| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Aroa Mañas-Ojeda | + 4913 word(s) | 4913 | 2020-08-14 04:11:44 | | | |
| 2 | Camila Xu | Meta information modification | 4913 | 2020-08-17 08:45:10 | | | | |
| 3 | Camila Xu | + 1 word(s) | 4914 | 2020-08-17 08:49:21 | | | | |
| 4 | Camila Xu | + 1 word(s) | 4914 | 2020-08-17 08:54:27 | | | | |
| 5 | Camila Xu | + 1 word(s) | 4914 | 2020-08-17 08:57:52 | | | | |
| 6 | Camila Xu | + 1 word(s) | 4914 | 2020-08-17 08:59:04 | | | | |
| 7 | Camila Xu | + 1 word(s) | 4914 | 2020-08-17 09:00:17 | | | | |
| 8 | Camila Xu | Meta information modification | 4914 | 2020-08-17 09:01:00 | | | | |
| 9 | Camila Xu | Meta information modification | 4914 | 2020-08-17 09:04:09 | | | | |
| 10 | Camila Xu | -134 word(s) | 4780 | 2020-10-27 05:04:32 | | | | |
| 11 | Francisco Olucha-Bordonau | + 51 word(s) | 4831 | 2021-01-30 23:40:17 | | |
Video Upload Options
Stress could be defined as an organism's entire response to environmental demands or strains. Exposure to highly stressful or traumatic events, depending on the stage of life in which stress exposure occurs, could severely affect limbic structures, including the amygdala, and lead to alterations in social and affective behaviors.
1. Introduction
Human beings are a highly social species. This does not make us special, since all mammals exhibit some degree of social behavior, such as cooperation, affiliation or aggression, that allows us to survive and thrive. However, socio-affective relations are so important for human health and well-being that many psychiatric and neurological disorders are characterized by prominent impairments in social or affective functioning. Autism spectrum disorder (ASD), schizophrenia, bipolar disorder, major depression and social anxiety disorder are just some examples[1][2] [3].
Social and affective behaviors occur at every stage of our lives, beginning in infancy with caregiver attachment, followed by peer interactions during childhood and adolescence and the formation of pair-bonds and paternal behaviors during adulthood and old age[4]. Our “social world”, that is, the environment and people around us, has a crucial role in the development and maintenance of our socio-affective behavior. While normative or positive stimuli are necessary for proper neurological and behavioral development, impairments in the quality of this “social world” can be exceptionally detrimental and lead to psychopathology[2][5].
The mammalian brain is extraordinarily plastic, capable of restructuring synaptic connections in response to a changing environment. In fact, during brain development, there are stages of heightened plasticity, the so-called critical periods or critical windows of plasticity, when environment is extremely effective in producing lifelong changes in the brain [6]. These critical periods are a time not only of opportunity, but also of great vulnerability. Exposure to supportive or enriching situations during these periods may favor the long-term remodeling of neurobehavioral trajectories so as to promote better stress-coping strategies [7]. Conversely, the exposure to stressful or traumatic situations during critical periods of plasticity may impair these trajectories and therefore contribute to the emergence of psychopathologies and abnormal behaviors later in life (affective disorders, schizophrenia, violence, addiction, etc.) [5][8][9]. In a global and simple definition "stress is any influence of internal and/or surrounding environment on living being which disrupt its homeostasis"[10] .Among the different age stages at which individuals are more vulnerable to the effects of stress, the majority of studies in animal models have focused on the early postnatal period [7]. Lately, the peri-pubertal period (which encompasses late childhood and adolescence) has also attracted significant attention. In fact, current evidence has identified this age stage as a period of enhanced vulnerability to the effects of stress, and also as a strong modulator of socio-affective behaviors in adulthood[5][11]. Moreover, many neuropsychiatric disorders are thought to emerge during this period [12].
The ability to cope with a stressor depends on several intrinsic factors, including age, as was just mentioned, but also on some properties of the stressor itself, such as duration, strength and unpredictability[13][14]. In fact, the response to an acutely stressful stimulus is considered to be beneficial, since it is an evolutionary mechanism of adaption to an environmental change. However, if a stressor becomes chronic, or if it is unpredictable or too severe, the ability to cope with the stressor can be impaired, leading to behavioral alterations or even to the emergence of psychopathologies later in life [14][15]. This is the so-called maladaptive response to stress[15] and will be the focus of the present review. This maladaptive response to stress, depending on the stage of life in which stress exposure occurs, could differently affect social and affective behavior [11][15][16] These effects can be persistent or reversible, and can be suffered just after stress exposure, later in life, or can even affect several offspring generations, in what is known as the transgenerational inheritance of stress [11][17][18][19][20].
Violence, particularly against women and children, is one of the most important social and public health problems worldwide, affecting up to 1 billion children[21] and nearly one-third of the world’s female population[22]. Many stressors have been associated with these high levels of violence, including social neglect [23] and socio-economic inequality [24]. When children are raised in disadvantaged social environments, they are at risk of developing severe mental health problems during adulthood, including anti-social and violent behaviors. These problems can even be inherited by future generations, as was mentioned above [25]. Social neglect is one of the important factors that can elicit this pathological aggression, especially when it occurs in early life[5][11][25], although the underlying neural bases of such phenomenon remain poorly understood.
In this regard, experimental animal models devoid of human cultural connotations constitute a valuable tool for studying anti-social behaviors and affective impairments related to stress. Animal models are also indispensable for investigating the molecular and structural changes in the brain related to these stressors (Figure 1). In addition, as numerous parallelisms have been identified between rodents and humans regarding age periods of heightened susceptibility to environmental stimulation, studies using rodent models of stress can complement studies in humans [7].
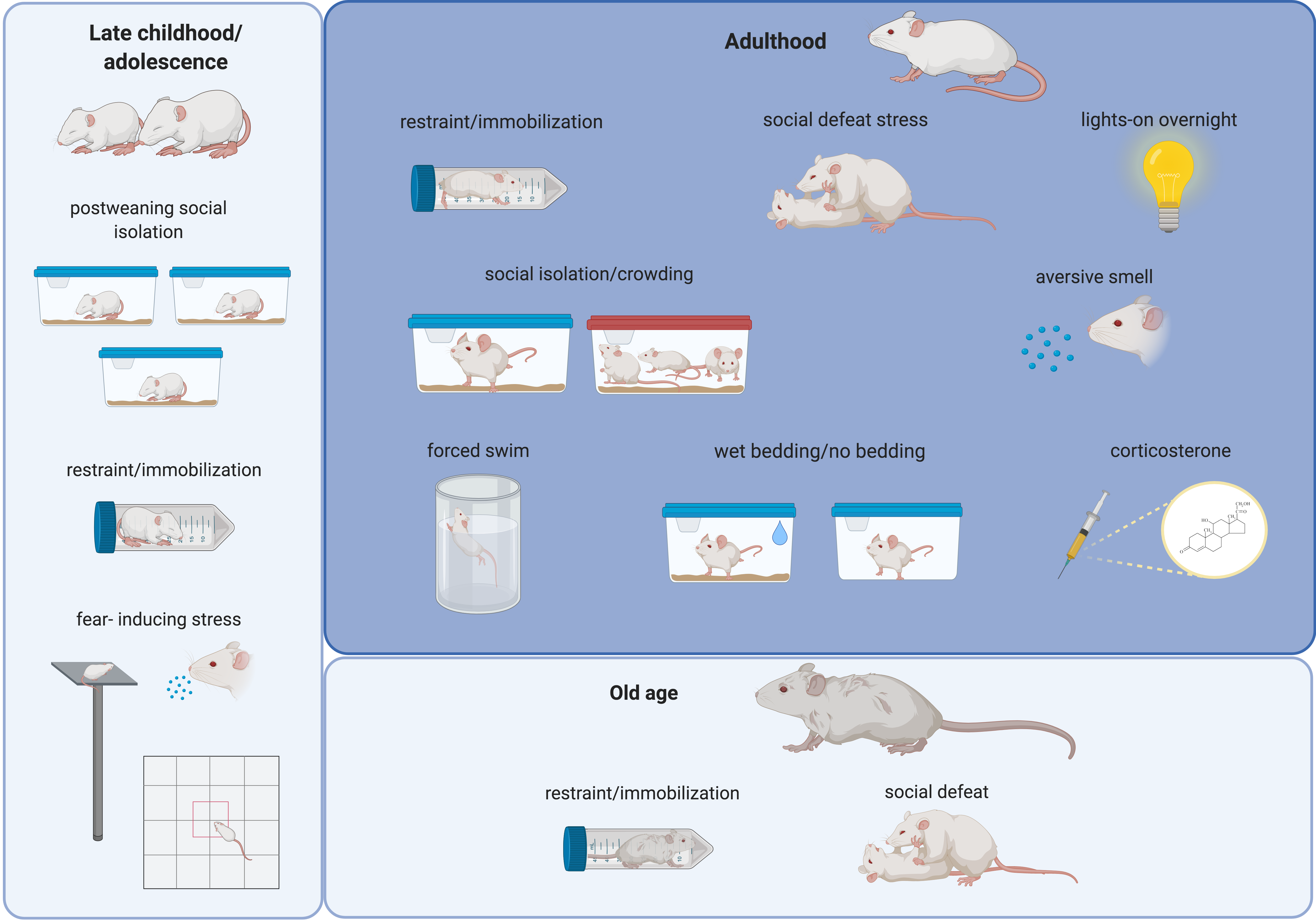
Figure 1. Schematic drawing summarizing the most frequently used stressors in rodents during different last periods of the lifespan. Alterations in the structure and function of the amygdala and in social and affective behaviors have been related to these stressors (see text). Created with BioRender.com.
