
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Charu Kothari | + 3531 word(s) | 3531 | 2020-08-13 06:22:14 | | | |
| 2 | Conner Chen | Meta information modification | 3531 | 2020-08-17 03:30:55 | | | | |
| 3 | Conner Chen | Meta information modification | 3531 | 2020-08-26 09:34:05 | | |
Video Upload Options
Breast is a dynamic organ mainly composed of adipose and fibroglandular tissues. The adipose tissue extends from the collarbone to the underarm and around the center of the ribcage. Adipose tissue as an endocrine organ constantly affects the dynamics of the breast. However, the role of adipose tissue in breast has been mostly studied in terms of obesity and cancer. In this review, we have discussed the role of breast adipose tissue in breast development from embryonic stage to mature breast. Further, we draw attention to the involvement of breast adipose tissue in pregnancy, lactation and involution associated breast changes. Finally, we depict how breast adipose tissue can affect breast cancer.
1. Introduction
Adipose tissue is a complex endocrine organ, with a role in obesity and cancer. Adipose tissue is generally linked to excessive body fat, and it is well known that the female breast is rich in adipose tissue. Hence, one can wonder: what is the role of adipose tissue in the breast and why is it required? Adipose tissue as an organ consists of adipocytes, an extracellular matrix (ECM) and immune cells, with a significant role in the dynamics of breast changes throughout the life span of a female breast from puberty, pregnancy, lactation and involution.
2. Adipose Tissue and Its Plasticity
The adipose organ, a dynamic tissue complex and an endocrine organ, consists of adipocytes, a stromal–vascular fraction consisting of lymphocytes, macrophages, endothelial cells, fibroblasts, pericytes, extracellular matrix and adipose precursor cells [1]. It is composed of three different adipose tissues: white, brown and beige. The white adipose tissue (WAT) is the main energy storage compartment consisting of a large cytoplasmic lipid droplet. It releases energy between meals. It is also known to produce many pro-inflammatory molecules, many adipokines related to inflammatory changes and has a low metabolic activity. WAT is characterized by the expression of leptin and S100B and by the lack of uncoupling protein 1 (UCP-1). The brown adipose tissue (BAT) is supposed to be only present in hibernating animals and newborns. However, BAT is also identified in small repositories near the neck and the interscapular region [2]. BAT is characterized by small droplets of lipids, iron-enriched large-spherical and packed mitochondria and a large number of capillaries, which are used for oxygen transport to BAT to produce energy and for the distribution of produced energy to the rest of the body. BAT is responsible for maintaining body temperature by thermogenesis using mitochondria enriched UCP1 protein. Beige/brite (brown-like) adipose tissue is characterized by a role in both energy storage as well as thermogenesis. It expresses UCP-1, PPARγ, leptin, and has a high mitochondrial content compared with WAT [3]. The conversion of WAT to beige/brite adipose tissue has been reported in response to cold or β3-adrenergic agonists [4]. This process is often referred to as browning and can happen after the exposure to the PR-domain containing 16 (PRDM16), fibroblast growth factor (FGF) 21, Peroxisome proliferator-activated receptor-γ (PPAR-γ), PPAR-γ coactivator α (PGCα), irisin, apelin, Cyclooxygenase 2 (Cox2), microRNA 196 (MIR196a) and MIR28 [5]. The conversion of BAT to beige/brite adipose tissue has also been documented. The process is often referred to as the whitening of BAT and is usually considered as BAT malfunctioning leading to death of BAT cells [6]. Furthermore, it has been reported that WAT can trans differentiate to BAT in a cold environment [7][8][9].
3. Adipose Tissue in Breast Development
Several studies have demonstrated the role of breast adipose tissue in the morphogenesis of mammary glands. Breast adipose tissue is a major endocrine system of the breast and secretes many growth factors and enzymes. It has been shown by in vitro experiments that breast adipose tissue plays a role in mammary epithelial cell differentiation [10][11]. Experiments using a co-transplantation system with breast stromal cells have shown that breast adipose tissue is responsible for characteristic morphogenesis of epithelial cells in the breast [12]. Further, it has been shown that loss of WAT of breast—using A-ZIP/F1 mouse model—results in reduced fertility and distends mammary ducts [13]. A study by Hu et al. 2002 showed the complete loss of ductal epithelium development when inherited loss of functional leptin occurs or in the absence of the leptin receptor, whereas the structure of ductal epithelium is restored with the re-establishment of leptin signaling [14]. On the other hand, abnormal mammary growth with underdeveloped ducts is observed in the presence of overexpression of adiponectin in mice [15][16][17]. Leptin and adiponectin are secreted by breast adipose tissue in the breast.
Landskroner-Eiger et al., 2010 showed that breast adipocytes play a crucial role in mammary gland development during prepuberty, puberty and adulthood. It is known that during the prepuberty to puberty phase, rapid ductal branching and terminal end bud (TEB) formation take place, whereas during adulthood, alveolar buds start to develop side branching (Figure 1) [18]. This study also highlights that the loss of mammary gland specific adipocytes results in a slowdown of all these processes, leading to fewer duct branching points, fewer TEBs, excessive lobulation and changes in proliferation and apoptosis of TEBs associated with epithelium, pointing towards the importance of breast adipose tissue in the development as well as the maintenance of the breast [18].
