| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Charalampos Proestos | + 6709 word(s) | 6709 | 2020-09-01 14:22:23 | | | |
| 2 | Peter Tang | -2239 word(s) | 4470 | 2020-09-07 12:42:56 | | | | |
| 3 | Peter Tang | Meta information modification | 4470 | 2020-10-27 10:55:43 | | |
Video Upload Options
Polyphenols are a diverse group of compounds possessing various health-promoting properties that are of utmost importance for many wine sensory attributes. Apart from genetic and environmental parameters, the implementation of specific oenological practices as well as the subsequent storage conditions deeply affect the content and nature of the polyphenols present in wine. However, polyphenols are effectively employed in authenticity studies. Provision of authentic wines to the market has always been a prerequisite meaning that the declarations on the wine label should mirror the composition and provenance of this intriguing product. Nonetheless, multiple cases of intentional or unintentional wine mislabeling have been recorded alarming wine consumers who demand for strict controls safeguarding wine authenticity. The emergence of novel platforms employing instrumentation of exceptional selectivity and sensitivity along with the use of advanced chemometrics such as NMR (nuclear magnetic resonance)- and MS (mass spectrometry)-based metabolomics is considered as a powerful asset towards wine authentication.
1. Introduction
Polyphenols constitute a diverse group of bioactive compounds occurring in both grapes and wines [1]. In plants, they have been found to exhibit key roles in growth, fertility, and reproduction. They present protective properties against abiotic stress conditions such as UV-light and biotic stresses such as pathogen and predator attacks [2][3]. Polyphenols exhibit a significant role in modern food technology and human nutrition [4][5] and are frequently key ingredients in functional foods. The benefits derived from the moderated wine consumption for human health have been well elaborated [6][7] with several groups of phenolic compounds including stilbenes [8], flavonols [9], and proanthocyanidins [10] found to exert various health-promoting properties [6].
Wine production is regulated by OIV (International Organization of Vine and Wine), global wine policies, and national governments. Provision of authentic wines to the market has always been a prerequisite meaning that the declarations on the wine label should mirror the composition and provenance of this intriguing product [11]. However multiple cases of intentional or unintentional wine mislabeling [12][13][14][15] have been recorded, alarming wine consumers who demand for strict controls safeguarding wine transparency.
Wine is a complex matrix composed of molecules of diverse nature, significantly influenced by environmental factors, as well as viticultural and oenological management approaches. Concerning the latter, various winemaking practices are implemented that play a key role in the composition of the final product. As a result, wine fraud detection can become a challenging task. It has been reported that specific phenolic compounds can be employed as markers in authenticity verification [16].
It is important to investigate the parameters that affect wine composition and to develop reliable methods for wine authenticity. Common applications of wet chemistry or basic chromatographic applications are widely used in routine analysis of phenolic compounds in wine. Nowadays, emerging platforms including mass spectrometry (MS)-based or nuclear magnetic resonance (NMR) metabolomics are considered the current trend in wine authenticity studies. The use of instrumentation of exceptional selectivity and sensitivity combined with advanced multivariate methods of analysis (MVA) for efficient data mining have permitted a thorough characterization of the wine chemical profile often employing polyphenolic compounds as discriminant markers among studied groups.
2. Structural Information
From a structural aspect, polyphenols can be divided into two main categories, the flavonoids that bear a common C6-C3-C6 skeleton and the non-flavonoids. Detailed presentations of the polyphenolic profile of grapes and wine can be found elsewhere [17][18][19][20].
2.1. Non-Flavonoid Polyphenols
Essentially non-flavonoid polyphenols are located in the grape pulp with the main classes comprising of phenolic acids and stilbenes as well as their derivatives. In wine, oak-derived non-flavonoids have also been detected including the classes of hydrolysable tannins (gallotannins and elagitannins), coumarins, and lignans [21][22].
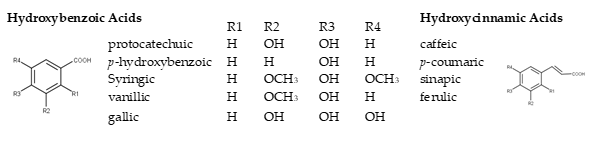
Phenolic acids have been successfully employed for white wine authentication purposes [16]. They are divided into two groups, the hydroxybenzoic (HBA) and the hydroxycinnamic (HCA) acids (phenolic acids). HBAs share a common C6-C1 structure, referring to a benzene ring with one carbon aliphatic chain substituent.
Figure 1. Basic structures of phenolic acids in wine.
Stilbenes are polyphenols featuring diverse biological properties and a complex structure that exhibits a limited but heterogeneous distribution in the plant kingdom [23]. Grapes are one of the richest sources of stilbenes that have also been detected in wines and oak wood. They share a common C6–C2–C6 skeleton, containing two benzene rings, usually bonded by an ethylene, or ethane chain. Studies have shown that resveratrol mimics effects of caloric restriction, exerts antioxidative anti-inflammatory properties, and is linked with the initiation and progression of many diseases through several mechanisms [24][25].
2.2. Flavonoid Compounds
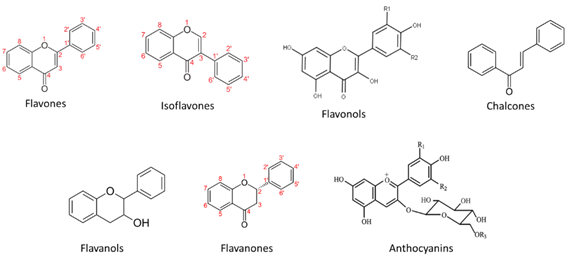
Flavonoids are secondary metabolites occurring in a wide variety of natural products such as vegetables, fruits, stems, cocoa, tea, grapes, and wine. Nowadays flavonoids are considered essential ingredients for various medicinal, nutraceutical, pharmaceutical, and cosmetic applications. This is due to their potent anti-oxidative, anti-carcinogenic, anti-inflammatory, and anti-mutagenic properties combined with the ability to modulate significant enzyme functions [26][27][28]. In plants they are involved in a series of processes related to defense against pathogens and pests, protection from ultraviolet (UV) radiation, allelopathy, pollen fertilization, auxin transport regulation, and pigmentation [29][30][31][32]. In wine, flavonoids have a fundamental role in the determination of its sensory attributes specifically wine color, flavor, astringency, and bitterness [33][34]. Total phenolic content in red wine ranges from 1200 to 1800 mg gallic acid equivalents/L, which is six to nine times more than the corresponding content in white wines [35].
Figure 2. Main flavonoid groups.
