactions of cancer cell and tumour extracellular matrix[22]. As the majority of cancers entosis correlates with poor outcome recurrence of disease[1][22], it can also be hypothesized that the inner entotic cell can survive unfavourable conditions caused by anticancer drugs within the host outer cell, protected by the environment of the entotic vacuole. Subsequently, the internal cell can leave the outer cell after a long time[2]. Thus, both cells can survive entosis, leading to a failure in the therapy and recurrence of cancer.
3. Entosis as a Potential Target for Novel Anticancer Strategies
Entosis is frequently found during histopathological examination of specimens obtained from various human cancers. Entotic cell death was postulated to act as a tumour suppressor, through elimination of aberrant cells. Indeed, some anticancer drugs induce entosis. However, the increased number of entotic structures correlates with tumour promotion and progression. Cancer tissues with numerous entotic figures usually represent a more malignant phenotype, which could result from the beneficial effects of entosis for malignant cells. For instance, internalised cells could be protected by the entotic vacuole formed inside the host outer cell, from harmful environmental factors like chemotherapy or other unfavourable conditions induced by anticancer drugs. Subsequently the inner entotic cell can leave the outer cell intact, after a long time. Such process can potentially result in chemotherapy failure or even cancer recurrence. Indeed, it was shown that, prostate cancer cells appear to develop entosis to survive treatment with the tyrosine kinase inhibitor nintedanib. Poor outcome and cancer recurrence associated with an increased number of entotic figures was proven in clinico-histopathological studies of head and neck in squamous cell carcinomas, anal carcinoma, lung adenocarcinomas, and pancreatic ductal carcinoma. All these findings open new perspectives to investigate entosis as a potential target for novel anticancer strategies.
Compared a fully adherent in vitro entosis model of BxPc3 cells with a semiadhesive model of MCF7. It could be found that both similarities and differences to entosis induced in MCF7. Importantly, in the BxPc3 model, most inner cells demonstrated no signs of degeneration or cell death. It's postulated that this model represents spontaneous or physiological entosis.
Due to the dual role of entosis in tumour progression, there is an unmet need to introduce a clear and reproducible criteria to identify and describe entotic cells to unify research of this phenomenon. In this context, unifying documentation of entosis with an easy and reproducible method, using the standard haematoxylin stain for light microscopy would be better. This allows counting the number of entotic figures as well as evaluation of the morphology of cell structures. It is a simple and inexpensive method, creating permanent specimens that could be viewed and evaluated repeatedly by different laboratories, as well as be easily used for investigation of different agents affecting entosis.
References
- Fais, S.; Overholtzer, M. Cell-in-cell phenomena in cancer. Rev. Cancer 2018, 18, 758–766.
- W.; Cibas, E.S.; Brugge, J.S. A nonapoptotic cell death process, entosis, that occurs by cell-in-cell invasion. Cell 2007, 131, 966–979.
- Mackay, H.L.; Muller, P.A.J. Biological relevance of cell-in-cell in cancers. Biochem. Soc. Trans. 2019, 47, 725–732.
- Florey, O.; Kim, S.E.; Sandoval, C.P.; Haynes, C.M.; Overholtzer, M. Autophagy machinery mediates macroendocytic processing and entotic cell death by targeting single membranes. Nat. Cell Biol. 2011, 13, 1335–1343.
- Kroemer, G.; Galluzzi, L.; Vandenabeele, P.; Abrams, J.; Alnemri, E.S.; Baehrecke, E.H.; Blagosklonny, M.V.; El-Deiry, W.S.; Golstein, P.; Green, D.R.; et al. Classification of cell death: Recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009, 16, 3–11.
- Zeng, C.; Zeng, B.; Dong, C.; Liu, J.; Xing, F. Rho-ROCK signaling mediates entotic cell death in tumor. Cell Death Discov. 2020, 6, 4.
- Hayashi, A.; Yavas, A.; McIntyre, C.A.; Ho, Y.J.; Erakky, A.; Wong, W.; Varghese, A.M.; Melchor, J.P.; Overholtzer, M.; O’Reilly, E.M.; et al. Genetic and clinical correlates of entosis in pancreatic ductal adenocarcinoma. Mod. Pathol. 2020.