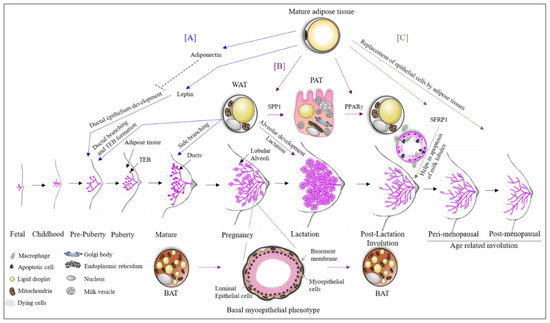
Figure 1. The role of adipose tissue in breast development. (A) Mature breast adipose tissue secretes leptin which is essential for ductal epithelial development. High levels of adiponectin inhibit this process. White adipose tissue (WAT) is essential for the formation of terminal end buds (TEBs) during prepuberty and puberty stages. After menarche, the breast starts to mature, and the duct starts its side branching, which requires WAT. (B) During pregnancy, WAT trans differentiates into pink adipose tissue (PAT). PAT has milk secretory potential. The process of trans differentiation from WAT to PAT is carried out by the transcription factor SPP1. Moreover, WAT is also essential for alveolar development during the lactation phase and also for the lactation process. During the phase of pregnancy and lactation, brown adipose tissue (BAT) trans differentiates to a basal myoepithelial phenotype helping in the alveolar development. Both trans differentiated cells revert to their original state after lactation with the aid of the transcription factor PPARγ. (C) There are two stages of breast involution (i) post-lactation and (ii) age-related. SFRP1 secreted by breast adipose tissue helps in these involution processes by the apoptosis of luminal epithelial cells. Furthermore, the epithelial cells are replaced by adipose tissue during the involution process.
4. Adipose Tissue Plasticity during Pregnancy, Lactation and Involution
The trans differentiation of adipose tissue underlines the extraordinary property of adipose tissue plasticity and is the result of physiological changes [19][20]. A recent study shows a broader view of this picture in pregnancy [21]. WAT in the breast has been shown to change during the second stage of pregnancy into milk producing glands containing lipid-enriched elements, an apical surface with microvilli and fully developed rough endoplasmic reticulum (Figure 1). The milk producing adipose glands are parenchymal cells of the breast adipose organ [21] and are named Pink adipose tissue (PAT). PAT arises exclusively in female subcutaneous depots during pregnancy and lactation. It is so-called this because during pregnancy, the mammary gland looks pink at the macroscopic level. The existence of PAT in the breast is also confirmed by the presence of the whey acidic protein (WAP) gene, a marker of the milk-producing epithelial mammary gland only present in pregnant women adipose tissue [22]. Perilipin 2 (Plin2) is also expressed in PAT, but is not present in WAT [23][24]. PAT does not express Plin1, which is typical of WAT [25]. The trans differentiation of WAT to PAT during pregnancy and lactation has been confirmed by the expression of these two genes (Plin1 and Plin2) on day 17–18 of pregnancy in compartmentalized adipocytes or early alveoli from the mammary gland section of mice [23]. The expression of Plin1 decreases as pregnancy progresses. Another study shows that 10–15% of early post-lactation adipocytes showed the expression of both WAP and S-100B confirming the PAT to WAT differentiation post-lactation [26]. A study by Couldrey et al., 2002, showed that the absence of WAT in the breast results in the inhibition of alveolar development and lactation in mice (Figure 1) [4]. Prokesch et al., 2014, showed that secreted phosphoprotein 1 (SPP1) signaling through integrin plays a role in WAT to PAT trans differentiation [23]. On the other hand, it has been shown that PPARγ is a key factor for PAT to WAT trans differentiation in the breast, which occurs post-lactation during involution (Figure 1) [21]. Another study reported that the downregulation of PPARγ expression results in a pro-breast tumorigenic environment [27], suggesting that PAT to WAT trans differentiation is an essential step in proper involution. Furthermore, it has been shown that partial involution directly results in the malignant transformation of normal breast cells [28]. In addition to partial post-lactation involution [29][30][31], an increase in breast adipose tissue (replacing the epithelial cells of glands) [32][33] results in excessive pro-inflammatory mediators, leading to tumorigenesis. The importance of breast adipose tissue in proper breast involution was also indicated in the study on Secreted Frizzled Related Protein 1 (SFRP1). SFRP1 is a known adipokine secreted by adipocytes, and it plays a role in adipogenesis, inflammation and apoptosis [34][35][36] processes that are all of significant importance in breast involution (Figure 1). Furthermore, SFRP1 overexpression was observed in the post-lactation period [37] indicating its role in involution. Gauger et al., 2012, also suggested that SFRP1 knockout mice show a ductal and lobular branching similar to mid-pregnancy mice [38]. This further indicates an important role of breast adipose tissue in breast involution and tumor development.
The role of BAT has also been studied in pregnancy. It was shown that BAT in the breast changes to a mammary basal myoepithelial phenotype during pregnancy and lactation (Figure 1) [39]. The lineage tracing experiments identified the expression of a gene signature resembling BAT and myoepithelial cells in 2.5% of the anterior dorsal interscapular mammary myoepithelial cell population. When traced during the involution process after lactation, 0.8% of BAT was trans differentiated from myoepithelial cells [39]. A study by Singh et al., 2017, reported a role for BAT and beige/brite adipose tissue in BC development [40]. Markers for BAT (UCP1, MYF5, EVA1 and OPLAH) and beige (UCP1, CD137/TNFRSF9 and TBX1) adipocytes were significantly high in BC xenografts [40].
These findings further strengthen the role of breast adipose tissue throughout the development of the mammary gland and is an essential factor in pregnancy, lactation and involution processes. A dysregulation in the proper functioning of the mammary adipose tissue has an adverse effect in breast development, leading to tumorigenesis. Furthermore, the multiplicity of breast adipose tissue subtypes and its association with breast further highlights the importance of personalized BC care.
5. Risk and Prognostic Factors for BC and Involvement of Adipose Tissue
There are several risk and prognostic factors associated with BC. In this section, we will discuss some, with respect to adipose tissue involvement (Figure 3).