Among all the regions that have been implicated in social and affective behavior, the amygdala seems to play a pivotal role[26]. Glucocorticoid receptors are highly expressed by neurons in the amygdala, what makes these neurons one of the main targets of the neuroendocrine mediator of stress, the hypothalamic pituitary adrenocortical (HPA) axis [27]. Research in humans and animal models has shown that the amygdala is a critical mediator of social behavior and affective processing [26][28][29]. In fact, alterations in this brain region have been found in many neuropsychiatric and behavioral disorders, including schizophrenia, ASD, major depression and social phobia, among others[2][26][28][29]. These studies in humans and animal models, together with classic studies based on amygdaloid lesions in nonhuman primates, have positioned the amygdala at the center of the socio-affective brain [28]. Excitatory and inhibitory amino acids, glucocorticoids and a growing list of intra- and extracellular mediators, which includes endocannabinoids (eCB) and brain-derived neurotrophic factor (BDNF), have also been identified to play key roles in social and affective processes [30][31]. Enviromental insults, including stressful events, result in an incessantly changing pattern in the expression of genes related to these aminoacids, glucocorticoids, eCB and BDNF. These changes may be mediated by epigenetic mechanisms, as we will also discuss.
This review summarizes the recent findings of stress research, focused on peripubertal, adult and elderly stress, and provides an overview of its age-dependent effects on the structure and function of the amygdala, which includes molecular and cellular changes, and how they can trigger deviant social and affective behaviors. The findings in this field may represent an advance both for medical science and for society, as they may help in the development of new therapeutic approaches and prevention strategies in neuropsychiatric disorders and pathological behaviors
2. Stress Exposure during Late Childhood and Adolescence: Peripubertal Stress
Puberty is defined as “the peak phase of maturation of the hypothalamo–pituitary–gonadal axis, when alterations in gonadotropin levels in circulation and elevated levels of sex steroids occur” [32]. The peripubertal period (or periadolescent period) is characterized by hormonal and neurophysiological changes that make peri-adolescent individuals unique in their species compared to younger or older members [5] [33]. In humans, this period includes late childhood and adolescence, and is broadly considered to range from 10–12 to 18–19 years of age [5][[34]. In rodents, the first observable signs of puberty have been reported around postnatal day 28 in mice [32] and postnatal day 41 in rats[34], so the peripubertal period can considered by researchers to range from the age of weaning (postnatal day 21) to late adolescence (7–8 weeks of age) [5][32][33].
In humans, substantial evidence indicates that exposure to highly stressful or traumatic events during late childhood and adolescence is a predisposing factor for developing psychopathologies and alterations in socio-affective behavior, including schizophrenia [35][36][37], major- depression and anxiety-related disorders [38][39], violence[40] [41][42][43] and impaired social function [35][36][44].
Stress susceptibility differs between the peri-pubertal and the adult brain in both humans and rodents. In fact, although the glucocorticoids levels in peri-adolescent individuals are similar to those of individuals at other age stages, when exposed to stress, both the duration and the amount of glucocorticoids are higher during puberty. This points to puberty as another critical period for shaping the HPA axis’ responsiveness[14]. The impact of stress in rodents also differs behaviorally between peri-puberty and adulthood (i.e., adolescent female rats exhibit “play and avoidant behaviors”, but not aggressive behaviors when facing a resident female, and exhibit less anxiety in response to social defeat stress). By contrast, the programming effects of stress during puberty are very similar to those observed in earlier age stages, thus adolescent rats exposed to stress show altered socio-affective behaviors in adulthood [5][14] .
Many different rodent models of peripubertal stress have been described (Figure 1 and Table 1) in an attempt to best mimic the effects of stress suffered during late childhood and adolescence in humans.
Table 1. Summary of the main molecular, cellular and behavioral alterations reported after peripubertal stress exposure in rodents.
| Stressor | Stress Protocol | Molecular/Cellular | Behavior | References | ||
|---|---|---|---|---|---|---|
| Assessment | Age | Assessment | Age | |||
| Post-weaning social isolation stress model (PWSI) | Individual cages from P21 to P82 | ↓ BDNF expression (amygdala) | P82 | pathological aggression, ↓ social communication |
P82 | [9][45] |
| Individual cages from P28 to P109 | ↑ BLA pyramidal cell excitability | P101–P115 | ↑ anxiety | P101–P115 | [46] | |
| Individual cages from P21 to P90 | ↑ GAD67 protein (CeM, MeA, BLA) | P90 | [47] | |||
| Individual cages from P21 to P90 | ↑ BLA, BMA, Ce volume ↑ number of PV+ interneurons (BLA, BMA) ↓ PSA-NCAM protein (amygdala) ↓ VGLUT1/VGAT (LA, BLA) ↑ mRNA CB1-R (amygdala) |
P90 | ↑ anxiety | P90 | [48] | |
| Unpredictable stress | fear-inducing stressors: open field, fox-odor, elevated platform (P28–P30, P34, P36, P40, P42) | ↑ mRNA NR1 (amygdala) ↓ mRNA GAD67 (amygdala) ↑ VGLUT1/VGAT (CeA) ↑ mRNA encoding GR and ↓ number of GR + cells (CeA) ↓ GAD and GABA-A receptor α3 (LA, BLA, BMA, MeA, CeA) |
P90 | ↓ sociability, ↑ anxiety, ↑ novelty reactivity, pathological aggression |
P90 | [34][49][50][51][52] |
| Repeated restraint stress | 20 min/day from P29 to P37 | ↓ GABAergic inhibition of LA projection neurons ↓ presynaptic GABA function and interneuron activity (LA) |
P39 | ↑ anxiety | P38 | [53] |
| 2 h/day restraint session + 40 tail shocks /day (from P22 to P24) | ↓ serotoninergic modulation of GABAergic transmission (BLA), amygdala hyperexcitability | P24–P25 | [54] | |||
Symbols and abbreviations: ↑ (increase), ↓ (decrease), P (postnatal day), BLA (basolateral nuclei of the amygdala), BMA (basomedial nucleus of the amygdala), LA (lateral nuclei of the amygdala), MeA (medial nucleus of the amygdala), CeA (central nucleus of the amygdala), CB1-R (cannabinoid receptor 1), Bdnf (brain derived neurotrophic factor, gene), BDNF (brain derived neurotrophic factor, protein), GAD (glutamic acid decarboxylase), GABA (gamma-amino butyric acid), GABA-A (GABA type A receptor), NR1 (subunit 1 of the N-methyl-D-aspartate-receptor), GR (glucocorticoid receptor), VGLUT (vesicular glutamate transporter), VGAT (vesicular GABA transporter).
The post-weaning social isolation stress model (PWSI) is one of the most widely used rodent models of social neglect [25]. In most laboratories, PWSI protocol consists of the housing of pups in individual cages from the first day of weaning (postnatal day 21) until adulthood, a time period covering the end of childhood and all adolescence [8][9][45][46][47][48]. Isolated animals are usually reared in the same room as other group-housed or isolated-reared rodents, so they have auditory, olfactory and sometimes visual but not physical contact with other conspecifics. Normal social and emotional development needs physical interactions with conspecifics from birth to early adulthood, thus it is not surprising that PWSI leads to alterations in social and emotional behavior, including pathological aggression [8][9], deficient social communication [9] and increased anxiety [46][47][48]. Interestingly, re-socialization, a laboratory model for behavioral therapy, fails to correct the PWSI-induced pathological aggression, suggesting that this stressor induces long-lasting changes in socio-affective behavior [45]. PWSI also induced a permanent decrease in BDNF expression in the amygdala, which was also demonstrated to be re-socialization-resistant [45]. Adult rodents subjected to PWSI have larger amygdala volumes, specifically in the BMA, BLA and CeA nuclei [48], and increased BLA pyramidal cell excitability, measured by means of electrophysiology[46]. Regarding the molecular and cellular effects of PWSI in the amygdala of adult rodents, many findings have been described, especially regarding the structure and plasticity of inhibitory networks[47] [48]. Rats subjected to PWSI showed increased GAD67 protein levels in the centromedial (CeM), MeA and BLA nuclei during adulthood[47]. Moreover, an increased number of parvalbumin-expressing interneurons was found in the BLA and BMA nuclei of PWSI-mice [48]. In the whole amygdala, a decreased expression of the PSA-NCAM, a plasticity-related molecule, was found, and in the La and BLA nuclei a decreased VGLUT1/VGAT ratio was reported[48]. In the same study, the level of mRNA encoding CB1-R was found to be increased in the amygdala of PWSI-mice [48], highlighting the influence of peripubertal stress on the eCB system.