Among phenolics, anthocyanins are regarded as the most successful compounds for red wine varietal authentication [36]. For instance, diglucoside anthocyanins displaying a second glyosidic bond at position 5′ with glucose characterize non-V.vinifera grapes and this has been successfully employed in chemotaxonomical studies [9]. The relative proportion of acylated vs non-acylated anthocyanins is characteristic of each grape variety and has been proposed for cultivar differentiation [37]; however, caution must be taken since these proportions can be modified during the vinification process with the use of pectolytic enzymes or specific maceration conditions [38].
3. Influence of Vinification Strategies on Wine Polyphenolic Profile
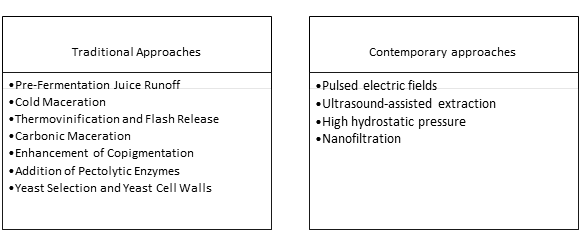
Polyphenol profile is influenced by genetic and environmental parameters including cultivar, vineyard management practices, seasonal variation, and time of harvest. The subsequent strategies in the early stages of the vinification process including maceration parameters (duration and intensity of turnovers, overall duration, temperature), type of additives used (i.e., enzymes, polysaccharide preparation, phenolic preparations, etc.), fermentation parameters (duration such as temperature and time) affect greatly the extractability and profile of the phenolic compounds having a profound effect on wine quality [19][39]. Current vinification practices aiming at enhanced pigment extraction and color stability are summarized in table 1.
Table 1: Vinification practices aiming at enhanced pigment extraction and color stability
The majority of the temperature-mediated processes above have the major drawback of high energy consumption. Significant limitations from the application of these processes have been reported including poor color stability, loss of varietal aromas, and limited aging aptitude of the resulting wines along with the need for starter culture additions, and the high energy demand [40]. Currently, several methods collectively characterized as non-thermal or physical methods have emerged that support polyphenol extraction.
Non-thermal strategies have additional benefits including the potential to synergistically reduce SO2 levels, to be used in various stages of the vinification process, and the possibility to conduct fermentations with wild yeast populations [40]. Evidently, synergies between (novel and traditional) extraction methods are expected to be widely applied in the near future.
4. Current Analytical Approaches to Wine Authenticity
4.1. Overview
Conventional methods of wet chemistry or basic applications of liquid chromatography are widely used in the determination of wine polyphenols as these procedures are part of the official methods of analysis [41]. However, the emergence of disciplines such as HRMS or NMR metabolomics combined with advanced chemometric techniques has been proven as a powerful tool to perform chemotaxonomic studies through identification of numerous grape and wine metabolites [42]. Metabolomics is a field involved in the study of multiple metabolites in a cell, a tissue, or an organism. The precise structure determination provided by NMR or the extensive metabolite coverage of MS-based metabolomics are among the properties that have made them the two most popular disciplines in food authenticity. Both, exhibit advantages and challenges as described in detail in previous reports [42][43][44].
4.2. Official Wine Analytical Methods
Traditional wine analytical methods involve multidisciplinary approaches in order to assess wine quality and authenticity. Official wine analytical methods include the determination of: alcoholic strength (ethanol), reducing substances, total and volatile acidity, total and free sulfur dioxide, volatile compounds by gas chromatography (GC), principal organic acid concentration by high-pressure capillary electrophoresis, mineral elements by inductive plasma atomic emission spectrometry, ethanol origin by isotope ratio mass spectrometry and ethanol deuterium distribution by NMR. Regulatory authorities also assess wine for the presence of artificial sweeteners or colorants, preservatives as well as fermentation inhibitors.
Regarding to the study of polyphenols in wine authenticity studies, the Folin-Ciocalteau index is the OIV reference method for the determination of all compounds with a phenolic structure (total phenols) and it is used in the European Union (EU) as the official method of analysis [45]. The official methods of analysis also include the determination of the five most important non-acylated anthocyanins and the four major acylated anthocyanins by reversed-phase liquid chromatography (RP-LC) HPLC [46] as well as the determination of the possible presence of malvidin diglycoside by fluorescence spectroscopy [47]. In any case, OIV encourages member states to continue research in the areas of interest to avoid any non-scientific evaluation of results.
4.3. Studies Focusing on Varietal or Geographical Origin Discrimination
Chemotaxonomical studies employing polyphenolic compounds mainly focus on (intra-, inter-) varietal/geographical origin characterization, discrimination as well fraud detection. As the data provided by the analytical platforms do not often solely target polyphenolic compounds, in modern studies polyphenolic substances are part of the compounds that contribute to class separation. Most recently, Arapitsas et al. (2020) provided the characterization of the metabolome of 11 single-cultivar, single-vintage Italian red wines with the use of untargeted HRMS metabolomics. In this significant study, among other biomarkers, quercetin was found more abundant in Sangiovese wines followed by Nebiolo and Nerello, isorhamnetin was more abundant in Nebiolo wines, while anthocyanin content was found higher in Teroldego wines [48]. Regarding grapes from the same variety, Locatelli et al. (2016) reported that Nebiollo wine samples evidenced significant differences in grape anthocyanin profile in comparison to Uva Rara and Vespolina cv. varieties enabling their classification [49].
Several studies have been conducted worldwide contributing to wine authenticity assessment. Concerning Argentinian wines, Pisano et al. (2015) employing HRMS metabolomics reported that three malvidin-derived anthocyanins contributed significantly to the geographical and varietal discrimination of 27 wines samples [50]. Rosso et al. (2018) also employing HRMS metabolomics proposed a method based on the calculation of secondary metabolite indexes namely (dihydro-)flavonols and anthocyanin ratios, to identify the unauthorized use of Primitivo and Negro Amaro grapes in the production of Valpolicella wines. They showed that the addition of Primitivo in the blend could be detected as it increased the indexes related to Laricitrin, Delphinidin, and Petunidin. [51]. Geana et al. (2016) reported that the abundance in the acylated glucoside of malvidin, as well as the ratios between the latter and the glucoside of malvidin along with the ratio between acylated and coumarilated monoglucosides of peonidin and malvidin were among the most significant variables, which enabled for varietal classification of 62 Romanian red wines. The same authors additionally reported that individual acylated and non-acylated anthocyanins as well as specific anthocyanin ratios contributed to vintage classification. Recently, Stoj et al. (2020) investigated the classification of 20 Polish red wines produced from Zweigelt (Vitis vinifera) and Rondo (non-Vitis vinifera) grape varieties based on the analysis of phenolic compounds by means of Ultra Performance Liquid Chromatography (UPLC) with a photo diode array detector (PDA) coupled to mass spectrometry. As expected, the non Vitis vinifera cultivar exhibited higher concentrations of anthocyanin diglucosides while in the V. vinifera variety the anthocyanin monoglucosides were found in greater abundance. An interesting finding of this study was that anthocyanin diglucosides were also found present even in low concentrations in the V. vinifera Zweigelt grape variety [52]. The latter evidence is in agreement with a relatively recent work from Xing et al. (2015) questioning earlier reports that denoted the absence of these compounds in V. vinifera varieties [9][19].