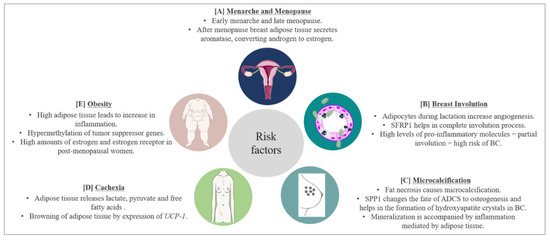
Figure 3. BC risk factors and prognostic factors and the role of adipose tissue. (A) Early menarche and late menopause increase the risk of BC by increasing the exposure of breast cells to estrogen hormones. Breast adipose tissue secretes aromatase which converts androgen to estrogen further complicating the scenario. (B) Breast adipose tissue secretes SFRP1 which helps in post-lactation involution age-related breast involution. Involution involves the inflammation process leading to apoptosis of epithelial cells. High levels of pro-inflammatory molecules secreted by breast adipose tissue lead to partial involution which increases the risk of BC. (C) Microcalcification is a predisposing factor for BC. Microcalcifications due to fat necrosis resemble malignant microcalcifications further complexifying prognosis of BC. Furthermore, SPP1 secreted by breast adipose tissue changes the fate of adipose derived stem cells (ADSC) to osteogenesis, increasing BC risk. Moreover, breast adipose tissue increases the inflammatory process accompanying mineralization. (D) During BC progression, BC cells signal lipolysis of surrounding adipose tissue to meet the energy requirement of BC. Adipose tissue releases lactate, pyruvate and free fatty acids. WAT also starts to express UCP-1, thereby trans differentiating to BAT and exhausting the energy source of the body. This leads to cachexia and eventually death. Around 30–50% of cancer-associated deaths are due to cachexia. (E) Obesity is a worldwide problem and known to increase BC risk. High adipose tissue increases the inflammatory process and hypermethylation (resulting in inhibition) of tumor suppressor genes. Obesity also increases the expression of estrogen and estrogen receptors in postmenopausal women, enhancing the risk of BC.
5.1. Menarche and Menopause
Early menarche has been considered as a risk factor for BC due to the increased exposure of breast cells to estrogens. The role of leptin in early menarche in obese patients has been discussed in the previous sections. Although early menopause means shorter exposure to estrogen, data suggest that after menopause, the adipose tissue becomes the main secretory organ for estrogens. In obese women, this becomes a major problem due to the abundance of adipose tissue [41]. Adipose tissue in the breast produces a high amount of aromatase which converts androgens secreted by the ovary in postmenopausal women [42][43]. Estrogen secreted in such a fashion in the breast increases the concentration of estrogen in the breast by 10 times in comparison with the concentration present in the circulation, thereby increasing the risk of BC [44]. Among BC patients, our group found higher levels of estradiol in breast adipose tissue of women diagnosed with ER-positive BC as compared to those with ER-negative BC [45].
5.2. Involution
The breast undergoes two kinds of involution: a post-lactation involution and an age-related lobular involution (around/after menopause). During pregnancy, breast stroma undergo a massive remodeling to make room for new growing epithelial cells. This remodeling sometimes results in pregnancy associated BC (PABC). PABC can occur during pregnancy, lactation, or post-lactation involution. PABC is usually ER and progesterone receptor (PR) negative, is often diagnosed at later stages and predominantly has a worse prognosis [46][47]. Data suggest a significant role of adipose tissue in PABC occurring during lactation or post-lactation involution. McCready et al., 2014, showed that breast adipocytes present during lactation promote tumorigenesis by increased vasculogenesis. This leads to an increase in vascular endothelial cells resulting in increased angiogenesis [48]. Furthermore, it has been shown that leptin can stimulate the growth of ER-negative BCs [49]. Post-lactation involution resembles wound healing, where an extensive immune response takes place. This leads to apoptosis of epithelial cells, which are replaced by stromal cells enriched in adipocytes [50]. The site of involution is enriched with TGFβ, VEGF, TNFα and IL6 [51], which have all been shown to promote cancer, and are also secreted by breast adipose tissue. On the other hand, a review from our group has highlighted that there could be a possible link between the post-lactation overexpression of SFRP1 and complete involution [34], as mice lacking expression of SFRP1 display a breast morphology that is similar to mid-pregnancy mice [38]. This could also suggest a protective role of breast adipose tissue against BC.
Age-related lobular involution (ARLI) is an irreversible change in the breast which is marked by a decrease in the number and size of breast lobules [52]. ARLI leads to a decrease in epithelial cells, which are replaced by stromal cells including adipocytes. The ARLI is inversely correlated with BC risk, as a very small amount of epithelial cells remains to be transformed into malignant cells after involution. On the other hand, no involution or partial involution increases the risk of BC [53][54]. A study from our group has shown that high levels of pro-inflammatory molecules in breast tissue can lead to partial involution and, therefore, could be correlated with high BC risk [55]. The increase in pro-inflammatory molecules could be attributed to the adipose tissue present in the breast. It has been reported that in the breast epithelium of premenopausal women, around 10% of epithelial cells are ER positive; however, 90% of the epithelial cells in postmenopausal women are ER positive [56], which increases the risk of BC in postmenopausal women. After lobular involution, the breast is enriched with adipose tissue, leading to an increased production of estrogen via androgens. This combination of increased ER and its ligand works in favor of BC after menopause and the complexity increases in obesity where there is a further increase in adipose tissue.