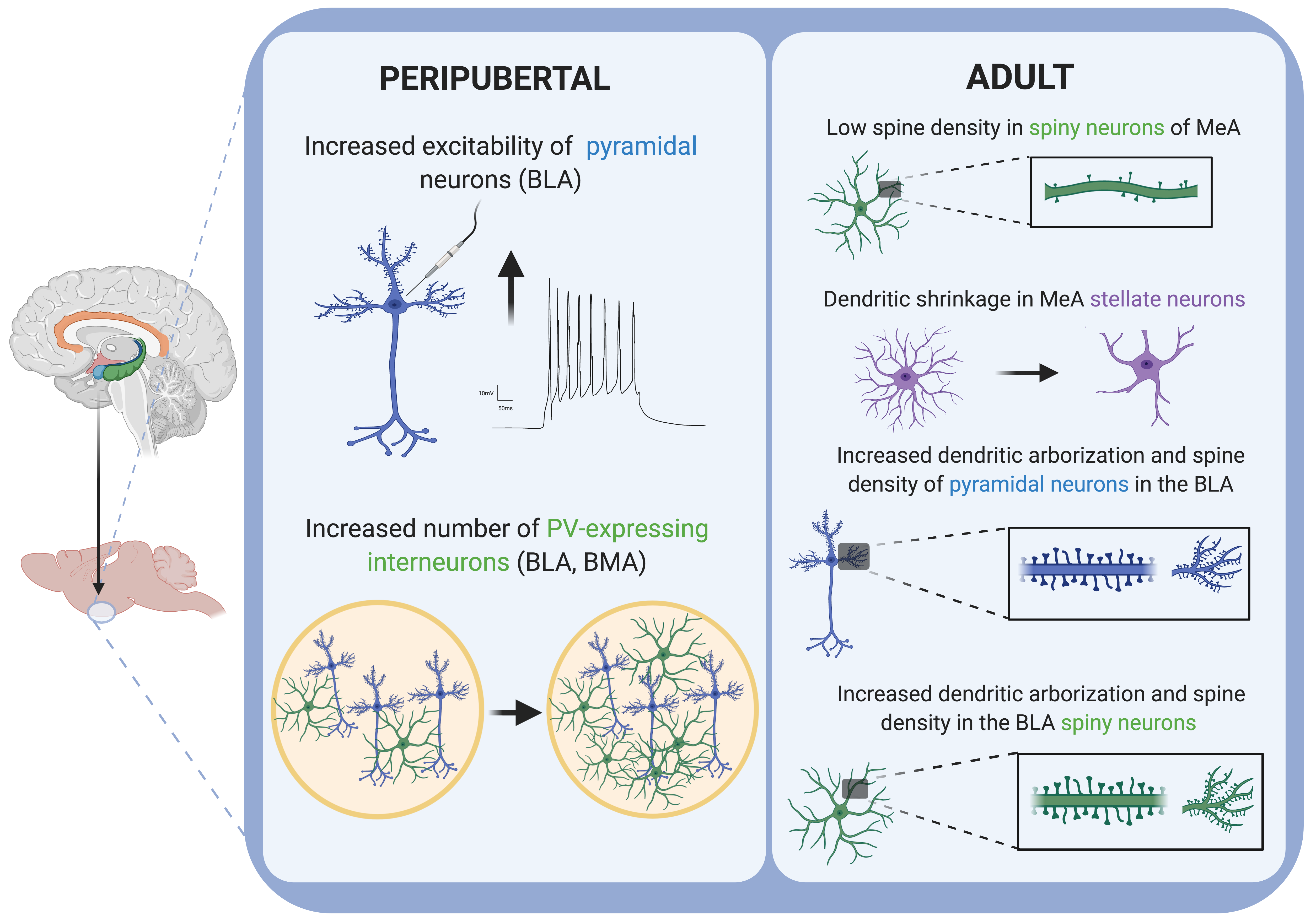
Figure 2. Schematic drawing summarizing the main cellular alterations reported in the amygdala of rodents after stress exposure in different periods of the lifespan. Created with BioRender.com.
Exposing rats to unpredictable stress by using fear-inducing stressors (open field, fox-odor, elevated platform) during the peripubertal period has been demonstrated to induce changes in several behavioral domains during adulthood, including pathological aggression, sociability deficits, and increased anxiety and novelty reactivity[34][49][50][51][52] .Moreover, hyperactivity in the amygdala has been observed in parallel with all these behavioral alterations. Peri-pubertal unpredictable stress also led to molecular changes in different nuclei of the amygdala [34][51][52]. Specifically, peri-pubertally stressed rats showed increased mRNA levels of the N-methyl D-aspartate receptor subunit 1 (NR1), reduced mRNA levels of the glutamic acid decarboxylase 67 enzyme (GAD67) and a heightened excitation/inhibition ratio (measured as the ratio between vesicular glutamate transporter 1 (VGLUT1) and vesicular GABA transporter (VGAT)) in the CeA nucleus of the amygdala [34]. An increased mRNA expression of the glucocorticoid receptor (GR) and a decreased number of GR-expressing cells in the CeA was also observed [52]. In the LA, BLA, BMA, MeA and CeA nuclei of the amygdala, reduced GAD67 and GABA-A receptor α3 was also reported [51]. Importantly, Dr. Sandi’s group also demonstrated that the peripubertal stress protocol they applied in all their studies[34][49][50][51][52] must be applied to its full extent in order to replicate the behavioral and neurobiological alterations they described, since the same protocol applied during the juvenile period only, or during the pubertal period, was demonstrated to be insufficient to produce the same effects [34].
As we have pointed out above, GABAergic inhibition is a key regulator of the activity of the amygdala, including the LA and BLA nuclei, and it has been demonstrated to have a critical influence over the behavioral and emotional sequelae resulting from stress [53][54]. Rats subjected to repeated restraint stress showed reduced GABAergic inhibition of the La projection neurons associated with increased anxiety [53]. When tail shock was added to the restraint stress protocol, animals also showed a severe impairment in the serotoninergic modulation of GABAergic transmission in the BLA, associated with amygdala hyperexcitability[54].
3. Stress Exposure during Adulthood: Adult Stress
Adulthood is biologically defined as the age at which sexual maturity is reached. This holds true for rodents or other animals, but in humans, adulthood is associated with several psychological and cultural concepts [64]. According to the World Health Organization (WHO), “an adult is a person older than 19 years of age unless national law delimits an earlier age”[55] . As mice reach sexual maturity at 8–12 weeks of age, mice older than 8 weeks of age are considered adult [32] .
It is well known that stress, a prevalent experience in modern society, is a major predisposing and triggering factor for mood disorders in humans. Patients suffering from different psychiatric and mental disorders, such as major depression, anxiety disorder or post-traumatic stress disorder (PTSD), often show functional abnormalities in limbic structures, including the amygdala [56][57].
Rodent models of adult stress (Figure 1) not only can recapitulate these abnormalities observed in patients, but can trigger the emergence of symptoms resembling those of human psychiatric disorders [58]. The great majority of the preclinical research on stress has focused for many years on its effects during adulthood, so it is not surprising that the majority of our knowledge concerning the impact of stress on the amygdala and socio-affective behavior derives from adult stress studies. Since many reviews on this topic are available [14] [15] [18] [31][59][60], we will just provide a summary of the principal consequences of adult stressors for the neuroarchitecture and function of the amygdala (Table 2).