Hu et al. (2020) employed proton-nuclear magnetic resonance instrumentation (1H-NMR) combined with multivariate statistical analysis to investigate the changes of metabolite levels in Cabernet Sauvignon, Merlot, and Cabernet Gernischt Chinese dry red wines. In this study, gallic acid was among the significant markers discriminating the grape varieties.
Savino et al. (2017) investigated the intra-varietal diversity of Aglianico cv. secondary metabolites including anthocyanins, flavonols, flavanols, and resveratrol, identifying significant differences among the accessions studied [53].
Reports on phenolic compounds mostly refer to red wines. However, polyphenolic compounds have also been found to contribute to rose’ or white wine classification. Gil et al. (2020) discriminated rosé wines using shotgun metabolomics with a genetic algorithm and MS ion intensity ratios. They focused on polyphenols and reported that the compounds used for discrimination were vanillic acid, peonidin 3-O-acetyl-Glucoside-(epi)catechin, peonidin 3-O-Glucoside, and (epi)catechin-ethyl-(epi)catechin isomers [54]. Roschetti et al. (2018), applying untargeted metabolomics coupled to multivariate methods of analysis, investigated the phenolic composition of Chardonnay wines from different origins [55]. Flavonoids and, in particular flavonols, were found to be the best markers in relation to geographical origin.
Long et al. (2019) investigated the distribution of a novel crown hexameric procyanidin and its tetrameric and pentameric congeners in Italian red and white wines by means of HPLC-HRMS/MS (High-Performance Liquid Chromatography-High Resolution Tandem Mass Spectrometry) [56]. They reported the presence of a crown hexameric procyanidin only in the red wines examined, while crown tetramer and pentamer procyanidins were also present in white wines. Regarding the white wines examined, cyclic pentameric procyanidin was absent in Gewürztraminer samples while in Sauvignon Blanc and Chardonnay samples, the pentameric procyanidin was found present solely in its cyclic form. The authors suggested that the proportions of crown 4-, 5-, and 6-mer procyanidins are grape variety dependent and demonstrated that crown procyanidins may act as a tool in wine authenticity studies.
4.4. Process Monitoring
The last decade emerging disciplines have enabled thorough monitoring of the various processes involved in viticulture and enology. Below we present examples of process/treatment monitoring related studies involving polyphenolic compounds in viticulture and wine. Wine evolution studies may offer valuable information regarding process-related authentication including age evaluation or proper storage verification.
There is an increasing demand for more sustainable management practices mainly organic and biodynamic farming protocols. “Eco”-friendly wines are frequently marketed at higher prices that are indirectly attributed to claimed health benefits. So far though, the results regarding the phenolic composition of wines from these “alternative” management protocols are contradictory. Tassoni et al. (2013) concluded that no significant differences were observed among samples coming from conventional, organic, and biodynamic management protocols [57]. Picone et al. (2016) reported that caffeic and coumaric acids, as well as other polyphenolic compounds, were in lower abundance in biodynamic grapes than in organic ones. Furthermore, it has been reported that concentration of total polyphenols and anthocyanins was found higher in organic wines in comparison to their biodynamic counterparts [58][59]. However, in a more recent report from Parpinello et al. (2019), it was stated that no statistically significant differences were observed regarding the concentrations of anthocyanins, flavonols, phenolic, and cinnamic acids between organic and biodynamic wines [60]. Nonetheless, it must be stated that polyphenol composition cannot be easily compared between different agronomical treatments as it is severely influenced by genetic and environmental factors [39].
A number of studies target wine evolution. Various pigments and tannins have been identified among discriminant biomarkers for micro-oxygenated Sangiovese wines versus non-micro-oxygenated ones [61]. In another study, Herbert-Pucheta et al. (2019) studied wine chemistry involved in aging processes, employing a set of mono-varietal Queretaro Merlot samples as model system. With the use of Ultraviolet Visible (UV-VIS) absorbance-transmittance coupled with excitation emission matrix fluorescence, they discovered a rich (poly)-phenolics aromatic region, which was subsequently confirmed with NMR experiments [62].
Proper storage conditions are considered fundamental for preserving wine sensory attributes and this is considered a prerequisite for wine enthusiasts and especially wine retailers. In this context, Arapitsas et al. (2016) developed a holistic metabolic profiling method using hydrophilic interaction chromatography (HILIC) mass spectrometry to study the effect of typical domestic storage conditions as compared to optimum cellar conditions for five Sangiovese red wines and for a period of 24 months. They reported that quercetin, catechin, malvidin 3-glucoside, and pyranomalvidin 3-glucoside were among the marker compounds affected by the different storage conditions. Quercetin was found richer for domestic storage that was derived from the hydrolysis of quercetin 3-glucoside in these conditions, while the latter three were more abundant in the case of optimum cellar storage [63].
The study of the evolution of anthocyanins and tannins during wine aging is considered a challenging task due to their vast structural diversity, low abundance, and the fact that many of these metabolites exhibit similar or identical mass spectral characteristics. Therefore, chromatographic separation is fundamental and the combination between separation modes may provide valuable insight. In this direction, Willemse et al. (2015) applied online HILIC × reversed-phase liquid chromatography (RP-LC) separation coupled to high-resolution mass spectrometry and characterized in detail the anthocyanin and derived pigments content of one and six year oldo Pinotage wine [64]. The authors reported the putative identification of ninety-four (94) anthocyanin-derived pigments and enhanced certainty in compound identification. It is expected that multi-dimensional platforms (i.e., GC × GC or LC × LC) will be widely applied in wine authenticity and metabolomic studies, as they increase the number of peaks and enhance resolution, selectivity, and sensitivity compared to conventional chromatographic approaches [65].
Roullier-Gall et al. (2019) combined electrochemical oxidation strategies and ultra-high-resolution Fourier transform ion cyclotron resonance mass spectrometry (FT-ICR-MS) in order to characterize from an untargeted molecular point of view, the antioxidant property of 7-year-old Chardonnay wines only differing in SO2 added after prior pressing. They concluded that apart from known metabolites such as catechin/epicatechin and caffeic acid, sulfur-containing compounds appeared to decrease with electrochemical oxidation, whereas nitrogen-containing compounds were mostly formed [66].