5.3. Microcalcifications
Microcalcifications in breast are recognized as a possible predisposing factor for BC. Usually, calcification is indicative of a benign process due to some injury or due to calcium deposition from serum. However, certain predisposing factors in microcalcification can indicate the possibility of an emerging cancer smaller in size (< 0.5 mm each). Microcalcifications have varying sizes and shapes, and are often worrying if they are branching, rod-like, or angular and if they are clustered in one area of the breast [57]. One cause of microcalcification is fat necrosis. Microcalcifications due to fat necrosis are pleomorphic, focally clustered and are often undistinguishable from microcalcifications associated with malignancy [58][59], leading to unnecessary intervention in many instances. However, the mineralization process which occurs during microcalcification leading to the production of calcium oxalate or calcium hydroxyapatite is increased by several processes including inflammatory response. The adipose tissue in breast has been reported to mediate acute inflammatory response in BC leading to cancer progression. Furthermore, a microcalcification event can be marked by changes in the transcription factors regulating ADSC, changing their fate to osteogenesis. Studies have shown that breast adipose tissue is an important site for the presence of ADSC [60][61]. Among the transcriptional regulators of ADSC is SPP1, which is also secreted by adipose tissue [62]. It has been shown that SPP1 is responsible for the formation of hydroxyapatite crystals in BC cells in response to an osteogenic cocktail [63]. Furthermore, the expression of SPP1 is significantly elevated in calcified BC [64], and the expression of SPP1 also aids in bone metastasis of BC cells [65]. Once BC cells metastasize to bone cells, they colonize in bone-marrow adipose tissue [66], where SPP1 modulates the ECM component of the bone microenvironment to promote BC progression [67]. Oyama et al., 2002, showed that infiltration of foam cells expressing SPP1 in the breast is responsible for breast microcalcification and atypical cystic lobule formation [222]. Foam cells are macrophages present in fat cells. Studies show that the presence of microcalcifications in BC leads to poor clinical outcome [68][69][70][71].
5.4. Cachexia
Cachexia is defined as a gradual decrease in the adipose tissue storage by the body and lean body mass. Around 30–50% of cancer-associated deaths are due to cachexia. During this process, metabolic changes occur; cancer cells behave like parasites, taking nutrition from the surrounding tissue leading to exhaustion of the body’s energy resources [72]. Cancer-associated cachexia not only increases the rate of mortality but is also responsible for treatment failure [73][74]. The adipose tissue and the muscle cells play a major role in this process, releasing lactate, pyruvate and free fatty acids upon the signals received by cancer cells [75]. In BC, it has been shown that exosomes released from cancer cells trigger cachexia [76]. Moreover, the adipose tissue adjacent to BC cells starts expressing the UCP-1 gene responsible for the browning of WAT [75], resulting in increased cachexia. Cachexia is also induced by several cytokines such as TNFα, IL-1, IL-6 and IFNγ [77]. A recent review by Rybinska et al., 2020, showed an increase in TNFα, IL-6, IL-1β, CCL2, adiponectin and collagen and a decrease in leptin in BC causing anorexia leading to cachexia. Furthermore, BC mediated cachexia is also associated with a decrease in adipogenesis transcription factors PPARγ, c/EBPα, GLUT4 and SREBP-1, and an increase in HSL, an enzyme responsible for lipolytic activity in cachexia [78].
5.5. Obesity
Obesity is defined as an excess accumulation of fat in the body, with great health risk to an individual. According to the world health organization, there is a three-fold increase in obesity since 1975 [79]. Obesity has been linked to an increase in the risk of BC and also with poor survival outcome [80][81]. Around 60% of women in the USA are overweight, and research has established a clear link between Body Mass Index (BMI) and BC [82]. However, BMI is not an absolute parameter, as a lean woman could also have a significant depot of visceral fat, which might not be reflected by BMI measurement. Therefore, the current standard followed in clinical settings also includes waist-to-hip ratio. Borugian et al., 2003, showed that waist-to-hip ratio measurement in combination with menopausal and ER status is a predictable marker of BC associated deaths [83]. Moreover, a study by our group suggests that the weight gained during adulthood could be associated with BC risk in women with more fatty breast; therefore, a minimum of breast fat may be needed to promote the development of BC [84]. Besides the known adverse effects of excess adipose tissue in BC, a systemic review from our group highlighted that the obesity linked hypermethylation of PTPRN2 and ABLIM2 genes in breast tissue could be associated with BC [85].
6. Conclusion
There are multiple layers in the biology of adipose tissue, which are not restricted to obesity. The presence of breast adipose tissue is of paramount importance in breast, due to its involvement in breast development, maturation and involution. However, breast adipose tissue also promotes breast cancer. The interaction between breast adipose tissue and cancer cells in the BC microenvironment is a complex network composed of both the autocrine and paracrine effects of secretory molecules from each component (breast adipose tissue and cancer cells), modulating each other’s function for a common goal, i.e. BC survival and proliferation. A thorough understanding of this crosstalk could give rise to potential therapeutic candidates which could improve the outcome of the present therapeutic options.
References
- Miana, V.V.; González, E.A.P. Adipose tissue stem cells in regenerative medicine. Ecancermedicalscience 2018, 12, 822.
- Wankhade, U.D.; Shen, M.; Yadav, H.; Thakali, K.M. Novel Browning Agents, Mechanisms, and Therapeutic Potentials of Brown Adipose Tissue. Biomed Res. Int. 2016, 2016, 2365609.
- Kajimura, S.; Spiegelman, B.M.; Seale, P. Brown and Beige Fat: Physiological Roles beyond Heat Generation. Cell Metab. 2015, 22, 546–559.
- Vitali, A.; Murano, I.; Zingaretti, M.C.; Frontini, A.; Ricquier, D.; Cinti, S. The adipose organ of obesity-prone C57BL/6J mice is composed of mixed white and brown adipocytes. J. Lipid Res. 2012, 59, 619–629.
