| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marina Al Daccache | + 2706 word(s) | 2706 | 2020-08-17 05:46:12 |
Video Upload Options
This work emphasized the apple fermentation process and showed how the fermentation can be affected by the first material composition and the used microorganisms.
Fermented apple beverages are produced all over the world with diverse characteristics associated with each country. Despite the diversifications, cider producers are confronted with similar issues and risks. The nature of the raw material, also known as the fermentation medium, plays a key role in fermentation. A well-defined composition of apples is, therefore, required to produce cider with good quality. In addition, ferment and its metabolism are important factors in the fermentation process. The producers of cider and other alcoholic beverages are looking in general for novel yeast strains or for the use of native strains to produce “authentic” and diversified beverages that are distinct from each other, and that attract more and more consumers. Research articles on cider production are infrequent compared to wine production, especially on the impact of the chemical composition and microbial diversity of apples on fermentation. Even though the processing of fermented beverages is close in terms of microbial interactions and production, the study of the specific properties of apples and the production challenges of cider production is advantageous and meaningful for cider producers.
- Introduction
Apples represent a very particular fruit known for their unique symbolic richness over time. Later, the different studies proved the importance of that fruit due to its chemical composition and specifically its antioxidant characteristics. The fruit belongs to the “Maloideae” subfamily and to the “Rosacea” family. It represents one of the most important deciduous tree fruits that are generally grown in temperate and tropical regions [1]. Apple is one of the most produced and consumed fruits in the world. It is ranked as the third-most fruit produced worldwide after bananas and watermelon with a production that reached 75 million tons in 2018–2019 [2]. China stands as the largest producer with increasing production of almost 33 million tons per year, followed by the European Union (EU) producing 15 million tons per year (Table 1). The United States comes in the third rank, producing 5.6 million tons of apples in 2019. The main apple producers in Europe are Poland, France, and Italy. Turkey and Iran produce around 3 million tons per year each while the production of Chile, Russia, Ukraine, and Brazil is around 1.2 million tons per year (Table 1).
Table 1. Global production and consumption of fresh apples per year in 2019 [2].
|
Country |
Apple Production (kt) |
Fresh Domestic Consumption (kt) |
|
China |
33,000 |
38,050 |
|
European Union |
15,442 |
7400.6 |
|
United States |
5564 |
2589.4 |
|
Turkey |
3306 |
2630.5 |
|
Iran |
3085 |
1813.9 |
|
Russia |
1656 |
1884.4 |
|
Chile |
1393 |
229.6 |
|
Ukraine |
1211 |
1066.2 |
|
Brazil |
1156 |
1325.9 |
Furthermore, apple juice is the main raw material for several beverages’ production. Vinegar, cider, calvados, and apple wine are obtained from apple juice fermentation, depending on the conditions applied. This review focuses on the alcoholic fermentation of apple juice to produce cider. Over the past years, different definitions were accorded to the word “fermentation”. The term was first applied to describe the production of wine and specifically the bubbling caused by the production of carbon dioxide. Nowadays, alcoholic fermentation is known as a biological complex process where yeasts convert sugars like glucose, fructose, and sucrose into cellular energy, ethanol, carbon dioxide, and other metabolic byproducts. Different parameters may affect the fermented product such as the composition of the raw materials, the microorganism used during the fermentation, and the process parameters and conditions.
- Cider-Making Process
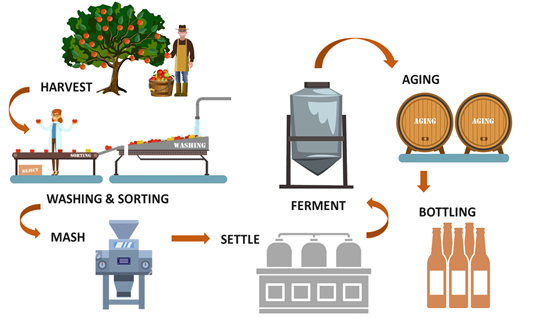
Different types of ciders exist in the market since every country has its specialty to produce traditional ciders. French cider is usually produced following a natural process without additives or other modern treatments, compared to the English cider. Due to the different production methods, French cider tends to be fruity while the English one is richer in alcohol. Even if the processes seem to be different, many key steps are common to all of these processes (Figure 1).