Gougeon et al. (2019) performed quantitative 1H NMR experiments on 224 commercial wines produced in the six major Bordeaux appellations and quantified 40 metabolites. Multivariate data analysis and advanced chemometrics allowed the discrimination of wines on different levels (young vs old wines; Bordeaux vs French wines; wines from different Bordeaux appellations) [67]. The authors stated that among the compounds responsible for vintage discrimination, catechin and epicatechin were more abundant in younger vintages while the opposite was true for caffeic and syringic acid. As stated in the previous sections, catechin and epicatechin are involved in a series of polymerization reactions with other compounds reducing their free form abundance explaining the latter finding.
Gougeon et al. (2019) performed a real case study on wine authentication, evaluating the complementarity of a q-NMR method with classical multidisciplinary approaches. The analyzed samples belonged to three categories (a) half bottled wines topped up with wine of different origin, (b) suspect wine samples from the foreign market, and (c) authentic samples [68]. They developed a similarity score index in order to compare authentic with suspect wine samples. Catechin, epicatechin, gallic, and syringic acids were among the phenolic compounds evaluated as possible authenticity markers. The authors also demonstrated the synergy between the methods examined. Table 2 depicts a collection of studies involving polyphenols that contribute to wine authenticity assessment.
Table 2. Collection of studies involving polyphenols that contribute to wine authenticity assessment.
|
No. |
Sample Type |
n |
Country |
An. Platform |
Research Aim |
VP |
GO |
AP |
Polyphenols as Significant Markers |
Statistical Analysis |
Ref |
|
1 |
Wine |
110 |
Italy |
UPLC-QTOF MS |
LC-MS metabolomic fingerprint of 11 mono-cultivar Italian red wines-metabolomic similarity-dissimilarity study. |
X |
X |
|
Holistic approach with specific flavonols (Quercetin, Isorhametin) and anthocyanins as markers |
PCA |
[48] |
|
2 |
Wine |
20 |
Poland |
UPLC-PDA-MS/MS |
Classification of Red Wines Produced from Zweigelt and Rondo Grape Varieties Based on the Analysis of Phenolic Compounds by UPLC-PDA-MS/MS |
X |
X |
|
Anthocyanin mono (malvidin 3-O-glucoside, delphinidin 3-O-glucoside, petunidin 3-O-glucoside)- and di- glucosides (malvidin diglucoside, peonidin diglucoside, delphinidin di glucoside), flavan-3ols |
PCA, HCA |
[52] |
|
3 |
Wine |
60 |
France |
UPLC-QTOF-MS |
Discrimination of rosé wines with a genetic algorithm and MS ion intensity ratios. |
|
X |
|
Vanillic acid, Peonidin 3-O-acetyl-glucoside-(epi)catechin, Peonidin 3-O-Glucoside and (epi) catechin-ethyl-(epi)catechin isomers |
GA, RF, LDA |
[54] |
|
4 |
Wine |
19 |
Italy |
HPLC -DAD-MS-MS |
Distribution of crown hexameric procyanidin and its tetrameric and pentameric congeners in red and white wines |
X |
|
|
Ratios between crown and non-cyclic procyanidins |
ANOVA, PCA |
[56] |
|
5 |
Grapes |
14 |
Italy |
HPLC -DAD |
Detection of Intra-Varietal Diversity of Aglianico cv based on differences in the accumulation of secondary metabolites. |
X |
|
|
Total anthocyanins, Total flavonoids, Flavonoids other than anthocyanins, Total flavonoids in seeds, Resveratrol and Flavonols |
ANOVA, PCA |
[53] |
|
6 |
Grapes |
14 |
Italy |
RP-HPLC/DAD |
Phenolic composition of Nebbiolo grape (Vitis vinifera L.) from Piedmont: characterization during ripening of grapes selected in different geographic areas and comparison with Uva Rara and Vespolina cv. |
X |
|
|
Major classes mentioned; Significant differences in Anthocyanin Profile (e.g., Peonidin 3–0 Glucoside) among varieties |
ANOVA, PCA, HCPC |
[49] |
|
7 |
Grape wine |
7 |
Italy |
UPLC-MS and HPLC-DAD |
A survey of red non-V. vinifera grape metabolites. |
X |
|
|
Anthocyanin mono - and di- glucosides, proanthocyanidins of non-V. vinifera genotypes rich in oligomers and short-chain polymers |
PCA |
[69] |
|
8 |
Grapes |
90 |
Greece |
HPLC-MS |
Discrimination of five Greek red grape varieties according to the anthocyanin and proanthocyanidin profiles of their skins and seeds |
X |
|
|
Selected skin anthocyanins and proanthocyanidins |
ANOVA, PCA |
[70] |
|
9 |
Wine |
45 |
Italy |
UPLC–QTOF MS |
The effect of storage conditions on the metabolite content of red wines. |
|
|
X |
Holistic approach, identified phenolic compounds: Quercetin, catechin, malvidin 3-glucoside and pyranomalvidin 3-glucoside |
PCA, OPLS-DA |
[63] |
|
10 |
Wines |
62 |
Romania |
HPLC-DAD |
Classification of red wines using suitable markers coupled with multivariate statistical analysis |
X |
|
|
Individual anthocyanins, ratio between anthocyanins to malvidin, ratios between acylated and coumarilated monoglucosides of peonidin and malvidin |
LDA |
[71] |
|
11 |
Grape |
3 |
Italy |
UPLC–QTOF MS |
HRMS metabolomic study of grape chemical markers to reveal use of not-allowed varieties in the production of Amarone and Recioto wines |
X |
|
|
Dihydroflavonols (laricitrin) and anthocyanin (delphinidin, petunidin) ratios |
PCA |
[51] |
|
12 |
Wines |
27 |
Argentina |
UPLC–QTOF MS |
Anthocyanins as markers for the classification of Argentinean wines according to botanical and geographical origin. Chemometric modeling of liquid chromatography–mass spectrometry data |
X |
X |
|
Malvidin derived pigments: malvidin-3-O-glu, malvidin-3-(6-O-acetylglucoside), malvidin-3-O-glucoside-4-vinylguaiacol or malvidin-3-(6-O-p- coumaroylglucoside |
MCR-ALS D-UPLS |
[50] |
|
13 |
Wine |
3 |
France |
FT-ICR-MS |
Electrochemical triggering of the Chardonnay wine metabolome. |
|
|
X |
Catechin, epicatechin, caffeic acid and sulfur-containing compounds |
ANOVA |
[66] |
|
14 |
Wine |
224 |
France |
NMR |
The metabolomic profile of Bordeaux red wines. |
X |
X |
|
Catechin, epicatechin, caffeic acid, syringic acid, galic acid |
OSC-PLS-D, PCA and ANOVA |
[67] |
|
15 |
Wine |
19 |
China |
NMR |
Wine Analysis and Authenticity Using 1H-NMR Metabolomics Data: Application to Chinese Wines
|
X |
X |
|
Gallic acid, Syringic acid |
ANOVA, PCA |