- Bartelt, A.; Heeren, J. Adipose tissue browning and metabolic health. Nat. Rev. Endocrinol. 2014, 10, 24–36.
- Kotzbeck, P.; Giordano, A.; Mondini, E.; Murano, I.; Severi, I.; Venema, W.; Cecchini, M.P.; Kershaw, E.E.; Barbatelli, G.; Haemmerle, G.; et al. Brown adipose tissue whitening leads to brown adipocyte death and adipose tissue inflammation. J. Lipid Res. 2018, 59, 784–794.
- Himms-Hagen, J.; Melnyk, A.; Zingaretti, M.C.; Ceresi, E.; Barbatelli, G.; Cinti, S. Multilocular fat cells in WAT of CL-316243-treated rats derive directly from white adipocytes. Am. J. Physiol. Cell Physiol. 2000, 279, C670–C681.
- Peres Valgas da Silva, C.; Hernández-Saavedra, D.; White, J.D.; Stanford, K.I. Cold and Exercise: Therapeutic Tools to Activate Brown Adipose Tissue and Combat Obesity. Biology 2019, 8, 9.
- Lee, Y.H.; Mottillo, E.P.; Granneman, J.G. Adipose tissue plasticity from WAT to BAT and in between. Biochim. Biophys. Acta 2014, 1842, 358–369.
- Howlett, A.R.; Bissell, M.J. The influence of tissue microenvironment (stroma and extracellular matrix) on the development and function of mammary epithelium. Epithel. Cell Biol. 1993, 2, 79–89.
- Zangani, D.; Darcy, K.M.; Shoemaker, S.; Ip, M.M. Adipocyte-epithelial interactions regulate the in vitro development of normal mammary epithelial cells. Exp. Cell Res. 1999, 247, 399–409.
- Sakakura, T.; Sakagami, Y.; Nishizuka, Y. Dual origin of mesenchymal tissues participating in mouse mammary gland embryogenesis. Dev. Biol. 1982, 91, 202–207.
- Couldrey, C.; Moitra, J.; Vinson, C.; Anver, M.; Nagashima, K.; Green, J. Adipose tissue: A vital in vivo role in mammary gland development but not differentiation. Dev. Dyn. 2002, 223, 459–468.
- Hu, X.; Juneja, S.C.; Maihle, N.J.; Cleary, M.P. Leptin—A growth factor in normal and malignant breast cells and for normal mammary gland development. J. Natl Cancer Inst. 2002, 94, 1704–1711.
- Combs, T.P.; Pajvani, U.B.; Berg, A.H.; Lin, Y.; Jelicks, L.A.; Laplante, M.; Nawrocki, A.R.; Rajala, M.W.; Parlow, A.F.; Cheeseboro, L.; et al. A transgenic mouse with a deletion in the collagenous domain of adiponectin displays elevated circulating adiponectin and improved insulin sensitivity. Endocrinology. 2004, 145, 367–383.
- Kim, J.Y.; van de Wall, E.; Laplante, M.; Azzara, A.; Trujillo, M.E.; Hofmann, S.M.; Schraw, T.; Durand, J.L.; Li, H.; Li, G.; et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J. Clin. Investig. 2007, 117, 2621–2637.
- Landskroner-Eiger, S.; Qian, B.; Muise, E.S.; Nawrocki, A.R.; Berger, J.P.; Fine, E.J.; Koba, W.; Deng, Y.; Pollard, J.W.; Scherer, P.E.. Proangiogenic contribution of adiponectin toward mammary tumor growth in vivo. Clin Cancer Res. 2009, 15, 3265–3276.
- Landskroner-Eiger, S.; Park, J.; Israel, D.; Pollard, J.W.; Scherer, P.E. Morphogenesis of the developing mammary gland: Stage-dependent impact of adipocytes. Dev. Biol. 2010, 344, 968–978.
- Granneman, J.G.; Li, P.; Zhu, Z.; Lu, Y. Metabolic and cellular plasticity in white adipose tissue I: Effects of beta3-adrenergic receptor activation. Am. J. Physiol. Endocrinol. Metab. 2005, 289, E608–E616.
- Cinti, S. Adipocyte differentiation and transdifferentiation: Plasticity of the adipose organ. J. Endocrinol. Investig. 2002, 25, 823–835.
- Giordano, A.; Smorlesi, A.; Frontini, A.; Barbatelli, G.; Cinti, S. White, brown and pink adipocytes: The extraordinary plasticity of the adipose organ. Eur. J. Endocrinol. 2014, 170, R159–R171.
- Robinson, G.W.; McKnight, R.A.; Smith, G.H.; Hennighausen, L. Mammary epithelial cells undergo secretory differentiation in cycling virgins but require pregnancy for the establishment of terminal differentiation. Development 1995, 121, 2079–2090.
- Prokesch, A.; Smorlesi, A.; Perugini, J.; Manieri, M.; Ciarmela, P.; Mondini, E.; Trajanoski, Z.; Kristiansen, K.; Giordano, A.; Bogner-Strauss, J.G.; et al. Molecular aspects of adipoepithelial transdifferentiation in mouse mammary gland. Stem Cells 2014, 32, 2756–2766.
- Kimmel, A.R.; Brasaemle, D.L.; McAndrews-Hill, M.; Sztalryd, C.; Londos, C. Adoption of PERILIPIN as a unifying nomenclature for the mammalian PAT-family of intracellular lipid storage droplet proteins. J. Lipid Res. 2010, 51, 468-471.
- Sztalryd, C.; Kimmel, A.R. Perilipins: Lipid droplet coat proteins adapted for tissue-specific energy storage and utilization, and lipid cytoprotection. Biochimie 2014, 96, 96–101.