Table 2. Summary of the main molecular, cellular and behavioral alterations reported after adult stress exposure in rodents.
| Stressor | Stress Protocol | Molecular/Cellular | Behavior | References | ||
|---|---|---|---|---|---|---|
| Assessment | Age | Assessment | Age | |||
| CRS | 6 h/day for 21 days | ↓ spine density of spiny neurons in the MeA (GABAergic) | P90 | depression-like behavior | P90 | [59][61] |
| 2 h/day for 21 days | Dendritic shrinkage in MeA stellate neurons (↓ arborization, ↓ dendrite length) | P77 | ↓ social interaction ↑ anxiety-like behavior |
P77 | [62] | |
| 1 h/day for 7 days | ↑ firing rate of BLA projecting neurons | P65 | ↑ anxiety-like behavior | P64 | [63] | |
| 1 h/day for 21 days | ↓ GAD67, synaptophysin and PSA-NCAM in the amygdala ↓ dendritic arborization of interneurons in the BLA |
P112 | [64] | |||
| 6 h/day for 10 days | ↑ number of PV + neurons in the BLA | P112 | [65] | |||
| 6 h/day for 21 days | ↓ CB1-R expression in the amygdala ↑ FAAH activity, ↓ AEA amygdala ↑ dendritic arborization, complexity and spine density of pyramidal neurons in the BLA |
P112 | ↑ anxiety-like behavior | P112 | [66] | |
| CUS (Forced swim, restraint, lights-on overnight, aversive smell, wet bedding, no bedding) | 2 weeks | ↑ dendritic arborization and spine density in the BLA spiny neurons (glutamatergic neurons) | P98 | depression-like behavior | P98 | [59][67] |
| 5 weeks | ↑ postsynaptic density-95 protein level in the amygdala and synaptic strengthening | P90 | ↑ behavioral emotionality | P90 | [68] | |
| Chronic social stress | Chronic defeat stress (5 min/day for 5 days) | ↑ POMC in the amygdala | P77 | [69] | ||
| Unpredictable chronic social instability (isolation and crowding, 3 h or 6 h/day for 28 days) | ↑ POMC, ↑ OXTR and ↓ AVPR1a in the amygdala |
P88 | ↑ anxiety-like behavior | P88 | [70] | |
| Chronic exposure to exogenous corticosterone | 10 mg/kg (s.c.) for 1 or 10 days | ↑ dendritic length ↑ spine density (pyramidal neurons BLA) |
P85 | ↑ anxiety-like behavior | P85 | [71] |
Symbols and abbreviations: ↑ (increase), ↓ (decrease), P (postnatal day), BLA (basolateral nuclei of the amygdala), BMA (basomedial nucleus of the amygdala), LA (lateral nuclei of the amygdala), MeA (medial nucleus of the amygdala), CeA (central nucleus of the amygdala), CB1-R (cannabinoid receptor 1), FAAH (fatty acid amine hydrolase), POMC (proopiomelanocortin), AEA (anandamide), OXTR (oxytocin receptor), AVPR1a (arginine vasopressin receptor 1a), GAD 67 (glutamic acid decarboxylase enzyme, 67 kDa), GABA (gamma-amino butyric acid), PSA-NCAM (polysialylated form of the neural cell adhesion molecule), PV (parvalbumin), s.c. (subcutaneous injection).
Several chronic stress procedures have been used in rodents to model the behavioral and neuroanatomical consequences of maladaptive-stress exposure in humans, seeking to achieve a measure of construct validity. The three most commonly employed models are chronic restraint stress (CRS), chronic unpredictable stress (CUS) and chronic social defeat stress (CSDS). All these models elicit depressive-like behavior and alterations in sociability [60]. Other models are based in artificially disrupting the animal’s glucocorticoid homeostasis (i.e., chronic glucocorticoid treatment, genetic mutant mice expressing abnormal levels of glucocorticoid receptors in the brain) [60].
In a recent review, Dr. Qiao and colleagues extensively discussed the effects of adult stress on the dendrites and spines of different brain regions, including the amygdala [59]. As a summary of their findings, one can say that rodent studies showed that chronic stress suffered during adulthood generally resulted in an increased spine density and dendritic arborization in the amygdala (Figure 2). Specifically, CRS caused a decrease in the spine density of spiny neurons in the MeA nucleus of the amygdala, which are GABAergic neurons [59][61]. By contrast, CUS caused an increase in dendritic arborization and spine density in the BLA spiny neurons, which are glutamatergic neurons [59][67] Interestingly, differently to hippocampal neurons, the dendritic hypertrophy described in spiny neurons from the BLA induced by CRS failed to be reversed after the same time period of stress-free recovery [59]. Moreover, although CRS showed no structural effect on BLA stellate neurons, a dendritic shrinkage was observed in MeA stellate neurons, together with a reduced social interaction and high vulnerability to social defeat stress [62]. Interestingly, it has also been demonstrated that, by modulating the duration of restraint stress in adult rodents, it is possible to induce the formation of new spines without remodeling dendrites in the BLA [72]. In the same way, repeated restraint stress (7 days) in adult rats increases the firing rate of BLA projecting neurons and increases anxiety-like behavior [63]. Similar to chronic stress protocols, both the acute (1 day) and chronic (10 days) exposure of mice to exogenous corticosterone increases dendritic length and spine density in the BLA. These changes were correlated with increased anxiety-like behavior after chronic or acute corticosterone treatment[71].
As we just mentioned, some studies have revealed dramatic alterations in the connectivity and structure of excitatory neurons in the amygdala after chronic stress in rodents [59]. However, chronic stress also induces changes in the structure and connectivity of interneurons in the amygdala [64][65]. At least some of these alterations seem to be facilitated by plasticity-related molecules associated to interneurons, such as the polysialylated form of the neural cell adhesion molecule (PSA-NCAM). Mice subjected to CRS (21 days) displayed decreased GAD67, synatophysin and PSA-NCAM protein levels in the amygdala and reduced dendritic arborization of interneurons in the BLA [64]. However, when a short CRS protocol (10 days) was applied to rats, an increased number of parvalbumin-expressing neurons was detected [65]. These findings were in line with results from the postmortem amygdala biopsies of major depression patients, which showed alterations in several synaptic and plasticity-related molecules. Specifically, researchers found decreased PSA-NCAM protein levels in the BLA and BM, decreased synaptophysin in the LA and BLA, and decreased GAD67 protein levels in the BMA, while the VGLUT-1 protein level was increased in the LA of depressed patients [73]. The unpredictable chronic mild stress protocol (UCMS) in mice has also been demonstrated to induce alterations in plasticity and synaptic strengthening in the amygdala[68]. Specifically, UCMS-induced elevated behavioral emotionality was found to correlate with the enlarged volume of the amygdala and an increased postsynaptic density-95 protein level[68].
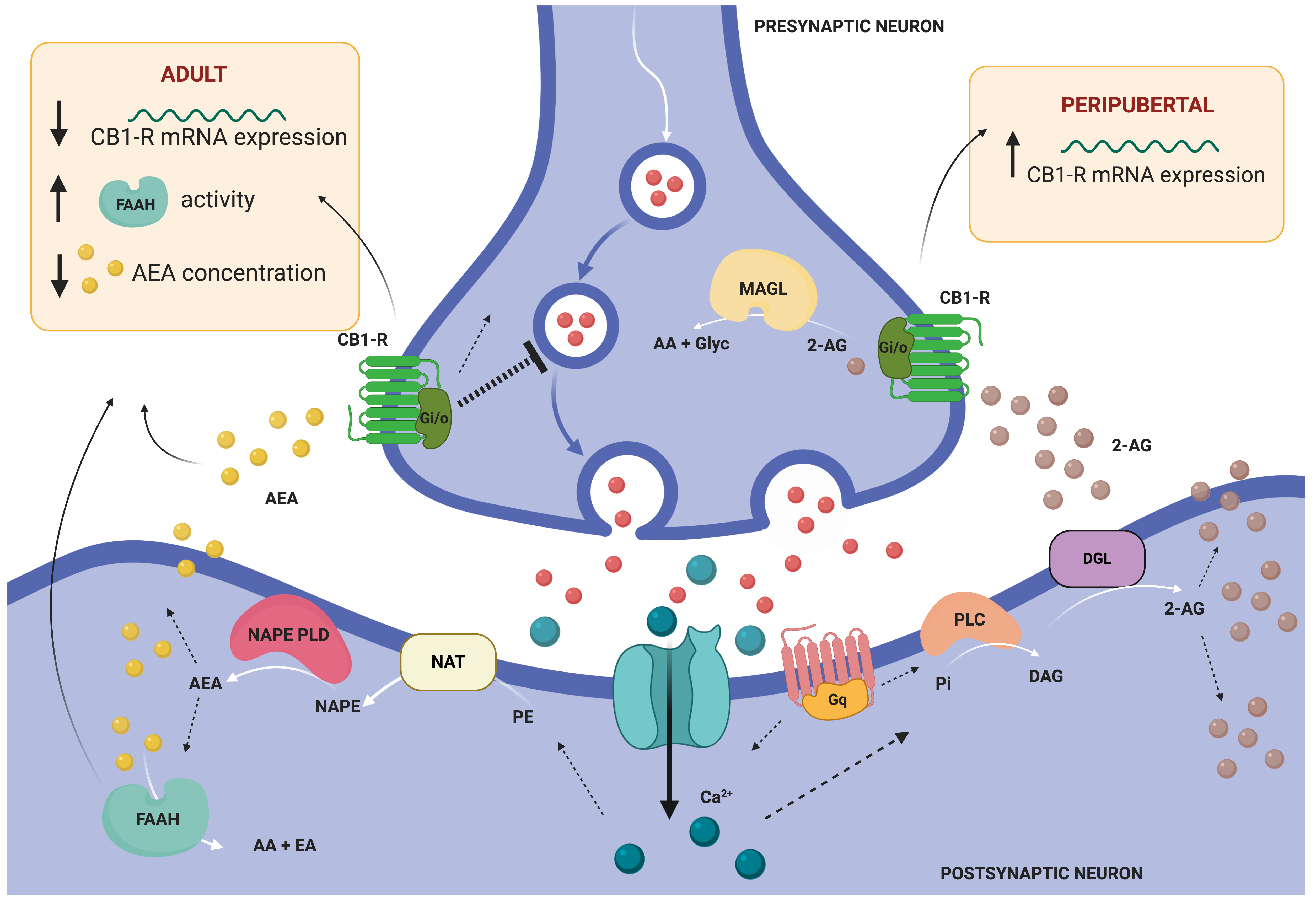
Chronic stress exposure during adulthood alters the eCB system in many regions involved in emotional processing, including the amygdala, as we have already discussed in previous sections for other age periods. Specifically, exposure to CRS downregulated CB1-R expression in the amygdala of mice. It also increased fatty acid amide hydrolase (FAAH) activity and decreased the amount of the eCB N-arachidonylethanolamine (AEA) within the amygdala, which would be expected to decrease eCB signaling at the level of ligand availability [66] (Figure 3). In the same study, authors reported increased anxiety-like behavior in CRS mice, together with increased dendritic complexity, arborization and spine density of the pyramidal neurons in the BLA[66].