[72] |
|
16 |
Wines |
37 |
France |
NMR |
Wine Authenticity by Quantitative 1H NMR Versus Multitechnique Analysis: A Case Study |
X |
X |
X |
|
PCA |
[68] |
|
17 |
Wine |
11 |
Mexico |
NMR |
Multivariate spectroscopy for targeting phenolic choreography in wine with A-TEEM TM and 1H NMR crosscheck non-targeted metabolomics |
X |
|
X |
Novel rich (poly)-phenolics region around 5.58–8.0 ppm within the 1H-NMR spectra of wine samples |
PARAFAC |
[62] |
|
18 |
Grapes |
50 |
Italy |
NMR |
1H NMR metabolomic study of biodynamic Sangiovese grapes in comparison to organic ones. |
|
|
X |
caffeic and coumaric acids |
PCA |
[58] |
|
19 |
Wine |
2 |
China (16) |
NMR |
1H NMR Metabolomic study Shanxi Cabernet Sauvignon and Shiraz wines. |
X |
|
|
gallic acid |
PCA and PLS-DA |
[73] |
|
20 |
Wine |
6 |
Italy |
NMR |
Study of effects of area, year and climatic factors on Barbera wine characteristics by the combination of 1H-NMR metabolomics and chemometrics |
X |
|
|
p-coumaric acid |
PCA |
[74] |
Abbreviations: VP: Varietal profiling; GO: Geographical Origin; AP: Ageing properties; UPLC-QTOF-MS: Ultra Performance Liquid Chromatography coupled with Quadrupole/Time Of Flight Mass Spectrometry; UPLC-PDA-MS/MS: UPLC- Photo diode Array detector coupled to Mass Spectrometry; HPLC-DAD: High-Performance Liquid Chromatography-DAD; FT-ICR-MS: Fourier transform ion cyclotron resonance mass spectrometry; NMR: Nuclear Magnetic Resonance. ANOVA: Analysis of Variance; PCA: Principal Components Analysis; HCA: Hierachical Cluster Analysis; GA: Genetic Algorithm; RF: Random Forest; LDA: Linear Discriminant Analysis; HCPC: Hierarchical Clustering on Principal Components; MCR-ALS: multivariate curve resolution-alternating least-squares; D-UPLS: unfolded partial least-squares in discriminant mode; OPLS-DA: Orthogonal Projections to Latent Structures Discriminant Analysis; OSC-PLS-DA: Partial Least Squares Discriminant Analysis combined to Orthogonal Signal Correction Filter; PARAFAC: Parallel Factor Analysis.
4.5. Data Processing and Interpretation
Emerging NMR and MS-based analytical platforms produce a tremendous amount of information, posing nowadays elevated challenges in handling, pre-processing, statistical analysis, visualization, and interpretation of frequently large datasets. The synergy between scientists belonging to different fields including bioinformatics, statistics, computational and data science has given rise to numerous tools and platforms, providing significant resources in this direction.
Metabolomics scientists are able to develop their tools with modern scripting languages such as the open source Python, R, Raku, Ruby, or the commercially available Matlab being considered as the most popular languages in which the scripts are written. Moreover, online platforms are publicly available such as the Metaboanalyst 4.0 (https://www.metaboanalyst.ca) or the Workflow4Metabolomics 3.0 (https://workflow4metabolomics.org), which provide GUI (graphical user interface) solutions for efficient data processing and interpretation of results. Numerous statistical packages are also currently available including EZInfo SIMCA-P (Umetrics, Umea, Sweden), Origin Statistical software (OriginLab Corporation, Northampton, USA) or SPSS (SPSS Inc., Chicago, IL, USA), and Minitab v.14 (Minitab Inc., State College, PA, USA). A detailed presentation of the tools and packages available in each step (pre-processing, statistical analysis, visualization, and interpretation) or approach (targeted-non targeted metabolomics) is beyond the scope of this work and can be found in recent reviews [75][76][77][78].
Regarding the statistical analysis of the datasets, tools such as the Hierarchical Cluster Analysis (HCA) and the omnipresent PCA have been traditionally employed for an unbiased search for differential or common trends among samples. However, these approaches are less favorable when searching for discriminative markers among groups. In this case, various supervised methods including partial least squares (PLS) and orthogonal partial least squares (OPLS) discriminant analysis, ANN (artificial neural networks), CVA (canonical variate analysis), SVM (support vector machine) have been previously employed [54][65].
It must be noted that taking into consideration the diverse experimental design often involved, no generalized standard operation approach for the statistical data mining of such experiments should be suggested [79].
5. Conclusion
Polyphenols are a diverse group of compounds of utter importance to wine quality. Current understanding of the polyphenolic composition in wine is well elaborated, however, recent studies continuously report novel findings involving them.
Apart from genetic and environmental factors, polyphenol composition in wine is significantly influenced by winemaking practices. This is greatly reflected upon the composition of the final product constituting wine authentication as a challenging task. In order to better understand the extraction mechanisms, an overview of the current technological practices involved was presented.
Emerging analytical approaches employing instrumentation of exceptional sensitivity combined with advanced chemometric techniques have shed unprecedented light on wine polyphenolic composition. MS-based as well as NMR metabolomics enable for thorough polyphenol profile characterization. Inter- and intra-varietal investigation as well as process monitoring from vine to wine are among the most studied current topics while selected polyphenols have been proved to be effective discriminant biomarkers. Individual phenolic compounds as well as specific phenolic compound ratios have been found to significantly contribute to class separation. It is evident that synergistic approaches between emerging analytical platforms in combination with advanced multivariate data analysis will be considered the spearheads toward fraud detection and the provision of authentic wines.
References
- Proestos, C.; Bakogiannis, A.; Komaitis, M. Determination of Phenolic Compounds in Wines. Int. J. Food Stud. 2012, 1.