- Kong, Y.; Liang, Y.; Wang, J. Foci of entotic nuclei in different grades of noninherited renal cell cancers. IUBMB Life 2015, 67, 139–144.
- Florey, O.; Gammoh, N.; Kim, S.E.; Jiang, X.; Overholtzer, M. V-ATPase and osmotic imbalances activate endolysosomal LC3 lipidation. Autophagy 2015, 11, 88–99.
- Huang, H.; Chen, Z.; Sun, Q. Mammalian cell competitions, cell-in-cell phenomena and their biomedical implications. Curr. Mol. Med. 2015, 15, 852–860.
- Lozupone, F.; Fais, S. Cancer cell cannibalism: A primeval option to survive. Curr. Mol. Med. 2015, 15, 836–841.
- Sierro, F.; Tay, S.S.; Warren, A.; Le Couteur, D.G.; McCaughan, G.W.; Bowen, D.G.; Bertolino, P. Suicidal emperipolesis: A process leading to cell-in-cell structures, T cell clearance and immune homeostasis. Curr. Mol. Med. 2015, 15, 819–827.
- Krajcovic, M.; Johnson, N.B.; Sun, Q.; Normand, G.; Hoover, N.; Yao, E.; Richardson, A.L.; King, R.W.; Cibas, E.S.; Schnitt, S.J.; et al. A non-genetic route to aneuploidy in human cancers. Cell Biol. 2011, 13, 324–330.
- Durgan, J.; Tseng, Y.Y.; Hamann, J.C.; Domart, M.C.; Collinson, L.; Hall, A.; Overholtzer, M.; Florey, O. Mitosis can drive cell cannibalism through entosis. eLife 2017, 6.
- Durgan, J.; Florey, O. Cancer cell cannibalism: Multiple triggers emerge for entosis. Biophys. Acta Mol. Cell Res. 2018, 1865, 831–841.
- Schenker, H.; Buttner-Herold, M.; Fietkau, R.; Distel, L.V. Cell-in-cell structures are more potent predictors of outcome than senescence or apoptosis in head and neck squamous cell carcinomas. Radiat. Oncol. 2017, 12, 21.
- Schwegler, M.; Wirsing, A.M.; Schenker, H.M.; Ott, L.; Ries, J.M.; Buttner-Herold, M.; Fietkau, R.; Putz, F.; Distel, L.V. Prognostic value of homotypic cell internalization by nonprofessional phagocytic cancer cells. Biomed. Res. Int. 2015, 2015, 359392.
- Khalkar, P.; Diaz-Argelich, N.; Antonio Palop, J.; Sanmartin, C.; Fernandes, A.P. Novel methylselenoesters induce programed cell death via entosis in pancreatic cancer cells. Int. J. Mol. Sci. 2018, 19, 2849.
- Liu, J.; Wang, L.; Zhang, Y.; Li, S.; Sun, F.; Wang, G.; Yang, T.; Wei, D.; Guo, L.; Xiao, H. Induction of entosis in prostate cancer cells by nintedanib and its therapeutic implications. Oncol. Lett. 2019, 17, 3151–3162.
- Cano, C.E.; Sandi, M.J.; Hamidi, T.; Calvo, E.L.; Turrini, O.; Bartholin, L.; Loncle, C.; Secq, V.; Garcia, S.; Lomberk, G.; et al. Homotypic cell cannibalism, a cell-death process regulated by the nuclear protein 1, opposes to metastasis in pancreatic cancer. EMBO Mol. Med. 2012, 4, 964–979.
- Miyatake, Y.; Kuribayashi-Shigetomi, K.; Ohta, Y.; Ikeshita, S.; Subagyo, A.; Sueoka, K.; Kakugo, A.; Amano, M.; Takahashi, T.; Okajima, T.; et al. Visualising the dynamics of live pancreatic microtumours self-organised through cell-in-cell invasion. Sci. Rep. 2018, 8, 14054.
- Wang, X.; Li, Y.; Li, J.; Li, L.; Zhu, H.; Chen, H.; Kong, R.; Wang, G.; Wang, Y.; Hu, J.; et al. Cell-in-cell phenomenon and its relationship with tumor microenvironment and tumor progression: A Review. Cell Dev. Biol. 2019, 7, 311.