Figure 3. General model illustrating retrograde endocannabinoid (eCB) signaling and summarizing the main findings of altered eCB system in the amygdala of rodents subjected to stress in different periods of the lifespan. This model illustrates the two primary biosynthetic pathways for anandamide (AEA) and 2-arachidonoyl glycerol (2-AG). AEA and 2-AG migrate from postsynaptic neurons to bind presynaptic-located cannabinoid type 1 receptors (CB1-R). Once activated, CB1-R couple through Gi/o proteins to inhibit adenylyl cyclase and regulate ion channels, and ultimately suppress neurotransmitter release. Endocannabinoid signaling is then terminated by degrading enzymes. AEA is mainly hydrolyzed to arachidonic acid (AA) and ethanolamine (EA) by fatty acid amide hydrolase (FAAH), located post-synaptically [74]. 2-AEG is mainly hydrolyzed pre-synaptically to AA and glycerol (Glyc) by monoacylglycerol lipase (MAGL). Abbreviations: DAG (diacylglycerol), DGL (DAG lipase), NAPE (N-arachidonoyl-PE), NAPE-PLD (NAPE-phospholipase D), NAT (N-acetyltransferase), PE (phosphatidylethanolamine), Pi (phosphatidylinositol), PLC (phospholipase C). Illustration created with BioRender.com.
The activation of the HPA axis could be studied by analyzing the altered expression of some genes, such as vasopressin and proopiomelanocortin (POMC), after stress exposure [75][76]. Indeed, anxiety-related behaviors and aggression are regulated by brain vasopressin and oxytocin [77]. Chronic defeat stress increased the expression of POMC in the amygdala of adult mice [69]. In the same way, chronic social instability stress increased amygdaloid expression of POMC and OXTR in the amygdala and reduced the expression of AVPR1a in the same region. These changes were correlated with increased anxiety behavior after the stress protocol [70].
4. Stress Exposure during Old Age: Elderly Stress
Old age is biologically defined as the age of a particular individual who reaches or surpasses the average lifespan of his species. In humans, old age is commonly defined as having a chronological age of 65 years or older [78]. To define old age in rodents, senescence biomarkers must be significantly identified, and this usually occurs from 18 months of age onwards [32].
Senescence has long been viewed as a period of decreased adaptiveness to stress, in part due to the fact that depressive symptoms are very common in older people, although healthy aging has been associated with a stable emotional state and weakened brain responses to negative stimuli [79][80][81]. In a study comparing the effects of acute stress on reactions to happy and fearful facial expressions between old age (aged 60–75 years) and young adulthood (aged 18–30 years) people, researchers found that acute stress impaired emotional processing in healthy aged people, which may in turn increase their vulnerability to affective disorders. Specifically, they found that, although the physiological and affective responses to the stressor were very similar between the two age groups, only elderly subjects showed a stress-related increase in the neural activity of the amygdala [81].
One of the most well-known hypotheses that has been formulated to explain the relation between aging and the response to stress is the so-called “glucocorticoid cascade hypothesis of aging” [79][80]. In agreement with this hypothesis, it has been demonstrated that, although aged rats were capable of appropriately initiating a corticosterone stress response after immobilization stress (Figure 1), their capacity to finish it was dramatically impaired. When old and young-adult rats were monitored during the recovery period after immobilization, the corticosterone concentrations in young rats returned to basal range within 60 min after the end of stress, but the corticosterone concentrations in aged rats remained elevated for 24 h post stress, due to continued secretion of the hormone [79]. This problem of corticosterone hypersecretion has been suggested to result from degenerative changes within the aging brain, specifically in the limbic system [14]. In fact, many studies in non-stressed rodents have demonstrated an age-related hypertrophy of the amygdala [14]. Specifically, increased dendritic branching, neuronal loss and decreased synaptic afferences could be found in the BLA of non-stressed old-aged rats when compared to non-stressed adult rats [82]. Interestingly, this “normal” hypertrophy of the BLA during aging has been related to age-associated changes in the stress response, including decreases in corticotrophin-releasing factor (CRF)-binding proteins in the BLA of aged rats. Thus, old-aged BLA neurons become more vulnerable to dendritic hypertrophy caused by stress due to a decreased capacity to regulate CRF levels [83].
However, despite the importance and high prevalence of socio-affective-related disorders among elderly people, and the apparent role of the amygdala in these disorders, preclinical studies addressing these questions are still scarce.
In a study comparing stress-induced behavioral changes in mice exposed to mild social defeat stress, researchers found that mice that were stressed during old age (24-month-old) exhibited similar reduced social interactions (social avoidance behavior) to mice that were stressed during adulthood (8–16-week-old) when compared to matched non-stressed control mice. However, only the old-aged stressed group showed a decreased preference towards sucrose and an attenuated defeat-induced increase in water intake. By contrast, the young stressed and control groups did not display such anhedonic behavior. Interestingly, these findings reveal that the positive stimuli of hedonic behavior in aged mice becomes more vulnerable to social defeat [84].
Finally, in a study applying an acute restraint stress protocol that lasted 3 h, researchers found that young-adult (3-month-old) and aged rats (21-month-old) displayed equivalent levels of distress, as well as higher but equivalent glucocorticoid blood levels 21 h after restraint. However, aged but not young rats proved to be less responsive to new-onset acute stress, which may negatively impact long-term stress adaptation[85].
5. Conclusions
As a process orchestrated by the brain, the stress response varies across the lifespan. The studies in humans and animal models reviewed here reveal that, while deficits in sociability and social interaction/communication are shared consequences of stress exposure across all stages of life, depression- and anxiety-like symptoms are more variable among age periods, and aggression is just documented as a consequence of peripubertal period. Early exposure to stressful events (peripubertal stress) can trigger immediate molecular and cellular changes in the amygdala, and thus re-shape the way the brain reacts to stress in adulthood towards maladaptive responses. Maladaptive responses can also be triggered by exposure to stress later in life, during adulthood or even old age. Particularly interesting is the stress response during old age; while elderly subjects are able to equally respond to a chronic or acute stressor, when a second stressor is presented, they show hypo-responsiveness, making them more vulnerable to the stressor, which may lead to psychopathologies. Regarding the molecular and cellular consequences of stress in the amygdala, the studies reviewed here show alterations in plasticity, eCB system and neuronal cytoarchitecture in late stages of life, suggesting that the amygdala remains vulnerable to stress throughout the whole of life, and therefore remains susceptible to being re-shaped.
Funding
Funded by: Fundación Alicia Koplowitz (Spain), grant number 19I436; Ministerio de Ciencia, Innovación y Universidades (Spain), grant number RTI2018-095698-B-I00; Generalitat Valenciana (Spain), grant number GV/2019/088 (E.C.-G) and Universitat Jaume I, grant number UJI-B2019-54 (F.E.O.-B.) and UJI-A2017-17 (F.R.-B.).