- Yun, J.W.; Lee, W.S.; Kim, M.J.; Lu, J.N.; Kang, M.H.; Kim, H.G.; Kim, D.C.; Choi, E.J.; Choi, J.Y.; Kim, H.G.; et al. Characterization of a profile of the anthocyanins isolated from Vitis coignetiae Pulliat and their anti-invasive activity on HT-29 human colon cancer cells. Food Chem. Toxicol. 2010, 48, 903–909.
- Watson, R.R. Polyphenols in Plants: Isolation, Purification and Extract Preparation; Elsevier Inc.: Amsterdam, Netherlands, 2018; ISBN 9780123979346.
- Rana, Z.H.; Alam, M.K.; Akhtaruzzaman, M. Nutritional Composition, Total Phenolic Content, Antioxidant and α-Amylase Inhibitory Activities of Different Fractions of Selected Wild Edible Plants. Antioxidants 2019, 8, 203.
- Alam, M.K.; Rana, Z.H.; Islam, S.N.; Akhtaruzzaman, M. Comparative assessment of nutritional composition, polyphenol profile, antidiabetic and antioxidative properties of selected edible wild plant species of Bangladesh. Food Chem. 2020, 320, 126646.
- Golan, R.; Gepner, Y.; Shai, I. Wine and Health–New Evidence. Eur. J. Clin. Nutr. 2019, 72, 55–59.
- Bose, S.; Sarkar, D.; Bose, A.; Mandal, S.C. Natural Flavonoids and Its Pharmaceutical Importance. Pharma Rev. 2018, 61–75.
- Ma, D.S.L.; Tan, L.T.H.; Chan, K.G.; Yap, W.H.; Pusparajah, P.; Chuah, L.H.; Ming, L.C.; Khan, T.M.; Lee, L.H.; Goh, B.H. Resveratrol-potential antibacterial agent against foodborne pathogens. Front. Pharmacol. 2018, 9, 102.
- Flamini, R.; Mattivi, F.; De Rosso, M.; Arapitsas, P.; Bavaresco, L. Advanced knowledge of three important classes of grape phenolics: Anthocyanins, stilbenes and flavonols. Int. J. Mol. Sci. 2013, 14, 19651–19669.
- Nunes, M.A.; Pimentel, F.; Costa, A.S.G.; Alves, R.C.; Oliveira, M.B.P.P. Cardioprotective properties of grape seed proanthocyanidins: An update. Trends Food Sci. Technol. 2016, 57, 31–39.
- European Commission REGULATION (EU) No 1308/2013 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCILof 17 December 2013establishing a common organisation of the markets in agricultural products and repealing Council Regulations (EEC) No 922/72, (EEC) No 234/79, (EC) No 1037/2001 a. Off. J. Eur. Union L. 2013, L347, 1–184.
- Holmberg, L. Wine fraud. Int. J. Wine Res. 2010, 2, 105–113.
- Muhammad, A.; Countryman, A.M. In Vino ‘No’ Veritas: impacts of fraud on wine imports in China. Aust. J. Agric. Resour. Econ. 2019, 63, 742–758.
- Fougere, E.; Kaplan, E.K.; Collins, C.A. Pricing uncertainty in wine markets following the Rudy Kurniawan scandal. J. Wine Res. 2020, 31, 1–5.
- Geana, E.I.; Popescu, R.; Costinel, D.; Dinca, O.R.; Stefanescu, I.; Ionete, R.E.; Bala, C. Verifying the red wines adulteration through isotopic and chromatographic investigations coupled with multivariate statistic interpretation of the data. Food Control 2016, 62, 1–9.
- Pasvanka, K.; Tzachristas, A.; Proestos, C. Quality tools in wine traceability and authenticity. In Quality Control in the Beverage Industry: Volume 17: The Science of Beverages; Elsevier Inc.: Amsterdam, Netherlands, 2019; Vol. 17, pp. 289–334 ISBN 9780128166819.
- Garrido, J.; Borges, F. Wine and grape polyphenols - A chemical perspective. Food Res. Int. 2013, 54, 1844–1858.
- Monagas, M.; Bartolomé, B.; Gómez-Cordovés, C. Updated knowledge about the presence of phenolic compounds in wine. Crit. Rev. Food Sci. Nutr. 2005, 45, 85–118.
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology, The Chemistry of Wine: Stabilization and Treatments: Second Edition; John Wiley & Sons, Ltd: Chichester, England, 2006; Vol. 2; ISBN 9780470010396.
- Palade, L.; Popa, M. Polyphenol Fingerprinting Approaches in Wine Traceability and Authenticity: Assessment and Implications of Red Wines. Beverages 2018, 4, 75.
- Rodríguez-García, C.; Sánchez-Quesada, C.; Toledo, E.; Delgado-Rodríguez, M.; Gaforio, J.J. Naturally lignan-rich foods: A dietary tool for health promotion? Molecules 2019, 24.
- Baderschneider, B.; Winterhalter, P. Isolation and characterization of novel benzoates, cinnamates, flavonoids, and lignans from Riesling wine and screening for antioxidant activity. J. Agric. Food Chem. 2001, 49, 2788–2798.
- Rivière, C.; Pawlus, A.D.; Mérillon, J.M. Natural stilbenoids: Distribution in the plant kingdom and chemotaxonomic interest in Vitaceae. Nat. Prod. Rep. 2012, 29, 1317–1333.
- Berman, A.Y.; Motechin, R.A.; Wiesenfeld, M.Y.; Holz, M.K. The therapeutic potential of resveratrol: a review of clinical trials. NPJ Precis. Oncol. 2017, 1, 1–9.
- Bavaresco, L.; Lucini, L.; Busconi, M.; Flamini, R.; De Rosso, M. Wine resveratrol: from the ground up. Nutrients 2016, 8, 222.
- Swaminathan, M.; Chee, C.F.; Chin, S.P.; Buckle, M.J.C.; Rahman, N.A.; Doughty, S.W.; Chung, L.Y. Flavonoids with M1 muscarinic acetylcholine receptor binding activity. Molecules 2014, 19, 8933–8948.
- Silva, A.R.; Grosso, C.; Delerue-Matos, C.; Rocha, J.M. Comprehensive review on the interaction between natural compounds and brain receptors: Benefits and toxicity. Eur. J. Med. Chem. 2019.
- Jakaria, M.; Azam, S.; Jo, S.-H.; Kim, I.-S.; Dash, R.; Choi, D.-K. Potential Therapeutic Targets of Quercetin and Its Derivatives: Its Role in the Therapy of Cognitive Impairment. J. Clin. Med. 2019, 8, 1789.