Figure 1. Main steps of the cider-making process.
Apples are first transported from the silo to be machine-washed in water. They are sorted by appearance to remove rotten fruits. The remaining apples are transferred for milling where they are crushed into small pieces. In the French cider process, the apple pulp is oxidized from 30 min to up to 5 h. The pulp is then pressed and left to settle. The fermentation step, which in France relies on natural flora, begins with an oxidative phase. Oxygen flow is highly beneficial for this flora at the beginning of fermentation, leading to limited growth of Saccharomyces during this step. This stage is considered very important because this is when fruity aromas are generated. The fermentation is conducted later by Saccharomyces for 1 to 3 months at a moderate agitation speed. As for wine production, malolactic fermentation can occur due to bacterial growth in cider. Maturation is the next step after fermentation when other yeasts, such as Brettanomyces anomalus can grow, which can have a negative influence on the aromatic quality of the cider. Later, a post-fermentation clarification step takes place, leading to a clear product without turbidity and deposits, and which stabilizes the cider and eliminates haziness caused by the action of proteins or tannins. This step can also eliminate microorganisms and ensure better bacterial stability in the final product. Clarification is done either by settling, centrifugation, or filtration. Finally, after blending and final filtration, the cider is bottled with either carbonation or additional yeast to trigger a second fermentation in the bottle.
Some research works have been conducted to investigate the impact of power ultrasound and pulsed electric fields (PEF) on apple juice fermentation for cider production. Ultrasound- and PEF-assisted fermentations [3][4] showed that the treatment of the yeast strain Hanseniaspora sp., isolated from a spontaneous fermented Lebanese “Ace Spur” apple juice [5], may contribute to shortening the fermentation time and to reducing the ethanol content in the fermented product, depending on the parameters applied. Further investigations are, nonetheless, required to study the impact of these emerging technologies on the sensory properties on cider.
- Impact of Apple Juice Composition and Microbial Diversity on Alcoholic Fermentation in the Cider Production Process
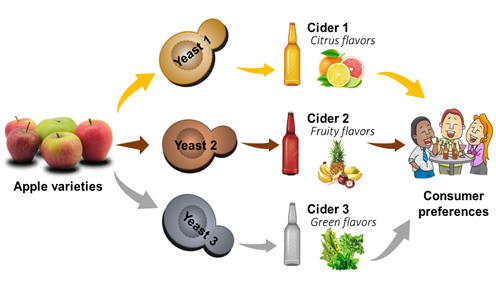
Fermentation is a complex metabolic process when sugars are transformed into ethanol, secondary metabolites, acids, alcohols, esters, and carbon dioxide. This transformation can be affected by several parameters related to the fermentation medium. Thus, the choice of apple varieties, as well as the yeast species carrying out the fermentation process, is important (Figure 2).
Figure 2. Impact of apple varieties and yeast type on cider production.
3.1. Impact of Apple Juice Composition on Fermentation
Sugar, acids, and polyphenols represent the three major compounds that affect apple juice fermentation [6]. Accordingly, apple selection is an important step, having a direct impact on the quality of the final product. In countries with ancient cider traditions, special varieties of apples known as “apple cider” are grown for their high levels of acids and phenolic compounds. However, nowadays, dessert apples are more and more frequently used, especially in Germany, Switzerland, and America. Consequently, in order to help cider producers to obtain an optimal mixture, acidity ratios, polyphenols, and alcohols derived from sugars or residual sugars in their products, a quantitative classification system was developed by Long Ashton Cider Research station in the UK[6]. Phenolic compounds have an important effect on the sensory properties of cider such as color, bitterness, and astringency balance, which provide the mouthfeel of cider [6][7]. The phenolic profile may differ from one apple variety to another, but it may also depend on the year