References
- McEwen,Bruce S.; Bowles, Nicole P.; Gray, Jason D.; Hill, Matthew N.; Hunter, Richard G; Karatsoreos, Ilia N.; Nasca, Carla; Mechanisms of stress in the brain. Nature Neuroscience 2015, 18, 1353-1363, 10.1038/nn.4086.
- Young, Simon N.; The neurobiology of human social behaviour: an important but neglected topic. J. Psychiatry Neurosci. 2008, 33, 391-392.
- Kennedy, Daniel P.; Adolphs, Ralph; The social brain in psychiatric and neurological disorders. Trends in Cognitive Sciences 2012, 16, 559-572, 10.1016/j.tics.2012.09.006.
- Lee, Nicole.S.; Beery, Annaliese K. Neural Circuits Underlying Rodent Sociality: A Comparative Approach. In Current Topics in Behavioral Neurosciences; Springer: Berlin/Heidelberg, Germany, 2019; Volume 43, pp. 211–238.
- Insel, Thomas R.; Fernald, Russell D.; How the brain processes social information: Searching for the Social Brain*. Annual Review of Neuroscience 2004, 27, 697-722, 10.1146/annurev.neuro.27.070203.144148.
- Kennedy, Daniel P.; Adolphs, Ralph; The social brain in psychiatric and neurological disorders. Trends in Cognitive Sciences 2012, 16, 559-572, 10.1016/j.tics.2012.09.006.
- Hensch, Takao K.; Bilimoria, Parizad M.; Re-opening Windows: Manipulating Critical Periods for Brain Development. Cerebrum : the Dana forum on brain science 2012, 2012, 11.
- Marco, Eva M.; Macrì, Simone; Laviola, Giovanni; Critical Age Windows for Neurodevelopmental Psychiatric Disorders: Evidence from Animal Models. Neurotoxicity Research 2010, 19, 286-307, 10.1007/s12640-010-9205-z.
- Tóth, Máté; Halász, József; Mikics, Eva; Barsy, Boglarka; Haller, John; Early social deprivation induces disturbed social communication and violent aggression in adulthood.. Behavioral Neuroscience 2008, 122, 849-854, 10.1037/0735-7044.122.4.849.
- Tóth, Máté; Mikics, Eva; Tulogdi, Aron; Aliczki, Manó; Haller, J; Post-weaning social isolation induces abnormal forms of aggression in conjunction with increased glucocorticoid and autonomic stress responses. Hormones and Behavior 2011, 60, 28-36, 10.1016/j.yhbeh.2011.02.003.
- Tzanoulinou, Stamatina; Sandi, Carmen. The Programming of the Social Brain by Stress During Childhood and Adolescence: From Rodents to Humans. In Social Behavior from Rodents to Humans; Springer: Cham, Switzerland, 2015; 411–429.
- Sandi, Carmen; Haller, József; Stress and the social brain: behavioural effects and neurobiological mechanisms. Nature Reviews Neuroscience 2015, 16, 290-304, 10.1038/nrn3918.
- Paus, Tomas; Keshavan, Matcheri; Giedd, Jay N.; Why do many psychiatric disorders emerge during adolescence?. Nature Reviews Neuroscience 2008, 9, 947-957, 10.1038/nrn2513.
- Bale, Tracy L.; Epperson, C Neill; Sex differences and stress across the lifespan. Nature Neuroscience 2015, 18, 1413-1420, 10.1038/nn.4112.
- Novais, Ashley; Monteiro, Susana; Roque, Susana; Correia-Neves, Margarida; Sousa, Nuno; How age, sex and genotype shape the stress response.. Neurobiology of Stress 2016, 6, 44-56, 10.1016/j.ynstr.2016.11.004.
- Sousa, Nuno; Almeida, O. F. X.; Disconnection and reconnection: the morphological basis of (mal)adaptation to stress. Trends in Neurosciences 2012, 35, 742-751, 10.1016/j.tins.2012.08.006.
- Beery, Annaliese K.; Kaufer, Daniela; Stress, social behavior, and resilience: Insights from rodents. Neurobiology of Stress 2015, 1, 116-127, 10.1016/j.ynstr.2014.10.004.
- McEwen, Bruce; Neurobiological and Systemic Effects of Chronic Stress. Chronic Stress 2017, 1, 1-5, 10.1177/2470547017692328.
- Yehuda, R; Schmeidler, James; Wainberg, Milton L.; Binder-Brynes, Karen; Duvdevani, Tamar; Vulnerability to Posttraumatic Stress Disorder in Adult Offspring of Holocaust Survivors. American Journal of Psychiatry 1998, 155, 1163-1171, 10.1176/ajp.155.9.1163.
- Franklin, Tamara B.; Russig, Holger; Weiss, Isabelle C.; Gräff, Johannes; Linder, Natacha; Michalon, Aubin; Vizi, Sándor; Mansuy, Isabelle M.; Epigenetic Transmission of the Impact of Early Stress Across Generations. Biological Psychiatry 2010, 68, 408-415, 10.1016/j.biopsych.2010.05.036.
- Matthews Sr., Stephen; Phillips, David I.; Transgenerational inheritance of stress pathology. Experimental Neurology 2012, 233, 95-101, 10.1016/j.expneurol.2011.01.009.
- Hillis, Susan; Mercy,James; Amobi, Adaugo; Kress, Howard; Global Prevalence of Past-year Violence Against Children: A Systematic Review and Minimum Estimates.. Pediatrics 2016, 137, e20154079-e20154079, 10.1542/peds.2015-4079.
- Stöckl, Heidi; March, Laura; Pallitto, Christina; García-Moreno, Claudia; WHO Multi-country Study Team; Intimate partner violence among adolescents and young women: prevalence and associated factors in nine countries: a cross-sectional study. BMC Public Health 2014, 14, 1-14, 10.1186/1471-2458-14-751.
- Burkle, Frederick M.; Argent, Andrew C.; Kissoon, N; The reality of pediatric emergency mass critical care in the developing world. Pediatric Critical Care Medicine 2011, 12, S169-S179, 10.1097/pcc.0b013e318234a906.
- Wilkinson, Richard G.; Why is Violence More Common Where Inequality is Greater?. Annals of the New York Academy of Sciences 2006, 1036, 1-12, 10.1196/annals.1330.001.
- De Boer, Sietse F.; Animal models of excessive aggression: implications for human aggression and violence. Current Opinion in Psychology 2018, 19, 81-87, 10.1016/j.copsyc.2017.04.006.
- Chen, Patrick; Hong, Weizhe; Neural Circuit Mechanisms of Social Behavior. Neuron 2018, 98, 16-30, 10.1016/j.neuron.2018.02.026.
- Tottenham, Nim; Sheridan, Margaret; A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Frontiers in Human Neuroscience 2009, 3, 68, 10.3389/neuro.09.068.2009.
- Wellman, Laurie L.; Forcelli, Patrick A.; Aguilar, Brittany L.; Malkova, Luise; Bidirectional Control of Social Behavior by Activity within Basolateral and Central Amygdala of Primates.. The Journal of Neuroscience 2016, 36, 8746-56, 10.1523/JNEUROSCI.0333-16.2016.
- McEwen, Bruce S.; Bowles, Nicole P.; Gray, Jason D.; Hill, Matthew N.; Hunter, Richard G; Karatsoreos, Ilia N.; Nasca, Carla; Mechanisms of stress in the brain. Nature Neuroscience 2015, 18, 1353-1363, 10.1038/nn.4086.
- McEwen,Bruce S.; Nasca, Carla; Gray, Jason D; Stress Effects on Neuronal Structure: Hippocampus, Amygdala, and Prefrontal Cortex. Neuropsychopharmacology 2015, 41, 3-23, 10.1038/npp.2015.171.
- Dutta, Sulagna; Sengupta, Pallav; Men and mice: Relating their ages. Life Sciences 2016, 152, 244-248, 10.1016/j.lfs.2015.10.025.
- Spear, Linda P.; The adolescent brain and age-related behavioral manifestations. Neuroscience & Biobehavioral Reviews 2000, 24, 417-463, 10.1016/s0149-7634(00)00014-2.
- Tzanoulinou, Stamatina; Riccio, O; De Boer, M W; Sandi, Carmen; Peripubertal stress-induced behavioral changes are associated with altered expression of genes involved in excitation and inhibition in the amygdala. Translational Psychiatry 2014, 4, e410-e410, 10.1038/tp.2014.54.