- Blancquaert, E.H.; Oberholster, A.; Ricardo-da-Silva, J.M.; Deloire, A.J. Grape Flavonoid Evolution and Composition Under Altered Light and Temperature Conditions in Cabernet Sauvignon (Vitis vinifera L.). Front. Plant Sci. 2019, 10, 1062.
- Dwivedi, S.; Malik, C.; Chhokar, V. Molecular structure, biological functions, and metabolic regulation of flavonoids. In Plant Biotechnology: Recent Advancements and Developments; Springer Singapore, 2017; pp. 171–188 ISBN 9789811047329.
- Nabavi, S.M.; Šamec, D.; Tomczyk, M.; Milella, L.; Russo, D.; Habtemariam, S.; Suntar, I.; Rastrelli, L.; Daglia, M.; Xiao, J.; et al. Flavonoid biosynthetic pathways in plants: Versatile targets for metabolic engineering. Biotechnol. Adv. 2020, 38, 107316.
- Baskar, V.; Venkatesh, R.; Ramalingam, S. Flavonoids (antioxidants systems) in higher plants and their response to stresses. In Antioxidants and Antioxidant Enzymes in Higher Plants; Springer International Publishing: New York, USA, 2018; pp. 253–268 ISBN 9783319750880.
- Gawel, R.; Smith, P.A.; Cicerale, S.; Keast, R. The mouthfeel of white wine. Crit. Rev. Food Sci. Nutr. 2018, 58, 2939–2956.
- Soares, S.; Brandão, E.; Mateus, N.; de Freitas, V. Sensorial properties of red wine polyphenols: Astringency and bitterness. Crit. Rev. Food Sci. Nutr. 2017, 57, 937–948.
- Kennedy, J.A.; Saucier, C.; Glories, Y. Grape and wine phenolics: History and perspective. Am. J. Enol. Vitic. 2006, 57, 239–248.
- Villano, C.; Lisanti, M.T.; Gambuti, A.; Vecchio, R.; Moio, L.; Frusciante, L.; Aversano, R.; Carputo, D. Wine varietal authentication based on phenolics, volatiles and DNA markers: State of the art, perspectives and drawbacks. Food Control 2017, 80, 1–10.
- Versari, A.; Laurie, V.F.; Ricci, A.; Laghi, L.; Parpinello, G.P. Progress in authentication, typification and traceability of grapes and wines by chemometric approaches; Elsevier Inc.: Amsterdam, Netherlands, 2014; Vol. 60; ISBN 3904573381.
- Cheynier, V.; Dueñas-Paton, M.; Salas, E.; Maury, C.; Souquet, J.M.; Sarni-Manchado, P.; Fulcrand, H. Structure and properties of wine pigments and tannins. Am. J. Enol. Vitic. 2006, 57, 298–305.
- Jackson, R.S. Wine science: principles and applications; Elsevier Inc.: Amsterdam, Netherlands, 2020; ISBN 0128165375.
- Maza, M.; Álvarez, I.; Raso, J. Thermal and Non-Thermal Physical Methods for Improving Polyphenol Extraction in Red Winemaking. Beverages 2019, 5, 47.
- OIV (Organisation International de la Vigne et du Vin) HPLC-Determination of nine major anthocyanins in red and rosé wine,Method OIV-MA-AS315-11. OIV method 2007, 2007, 1–13.
- Diamantidou, D.; Zotou, A.; Theodoridis, G. Wine and grape marc spirits metabolomics. Metabolomics 2018, 14, 159.
- Wishart, D.S. NMR metabolomics: A look ahead. J. Magn. Reson. 2019, 306, 155–161.
- Emwas, A.-H.M. The strengths and weaknesses of NMR spectroscopy and mass spectrometry with particular focus on metabolomics research. In Metabonomics; Springer, 2015; pp. 161–193.
- OIV Compendium of international methods of analysis: Folin-Ciocalteu Index. OIV-MA-AS2-10 Available online: http://www.oiv.int (accessed on Aug 12, 2020).
- OIV Compendium of international methods of analysis: Determination of nine major anthocyanins in red and rosé wines using HPLC (Oeno 22/2003, Oeno 12/2007). OIV-MA-AS315-11 Available online: http://www.oiv.int (accessed on Aug 12, 2020).
- OIV Compendium of international methods of analysis: Malvidin diglucoside. OIV-MA-AS315-03 Available online: http://www.oiv.int (accessed on Aug 12, 2020).
- Arapitsas, P.; Ugliano, M.; Marangon, M.; Piombino, P.; Rolle, L.; Gerbi, V.; Versari, A.; Mattivi, F. Use of untargeted LC-MS metabolome to discriminate Italian mono-varietal red wines, produced in their different terroirs. J. Agric. Food Chem. 2020.
- Locatelli, M.; Travaglia, F.; Coïsson, J.D.; Bordiga, M.; Arlorio, M. Phenolic composition of Nebbiolo grape (Vitis vinifera L.) from Piedmont: characterization during ripening of grapes selected in different geographic areas and comparison with Uva Rara and Vespolina cv. Eur. Food Res. Technol. 2016, 242, 1057–1068.
- Pisano, P.L.; Silva, M.F.; Olivieri, A.C. Anthocyanins as markers for the classification of Argentinean wines according to botanical and geographical origin. Chemometric modeling of liquid chromatography-mass spectrometry data. Food Chem. 2015, 175, 174–180.
- De Rosso, M.; Mayr, C.M.; Girardi, G.; Vedova, A.D.; Flamini, R. High-resolution mass spectrometry metabolomics of grape chemical markers to reveal use of not-allowed varieties in the production of Amarone and Recioto wines. Metabolomics 2018, 14, 1–10.
- Stój, A.; Kapusta, I.; Domagała, D. Classification of Red Wines Produced from Zweigelt and Rondo Grape Varieties Based on the Analysis of Phenolic Compounds by UPLC-PDA-MS/MS. Molecules 2020, 25, 1342.
- Savino, M.; Basile, T.; Alba, V.; Bolettieri, D.; Paradiso, F.; Tamborra, P.; Suriano, S.; Tarricone, L. Detection of Intra-Varietal Diversity Based on Differences in the Accumulation of Secondary Metabolites for Winemaking Management of High-Quality Red Wines. Beverages 2017, 3, 45.
- Gil, M.; Reynes, C.; Cazals, G.; Enjalbal, C.; Sabatier, R.; Saucier, C. Discrimination of rosé wines using shotgun metabolomics with a genetic algorithm and MS ion intensity ratios. Sci. Rep. 2020, 10, 1–7.
- Rocchetti, G.; Gatti, M.; Bavaresco, L.; Lucini, L. Untargeted metabolomics to investigate the phenolic composition of Chardonnay wines from different origins. J. Food Compos. Anal. 2018, 71, 87–93.