of harvest, variety, climate, maturity, storage, and processing [8][9][10][11]. Procyanidins, composed of high molecular compounds, play a major role in astringency, while molecules of lower weights are responsible for bitter taste. In addition, polyphenols can influence the sweetness and acidity, thus affecting overall aroma development during fermentation[12][13]. Not only nonvolatile phenolic compounds play a major role during fermentation, but also the volatile phenolic compounds formed by enzymatic reactions during fermentation contribute to the formation of the aromas of the final product. Another factor to consider is the composition and the concentration of the initial sugars. The nature of the sugar can also affect the fermentation process. Monosaccharides can produce carbon dioxide faster than disaccharides. Furthermore, many other factors can play a role in the progression of fermentation. The glucose and fructose concentrations may influence the yeast growth, i.e., a high sugar concentration will reduce the growth rate of certain yeast strains. For sugar concentrations between 200 and 300 g/L, a decrease in the growth rate of S. cerevisiae was observed[14][15]. Furthermore, high sugar levels increase the yeast demand for assimilable nitrogen, which can similarly inhibit the fermentation [16]. For low glucose concentrations, yeasts use sugars either by respiration or fermentation. Aeration induces an increase in the biomass formed (total and per unit of degraded sugar), and at the same time, a decrease in alcohol production and sugar consumption; Pasteur then retained that respiration inhibits fermentation. For high concentrations of glucose, S. cerevisiae metabolizes sugars only by fermentation. Even in the presence of oxygen, respiration is impossible. Al Daccache et al.[17] reported different fermentative behaviors of the yeast Hanseniaspora sp. during the fermentation of Lebanese “Ace spur” and French “Kermerrien” apple juices. The apples used had different chemical compositions, where the “Ace spur” apple juice had almost the double concentrations of sugars, compared to “Kermerrien” one. Different biomass and ethanol kinetics were obtained. In the presence of an excess of sugar, the yeast cells followed the fermentative pathway from the first hour of fermentation. For the fermentation of “Kermerrien” apple juice, the cells were in a respiratory mode generating biomass in the early hours of fermentation[17]. Some variables, such as temperature and pH, can influence yeast growth rates and the ecology and adaptation of yeast strains[14][15] . Rosend et al.[18] studied the impact of four apple varieties grown in Estonia, Antei, Melba, Kulikovskoye, and Orlovski Sinap, on cider fermentation. Alcoholic fermentation was carried out using the must from the apples at various stages of ripening (i.e., unripe, ripe, overripe) and commercially available yeast strains. The differences in volatile composition between the samples were assessed. The results showed that apple variety stands as the principal attribute influencing the quality and aroma properties of apple cider. The maturity of the fruit was variety-specific, the volatile profiles of Melba variety ciders were the least affected by the ripening stage of apples [18]. Organic acids are indicators of quality during cider fermentation. The dominant flavor of organic acids is sourness, but they also contribute to bitterness and astringency of cider [19]. Some yeasts can assimilate malic acid resulting in its reduction, fluctuating from 5 to 40% [20]. When a second bacterial fermentation occurs, its level is reduced mainly by lactic acid bacteria. During this fermentation, citric acid is transformed into acetic acid, whereas shikimic and quinic acids are metabolized to single phenols, like catechol and ethylcatechol, and other compounds. Organic acids may affect the yeast metabolism. The yeast enzymatic activity and the chemical alterations are also influenced by the juice acidity [21].