- Stain, Helen J.; Brønnick, Kolbjørn; Hegelstad, Wenche T.V.; Joa, Inge; Johannessen, Jan O.; Langeveld, Johannes; Mawn, Lauren; Larsen, Tor K.; Impact of Interpersonal Trauma on the Social Functioning of Adults With First-Episode Psychosis. Schizophrenia Bulletin 2013, 40, 1491-1498, 10.1093/schbul/sbt166.
- Palmier-Claus, Jasper; Berry, Katherine; Darrell-Berry, Hannah; Emsley, Richard; Parker,Sophie; Richard Drake; Bucci,Sandra; Childhood adversity and social functioning in psychosis: Exploring clinical and cognitive mediators. Psychiatry Research 2016, 238, 25-32, 10.1016/j.psychres.2016.02.004.
- Kilian,S; Asmal, L; Chiliza, B; Olivier, M.L; Phahladira, L.; Scheffler, F.; Seedat, S.; Marder, S.R.; Green, M. F.; Emsley, R.; et al. Childhood adversity and cognitive function in schizophrenia spectrum disorders and healthy controls: evidence for an association between neglect and social cognition. Psychological Medicine 2017, 48, 2186-2193, 10.1017/s0033291717003671.
- Heim, Christine M.; Binder, Elisabeth B.; Current research trends in early life stress and depression: Review of human studies on sensitive periods, gene–environment interactions, and epigenetics. Experimental Neurology 2012, 233, 102-111, 10.1016/j.expneurol.2011.10.032.
- Widom, Cathy Spatz; Dumont, Kimberly; Czaja, Sally J.; A Prospective Investigation of Major Depressive Disorder and Comorbidity in Abused and Neglected Children Grown Up. Archives of General Psychiatry 2007, 64, 49-56, 10.1001/archpsyc.64.1.49.
- Widom, Cathy Spatz; Maxfield, Michael G.; A Prospective Examination of Risk for Violence among Abused and Neglected Children. Annals of the New York Academy of Sciences 1996, 794, 224-237, 10.1111/j.1749-6632.1996.tb32523.x.
- Yeager, Catherine A.; Lewis, Dorothy Otnow; Mental illness, neuropsychologic deficits, child abuse, and violence.. Child and Adolescent Psychiatric Clinics of North America 2000, 9, 793-813, 10.1016/s1056-4993(18)30093-2.
- Van Der Kolk, Bessel A; The neurobiology of childhood trauma and abuse.. Child and Adolescent Psychiatric Clinics of North America 2003, 12, 293-317, 10.1016/s1056-4993(03)00003-8.
- Malvaso, Catia; Day, Andrew; Casey, Sharon; Corrado, Ray; Young Offenders, Maltreatment, and Trauma: A Pilot Study.. Psychiatry, Psychology and Law 2016, 24, 458-469, 10.1080/13218719.2016.1247682.
- Brydges, Nichola M.; Hall, Jessica; Best, Caroline; Rule, Lowenna; Watkin, Holly; Drake, Amanda J.; Lewis, Catrin; Thomas, Kerrie L.; Hall, Jeremy; Childhood stress impairs social function through AVP-dependent mechanisms. Translational Psychiatry 2019, 9, 1-12, 10.1038/s41398-019-0678-0.
- Mikics, Éva; Guirado, Ramon; Umemori, Juzoh; Tóth, Máté; Biro, Laszlo; Miskolczi, Christina; Balázsfi, Diána; Zelena, Dóra; Castren, Eero; Haller, József; et al.Karpova,Nina N. Social Learning Requires Plasticity Enhanced by Fluoxetine Through Prefrontal Bdnf-TrkB Signaling to Limit Aggression Induced by Post-Weaning Social Isolation. Neuropsychopharmacology 2017, 43, 235-245, 10.1038/npp.2017.142.
- Rau, Andrew R.; Chappell, Ann M.; Butler, Tracy R.; Ariwodola, Olusegun J.; Weiner, Jeffrey L.; Increased Basolateral Amygdala Pyramidal Cell Excitability May Contribute to the Anxiogenic Phenotype Induced by Chronic Early-Life Stress.. The Journal of Neuroscience 2015, 35, 9730-40, 10.1523/JNEUROSCI.0384-15.2015.
- Gilabert-Juan, Javier; Moltó, María Dolores; Nacher, Juan; Post-weaning social isolation rearing influences the expression of molecules related to inhibitory neurotransmission and structural plasticity in the amygdala of adult rats. Brain Research 2012, 1448, 129-136, 10.1016/j.brainres.2012.01.073.
- Castillo-Gómez, Esther; Pérez-Rando, Marta; Bellés, María; Gilabert-Juan, Javier; Llorens, José Vicente; Carceller, Hector; Bueno-Fernández, Clara; Garcia-Mompo, Clara; Ripoll-Martínez, Beatriz; Curto, Yasmina; et al.Sebastiá-Ortega, NoeliaMoltó, María DoloresSanjuan, JulioNacher, Juan Early Social Isolation Stress and Perinatal NMDA Receptor Antagonist Treatment Induce Changes in the Structure and Neurochemistry of Inhibitory Neurons of the Adult Amygdala and Prefrontal Cortex. eneuro 2017, 4, -17.2017, 10.1523/eneuro.0034-17.2017.
- Marquez, Cristina; Poirier, Guillaume L; Cordero, María Isabel; Larsen, M H; Groner, A; Marquis, J; Magistretti, Pierre Julius; Trono, D; Sandi, Carmen; Peripuberty stress leads to abnormal aggression, altered amygdala and orbitofrontal reactivity and increased prefrontal MAOA gene expression. Translational Psychiatry 2013, 3, e216-e216, 10.1038/tp.2012.144.
- Cordero, María Isabel; Poirier, Guillaume L; Marquez, Cristina; Veenit, V; Fontana, X; Salehi, B; Ansermet, F; Sandi, Carmen; Evidence for biological roots in the transgenerational transmission of intimate partner violence. Translational Psychiatry 2012, 2, e106-e106, 10.1038/tp.2012.32.
- Tzanoulinou, Stamatina; Garcia-Mompo, Clara; Castillo-Gómez, Esther; Veenit, Vandana; Nacher, Juan; Sandi, Carmen; Long-Term Behavioral Programming Induced by Peripuberty Stress in Rats Is Accompanied by GABAergic-Related Alterations in the Amygdala. PLoS ONE 2014, 9, e94666, 10.1371/journal.pone.0094666.
- Papilloud, Aurélie; Veenit, Vandana; Tzanoulinou, Stamatina; Riccio, Orbicia; Zanoletti, Olivia; De Suduiraut, Isabelle Guillot; Grosse, Jocelyn; Sandi, Carmen; Peripubertal stress-induced heightened aggression: modulation of the glucocorticoid receptor in the central amygdala and normalization by mifepristone treatment. Neuropsychopharmacology 2018, 44, 674-682, 10.1038/s41386-018-0110-0.
- Zhang, Wei; Rosenkranz, J Amiel; Effects of Repeated Stress on Age-Dependent GABAergic Regulation of the Lateral Nucleus of the Amygdala. Neuropsychopharmacology 2016, 41, 2309-2323, 10.1038/npp.2016.33.
- Jiang, Xiaolong; Xing, Guoqiang; Yang, Chunhui; Verma, Ajay; Zhang, Lei; Li, He; Stress Impairs 5-HT2A Receptor-Mediated Serotonergic Facilitation of GABA Release in Juvenile Rat Basolateral Amygdala. Neuropsychopharmacology 2008, 34, 410-423, 10.1038/npp.2008.71.
- WHO. Definition of Key Terms; WHO: Geneva, Switzerland, 2013; Available online: https://www.who.int/hiv/ pub/guidelines/arv2013/intro/keyterms/en/ (accessed on 5 July 2020).
- Ferrari, A. J.; Somerville, A. J.; Baxter, Amanda J.; Norman, R.; Patten, Scott B.; Vos, T.; Whiteford, Harvey A.; Global variation in the prevalence and incidence of major depressive disorder: a systematic review of the epidemiological literature. Psychological Medicine 2012, 43, 471-481, 10.1017/s0033291712001511.
- Tang,Shi; Lu, Lu; Zhang, Lianqing; Hu, Xinyu; Bu, Xuan; Li, Hailong; Hu, Xiaoxiao; Gao, Yingxue; Zeng, Zirui; Gong, Qiyong; et al.Huang, Xiaoqi Abnormal amygdala resting-state functional connectivity in adults and adolescents with major depressive disorder: A comparative meta-analysis. EBioMedicine 2018, 36, 436-445, 10.1016/j.ebiom.2018.09.010.
- Mothersill, O.; Donohoe, Gary; Neural effects of social environmental stress – an activation likelihood estimation meta-analysis. Psychological Medicine 2016, 46, 2015-2023, 10.1017/s0033291716000477.