- Longo, E.; Rossetti, F.; Jouin, A.; Teissedre, P.L.; Jourdes, M.; Boselli, E. Distribution of crown hexameric procyanidin and its tetrameric and pentameric congeners in red and white wines. Food Chem. 2019, 299, 125125.
- Tassoni, A.; Tango, N.; Ferri, M. Comparison of biogenic amine and polyphenol profiles of grape berries and wines obtained following conventional, organic and biodynamic agricultural and oenological practices. Food Chem. 2013, 139, 405–413.
- Picone, G.; Trimigno, A.; Tessarin, P.; Donnini, S.; Rombolà, A.D.; Capozzi, F. 1 H NMR foodomics reveals that the biodynamic and the organic cultivation managements produce different grape berries ( Vitis vinifera L. cv. Sangiovese). Food Chem. 2016, 213, 187–195.
- Parpinello, G.P.; Rombolà, A.D.; Simoni, M.; Versari, A. Chemical and sensory characterisation of Sangiovese red wines: Comparison between biodynamic and organic management. Food Chem. 2015, 167, 1–8.
- Parpinello, G.P.; Ricci, A.; Rombolà, A.D.; Nigro, G.; Versari, A. Comparison of Sangiovese wines obtained from stabilized organic and biodynamic vineyard management systems. Food Chem. 2019, 283, 499–507.
- Arapitsas, P.; Scholz, M.; Vrhovsek, U.; Di Blasi, S.; Biondi Bartolini, A.; Masuero, D.; Perenzoni, D.; Rigo, A.; Mattivi, F. A metabolomic approach to the study of wine micro-oxygenation. PLoS One 2012, 7.
- Herbert-Pucheta, J.E.; Padilla-Maya, G.; Milmo-Brittinham, D.; Lojero, D.; Gilmore, A.M.; Raventós-Llopart, L.; Hernández-Pulido, K.E.; Zepeda-Vallejo, L.G. Multivariate spectroscopy for targeting phenolic choreography in wine with A-TEEM TM and NMR crosscheck non-targeted metabolomics . BIO Web Conf. 2019, 15, 02006.
- Arapitsas, P.; Corte, A. Della; Gika, H.; Narduzzi, L.; Mattivi, F.; Theodoridis, G. Studying the effect of storage conditions on the metabolite content of red wine using HILIC LC–MS based metabolomics. Food Chem. 2016, 197, 1331–1340.
- Willemse, C.M.; Stander, M.A.; Vestner, J.; Tredoux, A.G.J.; De Villiers, A. Comprehensive Two-Dimensional Hydrophilic Interaction Chromatography (HILIC) × Reversed-Phase Liquid Chromatography Coupled to High-Resolution Mass Spectrometry (RP-LC-UV-MS) Analysis of Anthocyanins and Derived Pigments in Red Wine. Anal. Chem. 2015, 87, 12006–12015.
- Alañón, M.E.; Pérez-Coello, M.S.; Marina, M.L. Wine science in the metabolomics era. TrAC - Trends Anal. Chem. 2015, 74, 1–20.
- Roullier-Gall, C.; Kanawati, B.; Hemmler, D.; Druschel, G.K.; Gougeon, R.D.; Schmitt-Kopplin, P. Electrochemical triggering of the Chardonnay wine metabolome. Food Chem. 2019, 286, 64–70.
- Gougeon, L.; da Costa, G.; Guyon, F.; Richard, T. 1H NMR metabolomics applied to Bordeaux red wines. Food Chem. 2019, 301, 125257.
- Gougeon, L.; da Costa, G.; Richard, T.; Guyon, F. Wine Authenticity by Quantitative 1H NMR Versus Multitechnique Analysis: a Case Study. Food Anal. Methods 2019, 12, 956–965.
- Ruocco, S.; Stefanini, M.; Stanstrup, J.; Perenzoni, D.; Mattivi, F.; Vrhovsek, U. The metabolomic profile of red non- V. vinifera genotypes. Food Res. Int. 2017, 98, 10–19.
- Kyraleou, M.; Kallithraka, S.; Gkanidi, E.; Koundouras, S.; Mannion, D.T.; Kilcawley, K.N. Discrimination of five Greek red grape varieties according to the anthocyanin and proanthocyanidin profiles of their skins and seeds. J. Food Compos. Anal. 2020, 92, 103547.
- Geana, E.I.; Popescu, R.; Costinel, D.; Dinca, O.R.; Ionete, R.E.; Stefanescu, I.; Artem, V.; Bala, C. Classification of red wines using suitable markers coupled with multivariate statistic analysis. Food Chem. 2016, 192, 1015–1024.
- Gougeon, L.; Da Costa, G.; Le Mao, I.; Ma, W.; Teissedre, P.L.; Guyon, F.; Richard, T. Wine Analysis and Authenticity Using 1H-NMR Metabolomics Data: Application to Chinese Wines. Food Anal. Methods 2018, 11, 3425–3434.
- Zhu, J.; Hu, B.; Lu, J.; Xu, S. Analysis of metabolites in cabernet sauvignon and shiraz dry red wines from Shanxi by 1H NMR spectroscopy combined with pattern recognition analysis. Open Chem. 2018, 16, 446–452.
- Cassino, C.; Tsolakis, C.; Bonello, F.; Gianotti, V.; Osella, D. Effects of area, year and climatic factors on Barbera wine characteristics studied by the combination of 1 H-NMR metabolomics and chemometrics. J. Wine Res. 2017, 28, 259–277.
- Powers, R.; Riekeberg, E. New frontiers in metabolomics: From measurement to insight. F1000Research 2017, 6, 1148.
- O’Shea, K.; Misra, B.B. Software tools, databases and resources in metabolomics: updates from 2018 to 2019. Metabolomics 2020, 16, 36.
- Li, Z.; Lu, Y.; Guo, Y.; Cao, H.; Wang, Q.; Shui, W. Comprehensive evaluation of untargeted metabolomics data processing software in feature detection, quantification and discriminating marker selection. Anal. Chim. Acta 2018, 1029, 50–57.
- Blaženović, I.; Kind, T.; Ji, J.; Fiehn, O. Software tools and approaches for compound identification of LC-MS/MS data in metabolomics. Metabolites 2018, 8, 31.
- Kopka, J.; Walther, D.; Allwood, J.W.; Goodacre, R. Progress in Chemometrics and Biostatistics for Plant Applications, or: A Good Red Wine is a Bad White Wine. In Annual Plant Reviews online; John Wiley & Sons, Ltd: Chichester, UK, 2018; pp. 317–342.