3.2. Impact of Yeast on Fermentation
Yeast plays an essential role in the production of all alcoholic beverages, and the selection of an appropriate yeast strain is crucial to control the alcohol yield and to preserve the beverage’s sensory quality. Fermentative yeasts can use sugars anaerobically as electron donors, electron acceptors, and carbon sources. However, the yeast action during fermentation is not only limited to the transformation of sugars into alcohol. Yeast metabolism produces different other metabolites and by-products that may have an essential impact on the organoleptic quality of the fermented product [22]. Thus, the criteria to select yeast strains for their use in fermented beverages comprise their capability to dominate the media and to improve desired sensorial characteristics and their inability to produce undesired compounds such as biogenic amines or off odors [23]. During spontaneous fermentation, several yeast species may be present and could play a significant, complex, and unpredictable role [24]. Some yeast species may be present only during the first stage of fermentation, while others, more resistant to ethanol, are dominant during the later stages. This type of yeast is nowadays known as belonging to the Saccharomyces strains [25]. S. cerevisiae is largely used to produce alcoholic beverages due to its controlled and repetitive behavior as well as for the release of its aroma precursors [26][27][28][29]. Nevertheless, fermentation is the collaboration of different species of yeast and bacteria initially present in the product or found on the surface of the presses and fermenters. Mixed fermentations are suggested as a feasible way to improve the complexity and enhancing the particular and specific characteristics of the product [30]. The growth of each yeast species is characterized by a definite metabolic activity, which determines the concentrations of flavor compounds in the final product. Therefore, the role of non-Saccharomyces yeasts appears important during the fermentation process. The main yeasts present in the early stages of fermentation belong to the genera Hanseniaspora and Candida. These species are characterized by a low fermentation capacity and are sensitive to an alcohol concentration close to 5 or 6%. In addition, some changes in fermentation parameters may result in the presence of yeasts such as Brettanomyces, Kluyveromyces, Schizosaccharomyces, Torulaspora, Zygosaccharomyces, and Saccharomycodes [31][32][33]. From the above-cited yeasts, some of them may have a positive impact on fermentation by releasing favorable aromas, but others may release undesirable aromas known as off-flavors. Yeasts can affect primary aroma determined by the initial composition of the product and the secondary aromas that are created during the fermentation, as well as the tertiary aromas generated during the maturation of the finished product [34]. Hanseniaspora, Zygosaccharomyces, and Schizosaccharomyces pombe species produce high amounts of volatile fatty acids, such as acetic acid[35][36][37][38][39][40], and low concentrations of higher alcohols[41][42][43][44]. Esters and sulfur compounds are mainly produced by Candida, Hansenisapora, Torulaspora delbrueckii, and Kazachstania gamospora[42][45][46][47]. Lorenzini et al. [48] investigated the capacity of Torulaspora delbrueckii, Hanseniaspora osmophila, Hanseniaspora uvarum, Starmerella bacillaris, and Zygosaccharomyces bailii to ferment apple juice and found that Hanseniaspora uvarum was the greatest producer of hexyl and isoamyl acetate. The complex volatile profile of cider suggests the possible strain-specific effects on the aroma formation. Wei et al. [49]tried to enhance the flavor complexity of cider by different non-Saccharomyces species. The chemical composition and sensory properties of five different fermentations of mixed cultures of Pichia kluyveri, Hanseniaspora vineae, Hanseniaspora uvarum, and Torulaspora quercuum were studied for apple juice fermentation. The results indicated that the growth of P. kluyveri and H. vineae were interreacted and affected by H. uvarum and T. quercuum. Furthermore, H. vineae was able to consume more sugar than P. kluyveri. In general, the fermentations involving H. uvarum displayed high pH values, whereas those involving P. kluyveri and the mixed P. kluyveri and H. uvarum resulted in high levels of residual sugar, sugar/acid ratio, and glucose-fructose consumption ratio. The pair P. kluyveri and H. uvarum produced the highest concentration of glycerol. Noticeable variations in organic acids and polyphenols were observed between the different fermentations. The analysis showed that acetate esters contributed the most positively to the roasted and cooked aroma note in all ciders. This was the first study evaluating the simultaneous fermentation of two non-Saccharomyces yeasts to produce cider. A recent study described the antagonistic and fermentative properties of Starmerella bacillaris. The yeast proved to positively modulate cider volatile profile in the microfermentation trials [50]. Brettanomyces, Kluyveromyces, Schizosaccharomyces, Torulaspora, Zygosaccharomyces, and Saccharomycodes have a negative influence on the product [51]. Brettanomyces may produce 2-ethyltetrahydropyridine, 2-acetyltetrahydopyridine, and 2-acetylpyrroline, causing taste defects and unpleasant smell in beverages.
Non-Saccharomyces yeasts have high enzyme activity such as β-glucosidase, esterase, and β-lyase. This enzyme activity contributes to a higher concentration of terpenes and thiols that may add a positive fruity aroma and fragrance to the fermented product [52][53][54][55]. De Arruda Moura Pietrowski et al. [56] and Wosiacki et al. [57] noted that the strains of Hanseniaspora sp. have a positive impact on the aromatic profile of cider, thereby accentuating the beneficial role of these yeasts. Nowadays, modern oenology is searching for novel strategies to reduce the final ethanol content in fermented beverages. This trend is due to consumer demand for products with lower ethanol content. The use of non-Saccharomyces species reduces the initial ethanol content by approximately 1–2% (v/v), depending on the yeast species and fermentation conditions [58][59][60]. In addition, these yeasts can be used to regulate the acidity of drinks [61][62] as Saccharomyces yeasts have no significant influence on acidity [63][64], and conventional chemical methods consist of the addition of expensive and qualified products being of food quality.