- Cinti S, Cigolini M, Morroni M, Zingaretti MC. S-100 protein in white preadipocytes: An immunoelectronmicroscopic study. Anat. Rec. 1989, 224, 466-472.
- Apostoli, A.J.; Skelhorne-Gross, G.E.A.; Rubino, R.E.; Peterson, N.T.; Di Lena, M.A; Schneider, M.M.; SenGupta, S.K.; Nicol, C.J.B. Loss of PPARγ expression in mammary secretory epithelial cells creates a pro-breast tumorigenic environment. Int. J. Cancer 2014, 134, 1055–1066.
- Hughes, L.E.; Mansel, R.E.; Webster, D.J. Aberrations of normal development and involution (ANDI): A new perspective on pathogenesis and nomenclature of benign breast disorders. Lancet 1987, 2, 1316–1319.
- Schedin, P.; O'Brien, J.; Rudolph, M.; Stein, T.; Borges, V. Microenvironment of the involuting mammary gland mediates mammary cancer progression. J. Mammary Gland Biol. Neoplasia 2007, 12, 71–82.
- Radisky, D.C.; Hartmann, L.C. Mammary involution and breast cancer risk: Transgenic models and clinical studies. J. Mammary Gland Biol. Neoplasia 2009, 14, 181–191.
- McDaniel, S.M.; Rumer, K.K.; Biroc, S.L.; Metz, R.P.; Singh, M.; Porter, W.; Schedin, P. Remodeling of the mammary microenvironment after lactation promotes breast tumor cell metastasis. Am. J. Pathol. 2006, 168, 608–620.
- Morroni, M.; Giordano, A.; Zingaretti, M.C.; Boiani, R.; De Matteis, R.; Kahn, B.B.; Nisoli, E.; Tonello, C.; Pisoschi, C.; Luchetti, M.M.; et al. Reversible transdifferentiation of secretory epithelial cells into adipocytes in the mammary gland. Proc. Natl. Acad. Sci. USA 2004, 101, 16801–16806.
- Zwick, R.K.; Rudolph, M.C.; Shook, B.A.; Holtrup, B.; Roth, E.; Lei, V.; Van Keymeulen, A.; Seewaldt, V.; Kwei, S.; Wysolmerski, J.; et al. Adipocyte hypertrophy and lipid dynamics underlie Ref 71 is same with ref 74mammary gland remodeling after lactation. Nat. Commun. 2018, 9, 3592.
- Clemenceau, A.; Diorio, C.; Durocher, F. Role of Secreted Frizzled-Related Protein 1 in Early Mammary Gland Tumorigenesis and Its Regulation in Breast Microenvironment. Cells 2020, 9, 208.
- Lagathu, C.; Christodoulides, C.; Tan, C.Y; Virtue, S.; Laudes, M.; Campbell, M.; Ishikawa, K.; Ortega, F.; Tinahones, F.J.; Fernández-Real, J.M.; et al. Secreted frizzled-related protein 1 regulates adipose tissue expansion and is dysregulated in severe obesity. Int. J. Obes. (Lond). 2010, 34, 1695–1705.
- Ehrlund, A.; Mejhert, N.; Lorente-Cebrián, S.; Aström, G.; Dahlman, I.; Laurencikiene, J.; Rydén, M. Characterization of the Wnt inhibitors secreted frizzled-related proteins (SFRPs) in human adipose tissue. J. Clin. Endocrinol. Metab. 2013, 98, E503–E508.
- Zheng, X.; Ning, C.; Dong, Y.; Zhao, P.; Li, J.; Fan, Z.; Li, J.; Yu, Y.; Mrode, R.; Liu, J. Quantitative proteome analysis of bovine mammary gland reveals protein dynamic changes involved in peak and late lactation stages. Biochem. Biophys. Res. Commun. 2017, 494, 292–297.
- Gauger, K.J.; Shimono, A.; Crisi, G.M.; Schneider, S.S. Loss of SFRP1 promotes ductal branching in the murine mammary gland. BMC Dev. Biol. 2012, 12, 25.
- Li, L.; Li, B.; Li, M.; Niu, C.; Wang, G.; Li, T.; Król, E.; Jin, W.; Speakman, J.R. Brown adipocytes can display a mammary basal myoepithelial cell phenotype in vivo. Mol. Metab. 2017, 6, 1198–1211.
- Singh, R.; Parveen, M.; Basgen, J.M.; Fazel, S.; Meshesha, M.F.; Thames, E.C.; Moore, B.; Martinez, L.; Howard, C.B.; Vergnes, L.; et al. Increased Expression of Beige/Brown Adipose Markers from Host and Breast Cancer Cells Influence Xenograft Formation in Mice. Mol. Cancer Res. 2016, 14, 78–92.
- Munsell, M.F.; Sprague, B.L.; Berry, D.A.; Chisholm, G.; Trentham-Dietz, A. Body mass index and breast cancer risk according to postmenopausal estrogen-progestin use and hormone receptor status. Epidemiol. Rev. 2014, 36, 114–136.
- Judd, H.L.; Judd, G.E.; Lucas, W.E.; Yen, S.S. Endocrine function of the postmenopausal ovary: Concentration of androgens and estrogens in ovarian and peripheral vein Blood J. Clin. Endocrinol. Metab. 1974, 39, 1020–1024.
- Baird, D.T.; Uno, A.; Melby, J.C. Adrenal secretion of androgens and oestrogens. J. Endocrinol. 1969, 45, 135–136.
- van Landeghem AA, Poortman J, Nabuurs M, Thijssen JH. Endogenous concentration and subcellular distribution of androgens in normal and malignant human breast tissue. Cancer Res. 1985, 45, 2907–2912.