- Qiao, Hui; Li, Ming-Xing; Xu, Chang; Chen, Hui-Bin; An, Shu-Cheng; Ma, Xin-Ming; Dendritic Spines in Depression: What We Learned from Animal Models. Neural Plasticity 2016, 2016, 1-26, 10.1155/2016/8056370.
- Nestler, Eric J.; Hyman, Steven E.; Animal models of neuropsychiatric disorders.. Nature Neuroscience 2010, 13, 1161-9, 10.1038/nn.2647.
- Bennur, S.; Shankaranarayana Rao, B. S.; Pawlak, R.; Strickland, S.; McEwen, B.S.; Chattarji, Sumantra; Stress-induced spine loss in the medial amygdala is mediated by tissue-plasminogen activator. Neuroscience 2007, 144, 8-16, 10.1016/j.neuroscience.2006.08.075.
- Lau, T.; Bigio, B.; Zelli, D.; McEwen,Bruce S.; Nasca, Carla; Stress-induced structural plasticity of medial amygdala stellate neurons and rapid prevention by a candidate antidepressant.. Molecular Psychiatry 2016, 22, 227-234, 10.1038/mp.2016.68.
- Zhang, Wei; Rosenkranz, J. Amiel; Repeated restraint stress increases basolateral amygdala neuronal activity in an age-dependent manner. Neuroscience 2012, 226, 459-474, 10.1016/j.neuroscience.2012.08.051.
- Gilabert-Juan, Javier; Castillo-Gómez, Esther; Pérez-Rando, Marta; Moltó, María Dolores; Nacher, Juan; Chronic stress induces changes in the structure of interneurons and in the expression of molecules related to neuronal structural plasticity and inhibitory neurotransmission in the amygdala of adult mice. Experimental Neurology 2011, 232, 33-40, 10.1016/j.expneurol.2011.07.009.
- Pesarico, Ana Paula; Bueno-Fernandez, Clara; Guirado, Ramón; Gómez-Climent, María Ángeles; Curto, Yasmina; Carceller, Hector; Nacher, Juan; Chronic Stress Modulates Interneuronal Plasticity: Effects on PSA-NCAM and Perineuronal Nets in Cortical and Extracortical Regions.. Frontiers in Cellular Neuroscience 2019, 13, 197, 10.3389/fncel.2019.00197.
- Hill, Matthew N.; Kumar, Shobha Anil; Filipski, Sarah B.; Iverson, Moriah; Stuhr, Kara L.; Keith, John M.; Cravatt, Benjamin F.; Hillard, Cecilia J.; Chattarji, Sumantra; McEwen, Bruce S.; et al. Disruption of fatty acid amide hydrolase activity prevents the effects of chronic stress on anxiety and amygdalar microstructure.. Molecular Psychiatry 2012, 18, 1125-35, 10.1038/mp.2012.90.
- Sharma, Himanshu R.; Thakur, Mahendra K.; Correlation of ERα/ERβ expression with dendritic and behavioural changes in CUMS mice. Physiology & Behavior 2015, 145, 71-83, 10.1016/j.physbeh.2015.03.041.
- Nikolova, Yuliya S.; Misquitta, Keith A.; Rocco, Brad R.; Prevot, Thomas D.; Knodt, Annchen R.; Ellegood, Jacob; Voineskos, Aristotle N.; Lerch, Jason P.; Hariri, Ahmad R.; Sibille, Etienne; et al.Banasr, Mounira Shifting priorities: highly conserved behavioral and brain network adaptations to chronic stress across species. Translational Psychiatry 2018, 8, 26, 10.1038/s41398-017-0083-5.
- Sachs, Benjamin D.; Tran, Ha L.; Folse, Emily; Caron, Marc G.; Brain-region-specific Molecular Responses to Maternal Separation and Social Defeat Stress in Mice. Neuroscience 2018, 373, 122-136, 10.1016/j.neuroscience.2018.01.018.
- Nowacka-Chmielewska, Marta; Kasprowska-Liśkiewicz, Daniela; Barski, Jarosław-Jerzy; Obuchowicz, Ewa; Małecki, Andrzej; The behavioral and molecular evaluation of effects of social instability stress as a model of stress-related disorders in adult female rats. Stress 2017, 20, 549-561, 10.1080/10253890.2017.1376185.
- Mitra,Rupshi; Sapolsky, Robert M.; Acute corticosterone treatment is sufficient to induce anxiety and amygdaloid dendritic hypertrophy. Proceedings of the National Academy of Sciences 2008, 105, 5573-5578, 10.1073/pnas.0705615105.
- Mitra, Rupshi; Jadhav, Shantanu P.; McEwen, Bruce; Vyas, Ajai; Chattarji, Sumantra; Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proceedings of the National Academy of Sciences 2005, 102, 9371-9376, 10.1073/pnas.0504011102.
- Varea, Emilio; Guirado, Ramon; Gilabert-Juan, Javier; Martí, Ulisses; Castillo-Gómez, Esther; Blasco-Ibáñez, José Miguel; Crespo, Carlos; Nacher, Juan; Expression of PSA-NCAM and synaptic proteins in the amygdala of psychiatric disorder patients. Journal of Psychiatric Research 2012, 46, 189-197, 10.1016/j.jpsychires.2011.10.011.
- Morena, Maria; Patel, Sachin; Bains, Jaideep S.; Hill, Matthew N.; Neurobiological Interactions Between Stress and the Endocannabinoid System. Neuropsychopharmacology 2015, 41, 80-102, 10.1038/npp.2015.166.
- Ancelin, Marie-Laure; Scali, J; Norton, Joanna; Ritchie, Karen; Dupuy, Anne-Marie; Chaudieu, Isabelle; Ryan, Joanne; Heterogeneity in HPA axis dysregulation and serotonergic vulnerability to depression. Psychoneuroendocrinology 2017, 77, 90-94, 10.1016/j.psyneuen.2016.11.016.
- Du, Xin; Pang, Terence Y.; Is Dysregulation of the HPA-Axis a Core Pathophysiology Mediating Co-Morbid Depression in Neurodegenerative Diseases?. Frontiers in Psychology 2015, 6, e34, 10.3389/fpsyt.2015.00032.
- Lukas, Michael; Neumann, Inga D.; Oxytocin and vasopressin in rodent behaviors related to social dysfunctions in autism spectrum disorders. Behavioural Brain Research 2013, 251, 85-94, 10.1016/j.bbr.2012.08.011.
- WHO.Proposed Working Definition of an Older Person in Africa for the MDS Project;WHO:Geneva,Switzerland, 2016; Available online: https://www.who.int/healthinfo/survey/ageingdefnolder/en/ (accessed on 7 July 2020).
- Sapolsky, Robert M.; Krey, Lewis C.; McEwen, Bruce S.; The Neuroendocrinology of Stress and Aging: The Glucocorticoid Cascade Hypothesis*. Endocrine Reviews 1986, 7, 284-301, 10.1210/edrv-7-3-284.
- McEwen, Bruce S.; Re-examination of the glucocorticoid hypothesis of stress and aging. Progress in Brain Research 1992, 93, 365-383, 10.1016/s0079-6123(08)64585-9.
- Everaerd, Daphne S.; Klumpers, Floris; Voshaar, Richard Oude; Fernández, Guillén; Tendolkar, Indira; Acute Stress Enhances Emotional Face Processing in the Aging Brain. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 2017, 2, 591-598, 10.1016/j.bpsc.2017.05.001.
- Rubinow, Marisa J.; Drogos, Lauren; Juraska, Janice M.; Age-related dendritic hypertrophy and sexual dimorphism in rat basolateral amygdala. Neurobiology of Aging 2009, 30, 137-146, 10.1016/j.neurobiolaging.2007.05.006.
- Xiao, C.; Sartin, J.; Mulchahey, J.J.; Segar, T.; Sheriff,S.; Herman, James P.; Kasckow, John; Aging associated changes in amygdalar corticotropin-releasing hormone (CRH) and CRH-binding protein in Fischer 344 rats. Brain Research 2006, 1073, 325-331, 10.1016/j.brainres.2005.12.063.
- Oizumi, Hiroaki; Kuriyama, Nae; Imamura, Sachiko; Tabuchi, Masahiro; Omiya, Yuji; Mizoguchi, Kazushige; Kobayashi, Hiroyuki; Influence of aging on the behavioral phenotypes of C57BL/6J mice after social defeat. PLoS ONE 2019, 14, e0222076, 10.1371/journal.pone.0222076.
- Adolphs, Ralph; What does the amygdala contribute to social cognition?. Annals of the New York Academy of Sciences 2010, 1191, 42-61, 10.1111/j.1749-6632.2010.05445.x.