References
- Ferree, D.C.; Warrington, I.J. Apples Botany, Production and Uses; CABI Publishing: Oxfordshire, UK, 2015; Volume 1; ISBN 9788578110796
- United States Department of Agriculture. Fresh Apples Fresh Domestic Consumption by Country in MT; U.S. Department of Agriculture: Washington, DC, USA, 2019
- Al Daccache, M.; Koubaa, M.; Salameh, D.; Maroun, R.G.; Louka, N.; Vorobiev, E. Ultrasound‐assisted fermentation for cider production from Lebanese apples. Ultrason. Sonochem. 2020, 63, 104952, doi:10.1016/j.ultsonch.2019.104952.
- Al Daccache, M.; Koubaa, M.; Salameh, D.; Vorobiev, E.; Maroun, R.G.; Louka, N. Control of the sugar/ethanol conversion rate during moderate pulsed electric field‐assisted fermentation of a Hanseniaspora sp. strain to produce low‐alcohol cider. Innov. Food Sci. Emerg. Technol. 2020, 59, 102258, doi:10.1016/j.ifset.2019.102258.
- AL Daccache, M.; Salameh, D.; Chamy, L.E.L.; Koubaa, M.; Maroun, R.G.; Vorobiev, E.; Louka, N. Evaluation of the fermentative capacity of an indigenous Hanseniaspora sp. strain isolated from Lebanese apples for cider production. FEMS Microbiol. Lett. 2020, 367, fnaa093, doi:10.1093/femsle/fnaa093
- Andrew, L.G.H.; Piggott, J.R. Cidermaking. In Fermented Beverage Production; Kluver Academic/Plenum: New York, NY, USA, 2003; ISBN 9780306477065.
- Alonso‐Salces, R.M.; Guyot, S.; Herrero, C.; Berrueta, L.A.; Drilleau, J.F.; Gallo, B.; Vicente, F. Chemometric characterisation of Basque and French ciders according to their polyphenolic profiles. Anal. Bioanal. Chem. 2004, 379, 464–475
- Mangas, J.J.; Rodríguez, R.; Suárez, B.; Picinelli, A.; Dapena, E. Study of the phenolic profile of cider apple cultivars at maturity by multivariate techniques. J. Agric. Food Chem. 1999, 47, 4046–4052.
- Nogueira, A.; Guyot, S.; Marnet, N.; Lequéré, J.M.; Drilleau, J.F.; Wosiacki, G. Effect of alcoholic fermentation in the content of phenolic compounds in cider processing. Braz. Arch. Biol. Technol. 2008, 51, 1025–1032.
- Lata, B. Relationship between apple peel and the whole fruit antioxidant content: Year and cultivar variation. J. Agric. Food Chem. 2007, 55, 663–671.
- van der Sluis, A.A.; Dekker, M.; de Jager, A.; Jongen, W.M. Activity and concentration of polyphenolic antioxidants in apple: Effect of cultivar, harvest year, and storage conditions. J. Agric. Food Chem. 2001, 49, 3606–3613.
- Symoneaux, R.; Baron, A.; Marnet, N.; Bauduin, R.; Chollet, S. Impact of apple procyanidins on sensory perception in model cider (part 1): Polymerisation degree and concentration. LWT Food Sci. Technol. 2014, 57, 22–27.
- Park, J. Characterizing and Improving the Oral Sensations and Preference of Polyphenol‐Rich Aronia Berry Juice; Honors Scholar Theses.348; University of Connecticut: Storrs, CT, USA, 2014.
- Arroyo‐López, F.N.; Orlić, S.; Querol, A.; Barrio, E. Effects of temperature, pH and sugar concentration on the growth parameters of Saccharomyces cerevisiae, S. kudriavzevii and their interspecific hybrid. Int. J. Food Microbiol. 2009, 131, 120–127.