- Laforest, S.; Pelletier, M.; Denver, N.; Poirier, B.; Nguyen, S.; Walker, B.R.; Durocher, F.; Homer, N.Z.M; Diorio, C.; Andrew, R.; et al. Estrogens and Glucocorticoids in Mammary Adipose Tissue: Relationships with Body Mass Index and Breast Cancer Features. J. Clin. Endocrinol. Metab. 2020, 105, e1504–e1516.
- Keinan-Boker, L.; Lerner-Geva, L.; Kaufman, B.; Meirow, D. Pregnancy-associated breast cancer. Isr. Med. Assoc. J. 2008, 10, 722–727.
- Litwiniuk M, Breborowicz E, Breborowicz D, Filas V, Breborowicz J: Steroid hormone status and HER2/neu expression in pregnancy-associate breast cancer. J. Clin. Oncol. 2007, 25, 21115–21115.
- McCready, J.; Arendt, L.M.; Glover, E.; Iyer, V.; Briende, J.L.; Lyle, S.R.; Naber, S.P.; Jay, D.G.; Kuperwasser, C. Pregnancy-associated breast cancers are driven by differences in adipose stromal cells present during lactation. Breast Cancer Res. 2014, 16, R2.
- Vona-Davis, L.; Rose, D.P. Adipokines as endocrine, paracrine, and autocrine factors in breast cancer risk and progression. Endocr. Relat. Cancer 2007, 14, 189–206.
- Alexander, C.M.; Selvarajan, S.; Mudgett, J.; Werb, Z. Stromelysin-1 regulates adipogenesis during mammary gland involution. J. Cell Biol. 2001, 152, 693–703.
- McCready, J.; Arendt, L.M.; Rudnick, J.A.; Kuperwasser, C. The contribution of dynamic stromal remodeling during mammary development to breast carcinogenesis. Breast Cancer Res. 2010, 12, 205.
- Russo, J.; Lynch, H.; Russo, I.H. Mammary gland architecture as a determining factor in the susceptibility of the human breast to cancer. Breast J. 2001, 7, 278–291.
- Milanese, T.R.; Hartmann, L.C.; Sellers, T.A.; Frost, M.H.; Vierkant, R.A.; Maloney, S.D.; Pankratz, V.S.; Degnim, A.C.; Vachon, C.M.; Reynolds, C.A.; et al. Age-related lobular involution and risk of breast cancer. J. Natl. Cancer Inst. 2006, 98, 1600–1607.
- Figueroa, J.D.; Pfeiffer, R.M.; Brinton, L.A.; Palakal, M.M.; Degnim, A.C.; Radisky, D.; Hartmann, L.C.; Frost, M.H.; Mann, M.L.S.; Papathomas, D.; et al. Standardized measures of lobular involution and subsequent breast cancer risk among women with benign breast disease: A nested case-control study. Breast Cancer Res. Treat. 2016, 159, 163–172.
- Hanna, M.; Dumas, I.; Orain, M.; Jacob, S.; Têtu, B.; Sanschagrin, F.; Bureau, A.; Poirier, B.; Diorio, C. Association between local inflammation and breast tissue age-related lobular involution among premenopausal and postmenopausal breast cancer patients. PLoS ONE 2017, 12, e0183579.
- Shoker, B.S.; Jarvis, C.; Sibson, D.R.; Walker, C.; Sloane, J.P. Oestrogen receptor expression in the normal and pre-cancerous breast. J. Pathol. 1999, 188, 237–244.
- Gatta, G.; Pinto, A.; Romano, S.; Ancona, A.; Scaglione, M.; Volterrani, L. Clinical, mammographic and ultrasonographic features of blunt breast trauma. Eur. J. Radiol. 2006, 59, 327–330.
- Baber, C.E.; Libshitz, H.I. Bilateral fat necrosis of the breast following reduction mammoplasties. AJR Am. J. Roentgenol. 1977, 128, 508–509.
- Bassett, L.W.; Gold, R.H.; Cove, H.C. Mammographic spectrum of traumatic fat necrosis: The fallibility of "pathognomonic" signs of carcinoma. AJR Am. J. Roentgenol. 1978, 130, 119–122.
- Di Stefano, A.B.; Grisafi, F.; Castiglia, M.; Perez, A.; Montesano, L.; Gulino, A.; Toia, F.; Fanale, D.; Russo, A.; Moschella, F.; et al. Spheroids from adipose-derived stem cells exhibit a miRNA profile of highly undifferentiated cells. J. Cell Physiol. 2018, 233, 8778–8789.
- Mandel, K.; Yang, Y.; Schambach, A.; Glage, S.; Otte, A.; Hass, R. Mesenchymal stem cells directly interact with breast cancer cells and promote tumor cell growth in vitro and in vivo. Stem. Cells Dev. 2013, 22, 3114–3127.
- Tardelli, M.; Zeyda, K.; Moreno-Viedma, V.; Wanko, B.; Grün, N.G.; Staffler, G.; Zeyda, M.; Stulnig1, T.M. Osteopontin is a key player for local adipose tissue macrophage proliferation in obesity. Mol. Metab. 2016, 5, 1131–1137.
- Rizwan, A.; Paidi, S.K.; Zheng, C.; Cheng, M.; Barman, I.; Glunde, K. Mapping the genetic basis of breast microcalcifications and their role in metastasis. Sci. Rep. 2018, 8, 11067.
- Huan, J.L.; Xing, L.; Qin, X.J.; Gao, Z.G.; Pan, X.F.; Zhao, Z.D. Expression and clinical significance of osteopontin in calcified breast tissue. Asian Pac. J. Cancer Prev. 2012, 13, 5219–5223.