- D’Amato, D.; Corbo, M.R.; Del Nobile, M.A.; Sinigaglia, M. Effects of temperature, ammonium and glucose concentrations on yeast growth in a model wine system. Int. J. Food Sci. Technol. 2006, 41, 1152–1157
- Boudreau, T.F.; Peck, G.M.; O’Keefe, S.F.; Stewart, A.C. Free amino nitrogen concentration correlates to total yeast assimilable nitrogen concentration in apple juice. Food Sci. Nutr. 2018, 6, 119–123.
- Al Daccache, M.; Koubaa, M.; Maroun, R.G.; Salameh, D.; Louka, N.; Vorobiev, E. Suitability of the Lebanese “Ace Spur” apple variety for cider production using Hanseniaspora sp. yeast. Fermentation 2020, 6, 32
- Rosend, J.; Kuldjarv, R.; Rosenvald, S.; Paalme, T. The effects of apple variety, ripening stage, and yeast strain on the volatile composition of apple cider. Heliyon 2019, 5, e01953
- Lawless, H.T.; Horne, J.; Giasi, P. Astringency of organic acids is related to pH. Chem. Senses 1996, 21, 397– 403
- Whiting, G.C. Organic acid metabolism of yeasts during fermentation of alcoholic beverages—A review. J. Inst. Brew. 1976, 82, 84–92.
- Zhang, H.; Zhou, F.; Ji, B.; Nout, R.M.J.; Fang, Q.; Yang, Z. Determination of organic acids evolution during apple ciderfermentation using an improved HPLC analysis method. Eur. Food Res. Technol. 2008, 227, 1183– 1190
- Walker, G.M.; Stewart, G.G. Saccharomyces cerevisiae in the production of fermented beverages. Beverages 2016, 2, 30.
- Dalia, E. Miranda Castilleja, J.A.A.T.; Medrano, S.M.A.; Tapia; Medrano, S.M.A.; Iturriaga, M.H.; Iturriaga, H.; Muñoz, L.S.; Peniche; Peniche, R.Á. Growth kinetics for the selection of yeast strains for fermented beverages. In Yeast—Industrial Applications Conversion; InTech: London, UK, 2017; pp. 67–87.
- Ciani, M.; Comitini, F.; Mannazzu, I.; Domizio, P. Controlled mixed culture fermentation: A new perspective on the use of non‐Saccharomyces yeasts in winemaking. Fems Yeast Res. 2010, 10, 123–133.
- Barnett, J.A. A history of research on yeasts 2: Louis Pasteur and his contemporaries, 1850–1880. Yeast 2000, 16, 755–771.
- Ubeda, J.; Briones, A. Characterization of differences in the formation of volatiles during fermentation within synthetic and grape musts by wild Saccharomyces strains. LWT Food Sci. Technol. 2000, 33, 408 414.
- Dubourdieu, D.; Tominaga, T.; Masneuf, I.; Peyrot des Gachons, C.; Murat, M.L. The role of yeast in grape flavour development during fermentation: The example of Sauvignon blanc. Am. J. Enol. Vitic. 2006, 57, 81– 88.
- Ugliano, M.; Bartowsky, E.J.; McCarthy, J.; Moio, L.; Henschke, P.A. Hydrolysis and transformation of grape glycosidically bound volatile compounds during fermentation with three Saccharomyces yeast strains. J. Agric. Food Chem. 2006, 54, 6322–6331.
- Pretorius, I. The Genetic Analysis and Tailoring of Wine Yeasts. In Functional Genetics of Industrial Yeasts; de Winde, J.H., Ed.; Springer: Berlin, Heidelberg, Germany, 2008; pp. 99–142
- Pretorius, I.S.; van der Westhuizen, T.J.; Augustyn, O.P.H. Yeast biodiversity in vineyards and wineries and its importance to the South African wine industry. South Afr. J. Enol. Vitic. 1999, 20, 61–75.
- Combina, M.; Elía, A.; Mercado, L.; Catania, C.; Ganga, A.; Martinez, C. Dynamics of indigenous yeast populations during spontaneous fermentation of wines from Mendoza, Argentina. Int. J. Food Microbiol. 2005, 99, 237–243.