- Bellahcène, A.; Castronovo, V. Increased expression of osteonectin and osteopontin, two bone matrix proteins, in human breast cancer. Am. J. Pathol. 1995, 146, 95–100.
- Templeton, Z.S.; Lie, W.R.; Wang, W.; Rosenberg-Hasson, Y.; Alluri, R.V.; Tamaresis, J.S.; Bachmann, M.H.; Lee, K.; Maloney, W.J.; Contag, C.H.; et al. Breast Cancer Cell Colonization of the Human Bone Marrow Adipose Tissue Niche. Neoplasia 2015, 17, 849‐861.
- Pang, X.; Gong, K.; Zhang, X.; Wu, S.; Cui, Y.; Qian, B.Z. Osteopontin as a multifaceted driver of bone metastasis and drug resistance. Pharmacol. Res. 2019, 144, 235‐244.
- Oyama, T.; Sano, T.; Hikino, T.; Xue, Q.; Iijima, K.; Nakajima, T.; Koerner, F. Microcalcifications of breast cancer and atypical cystic lobules associated with infiltration of foam cells expressing osteopontin. Virchows Arch. 2002, 440, 267–273.
- Tabár, L.; Chen, H.H.; Duffy, S.W.; Yen, M.F.; Chiang, C.F.; Dean, P.B. A novel method for prediction of long-term outcome of women with T1a, T1b, and 10–14 mm invasive breast cancers: A prospective study [published correction appears in Lancet 2000 Apr 15; 355, 1372]. Lancet 2000, 355, 429–433.
- Thurfjell, E.; Thurfjell, M.G.; Lindgren, A. Mammographic finding as predictor of survival in 1–9 mm invasive breast cancers. worse prognosis for cases presenting as calcifications alone. Breast Cancer Res. Treat. 2001, 67, 177–180.
- Ling, H.; Liu, Z.B.; Xu, L.H.; Xu, X.L.; Liu, G.Y.; Shao, Z.M. Malignant calcification is an important unfavorable prognostic factor in primary invasive breast cancer [published correction appears in Asia Pac J. Clin. Oncol. 2013 Dec; 9, 383]. Asia Pac. J. Clin. Oncol. 2013, 9, 139–145.
- Zhang, X.M.; Dou, Q.L.; Zeng, Y.; Yang, Y.; Cheng, A.S.K.; Zhang, W.W. Sarcopenia as a predictor of mortality in women with breast cancer: A meta-analysis and systematic review. BMC Cancer 2020, 20, 172.
- Bruggeman, A.R.; Kamal, A.H.; LeBlanc, T.W.; Ma, J.D.; Baracos, V.E.; Roeland, E.J. Cancer Cachexia: Beyond Weight Loss. J. Oncol. Pract. 2016, 12, 1163–1171.
- Vigano, A.A.L.; Morais, J.A.; Ciutto, L.; Rosenthall, L.; di Tomasso, J.; Khan, S.; Olders, H.; Borod, M.; Kilgour, R.D. Use of routinely available clinical, nutritional, and functional criteria to classify cachexia in advanced cancer patients. Clin. Nutr. 2017, 36, 1378–1390.
- Wu, Q.; Sun, S.; Li, Z.; Yang, Q.; Li, B.; Zhu, S.; Wang, L.; Wu, J.; Yuan, J.; Wang, C.; et al. Breast cancer-released exosomes trigger cancer-associated cachexia to promote tumor progression. Adipocyte 2019, 8, 31–45.
- Wang, F.; Gao, S.; Chen, F.; Fu, Z.; Yin, H.; Lu, X.; Yu, J.; Lu, C. Mammary fat of breast cancer: Gene expression profiling and functional characterization. PLoS ONE 2014, 9, e109742.
- Argilés, J.M.; López-Soriano, F.J. The role of cytokines in cancer cachexia. Med. Res. Rev. 1999, 19, 223–248.
- Rybinska, I.; Agresti, R.; Trapani, A.; Tagliabue, E.; Triulzi, T. Adipocytes in Breast Cancer, the Thick and the Thin. Cells 2020, 9, 560.
- Obesity and Overweight. World Health Organization. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 7 August 2020).
- Picon-Ruiz, M.; Morata-Tarifa, C.; Valle-Goffin, J.J.; Friedman, E.R.; Slingerland, J.M. Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention. CA Cancer J. Clin. 2017, 67, 378–397.
- Oudanonh, T.; Nabi, H.; Ennour-Idrissi, K.; Lemieux, J.; Diorio, C. Progesterone receptor status modifies the association between body mass index and prognosis in women diagnosed with estrogen receptor positive breast cancer. Int. J. Cancer 2020, 146, 2736–2745.
- Breastcancer.org. Breast Cancer Risk Seems More Affected by Total Body Fat Than Abdominal Fat. Available online: June 27, 2017. https://www.breastcancer.org/research-news/total-body-fat-affects-risk-more-than-belly-fat (27 July 2020).
- Borugian, M.J.; Sheps, S.B.; Kim-Sing, C.; Olivotto, I.A.; Patten, C.V.; Dunn, B.P.; Coldman, A.J.; Potter, J.D.; Gallagher, R.P.; Hislop, T.G. Waist-to-hip ratio and breast cancer mortality. Am. J. Epidemiol. 2003, 158, 963–968.
- Soguel, L.; Diorio, C. Anthropometric factors, adult weight gain, and mammographic features. Cancer Causes Control. 2016, 27, 333–340.
- Dragic, D.; Ennour-Idrissi, K.; Michaud, A.; Chang, S.L.; Durocher, F.; Diorio, C. Association Between BMI and DNA Methylation in Blood or Normal Adult Breast Tissue: A Systematic Review. Anticancer. Res. 2020, 40, 1797–1808.