- Fleet, G.H.; Lafon‐Lafourcade, S.; Ribereau‐Gayon, P. Evolution of yeasts and lactic acid bacteria during fermentation and storage of Bordeaux wines. Appl. Environ. Microbiol. 1984, 48, 1034–1038.
- Martínez, J.; Millán, C.; Ortega, J.M. Growth of natural flora during fermentation of inoculated musts from “Pedro Ximenez” grapes. South Afr. J. Enol. Vitic. 1989, 10, 31–35
- Padilla, B.; Gil, J.V.; Manzanares, P. Past and future of non‐Saccharomyces yeasts: From spoilage microorganisms to biotechnological tools for improving wine aroma complexity. Front. Microbiol. 2016, 7, 1–20.
- Ciani, M.; Maccarelli, F. Oenological properties of non‐Saccharomyces yeasts associated with wine‐making. World J. Microbiol. Biotechnol. 1998, 14, 199–203.
- Loureiro, V.; Malfeito‐Ferreira, M. Spoilage yeasts in the wine industry. Int. J. Food Microbiol. 2003, 86, 23–50.
- Romano, P.; Fiore, C.; Paraggio, M.; Caruso, M.; Capece, A. Function of yeast species and strains in wine flavour. Int. J. Food Microbiol. 2003, 86, 169–180.
- Francesca Comitini; Gobbi, M.; Domizio, P.; Romanib, C.; Lencioni, L.; Mannazzud, I.; Ciani, M. Selected non‐Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae on alcoholic fermentation behaviour and wine aroma of cherry wines. Food Microbiol. 2011, 44, 15–23
- Rantsiou, K.; Dolci, P.; Giacosa, S.; Torchio, F.; Tofalo, R.; Torriani, S.; Suzzi, G.; Rolle, L.; Cocolina, L.
- Candida zemplinina can reduce acetic acid produced by Saccharomyces cerevisiae in sweet wine fermentations. Appl. Environ. Microbiol. 2012, 78, 1987–1994.
- Romano, P.; Suzzi, G. Higher alcohol and acetoin production by Zygosaccharomyces wine yeasts. J. Appl. Bacteriol. 1993, 75, 541–545.
- Rojas, V.; Gil, J.V.; Piñaga, F.; Manzanares, P. Acetate ester formation in wine by mixed cultures in laboratory fermentations. Int. J. Food Microbiol. 2003, 86, 181–188.
- Clemente‐Jimenez, J.M.; Mingorance‐Cazorla, L.; Martínez‐Rodríguez, S.; Las Heras‐Vázquez, F.J.; Rodríguez‐Vico, F. Molecular characterization and oenological properties of wine yeasts isolated during spontaneous fermentation of six varieties of grape must. Food Microbiol. 2004, 21, 149–155.
- Moreira, N.; Mendes, F.; Guedes de Pinho, P.; Hogg, T.; Vasconcelos, I. Heavy sulphur compounds, higher alcohols and esters production profile of Hanseniaspora uvarum and Hanseniaspora guilliermondii grown as pure and mixed cultures in grape must. Int. J. Food Microbiol. 2008, 124, 231–238
- Moreira, N.; Mendes, F.; Hogg, T.; Vasconcelos, I. Alcohols, esters and heavy sulphur compounds production by pure and mixed cultures of apiculate wine yeasts. Int. J. Food Microbiol. 2005, 103, 285 294.
- Rojas, V.; Gil, J.V.; Piñaga, F.; Manzanares, P. Studies on acetate ester production by non Saccharomyces wine yeasts. Int. J. Food Microbiol. 2001, 70, 283–289.
- Viana, F.; Gil, J.V.; Genovés, S.; Vallés, S.; Manzanares, P. Rational selection of non‐ Saccharomyces wine yeasts for mixed starters based on ester formation and enological traits. Food Microbiol. 2008, 25, 778–785.
- Lorenzini, M.; Simonato, B.; Slaghenaufi, D.; Ugliano, M.; Zapparoli, G. Assessment of yeasts for apple juice fermentation and production of cider volatile compounds. LWT 2019, 99, 224–230.
- Wei, J.; Zhang, Y.; Wang, Y.; Ju, H.; Niu, C. Assessment of chemical composition and sensorial properties of ciders fermented with different non‐Saccharomyces yeasts in pure and mixed fermentations. Int. J. Food Microbiol. 2020, 318, 108471.
- Lemos Junior, W.J.F.; Binati, R.L.; Felis, G.E.; Slaghenaufi, D.; Ugliano, M.; Torriani, S. Volatile organic compounds from Starmerella bacillaris to control gray mold on apples and modulate cider aroma profile. Food Microbiol. 2020, 89, 103446.
- Shinohara, T.; Kubodera, S.; Yanagida, F. Distribution of phenolic yeasts and production of phenolic off‐flavors in wine fermentation. J. Biosci. Bioeng. 2000, 90, 90–97.
- Cûs, F.; Jenko, M. The influence of yeast strains on the composition and sensory quality of Gewürztraminer wine. Food Technol. Biotechnol. 2013, 51, 547–553.
- López, M.C.; Mateo, J.J.; Maicas, S. Screening of β‐Glucosidase and β‐Xylosidase activities in four non‐Saccharomyces yeast isolates. J. Food Sci. 2015, 80, C1696–C1704.
- Rosi, I.; Vinella, M.; Domizio, P. Characterization of beta‐glucosidase activity in yeasts of enological origin. J. Appl. Bacteriol. 1994, 77, 519–527.
- Spagna, G.; Barbagallo, R.N.; Palmeri, R.; Restuccia, C.; Giudici, P. Properties of endogenous beta—glucosidase of a Saccharomyces cerevisiae strain isolated from Sicilian musts and wines. Enzym. Microb. Technol. 2002, 31, 1030–1035.
- de Arruda Moura Pietrowski, G.; dos Santos, C.M.E.; Sauer, E.; Wosiacki, G.; Nogueira, A. Influence of fermentation with Hanseniaspora sp. yeast on the volatile profile of fermented apple. J. Agric. Food Chem. 2012, 60, 9815–9821.
- Wosiacki, G.; Nogueira, A.; Silva, N.C.C.; Denardi, F.; Vieira, R.G. Quality profile of samples of 139 apples. Acta Aliment. 2008, 37, 9–22.
- Ciani, M.; Morales, P.; Comitini, F.; Tronchoni, J.; Canonico, L.; Curiel, J.A.; Oro, L.; Rodrigues, A.J.; Gonzalez, R. Non‐conventional yeast species for lowering ethanol content of wines. Front. Microbiol. 2016, 7, 642.
- Röcker, J.; Strub, S.; Ebert, K.; Grossmann, M. Usage of different aerobic non‐ Saccharomyces yeasts and experimental conditions as a tool for reducing the potential ethanol content in wines. Eur. Food Res. Technol. 2016, 242, 2051–2070
- Contreras, A.; Hidalgo, C.; Henschke, P.A.; Chambers, P.J.; Curtin, C.; Varela, C. Evaluation of non‐Saccharomyces yeasts for the reduction of alcohol content in wine. Appl. Environ. Microbiol. 2014, 80, 1670–1678.
- Benito, Á.; Calderón, F.; Benito, S. Mixed alcoholic fermentation of Schizosaccharomyces pombe and Lachancea thermotolerans and its influence on mannose‐containing polysaccharides wine composition. Amb Express 2019, 9, 17.
- Benito, S. The impacts of Lachancea thermotolerans yeast strains on winemaking. Appl. Microbiol. Biotechnol. 2018, 102, 6775–6790.
- Redzepovic, S.; Orlic, S.; Majdak, A.; Kozina, B.; Volschenk, H.; Viljoen‐Bloom, M. Differential malic acid degradation by selected strains of Saccharomyces during alcoholic fermentation. Int. J. Food Microbiol. 2003, 83, 49–61.
- Zelle, R.M.; de Hulster, E.; van Winden, W.A.; de Waard, P.; Dijkema, C.; Winkler, A.A.; Geertman, J.‐M.A.; van Dijken, J.P.; Pronk, J.T.; van Maris, A.J.A. Malic acid production by Saccharomyces cerevisiae: Engineering of pyruvate carboxylation, oxaloacetate reduction, and malate export. Appl. Environ. Microbiol. 2008, 74, 2766–2777.