
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Harald Neumann | + 1128 word(s) | 1128 | 2020-08-08 03:14:20 |
Video Upload Options
This article gives a very short overview on sialic acids and is an excerpt of the introduction from the publication ‘Control of Innate Immunity by Sialic Acids in the Nervous Tissue’ by Liao H, Klaus C, Neumann H. in the Int J Mol Sci. 2020 Jul 31;21(15):E5494. doi: 10.3390/ijms21155494
1. Introduction
Glycosylation extends the functional diversity of proteins and lipids tremendously. It is one of the most complicated posttranslational modifications, which is added in the endoplasmic reticulum after the folding of glycoproteins or build-up of glycolipids. Thereby, proteins and lipids are elongated by various saccharides leading to high glycan diversity. The added saccharides mainly contribute new functional properties related to complex cell-cell and cell-matrix interactions [1]. Here, sialic acids (Sias) play a unique role. While Sias are displayed on the glycocalyx of few pathogens, such as Campylobacter jejuni and Neisseria spp. [2], they are abundantly present on most extracellular proteins and lipids in mammals. Sias mostly form terminal caps on the glycoconjugates of the mammalian glycocalyx. In addition, mammals have a huge repertoire of immune receptors to recognize Sias in conjunction with the underlying structures. Therefore, Sias have been introduced as essential determinants of self-recognition by the immune system [3]. Particularly, the innate immune system has developed several receptors for recognition of Sias on glycoconjugates as self-patterns. Both complement regulator factor H (CFH) and Sia-binding immunoglobulin-like lectins (SIGLECS) have been recognized as sensors of Sia-containing self-patterns [4][5].
Sias are nine carbon monosaccharides. They form the terminal caps on the glycocalyx of cell surfaces and secreted glycoproteins of vertebrates [6]. There are three main types of Sias in vertebrates, namely N-acetylneuraminic acid (Neu5Ac), N-glycolylneuraminic acid (Neu5Gc), and deaminoneuraminic acid (Kdn). While Neu5Ac is the most abundant Sia in humans, Neu5Gc and Kdn only are detected as trace amounts in human tissue, most likely derived from food sources [7][8][9][10]. The three major Sias are further subject to a number of modifications, such as methylation, sulfation, lactylation, acetylation, and lactonization and form a diverse family consisting of more than 50 members with different structures [11]. Sias are highly abundant on the surface of nervous and immune cells of mammals. For example, Sias have an estimated concentration of more than 100 mM at the surface of a lymphocyte [12].
2. Structure, Diversity, Biosynthesis Related Key Enzymes and Sialidases
The biosynthesis of Sia takes place in the cytosol and involves four steps and three enzymes. Among the three enzymes, the bifunctional glucosamine (UDP-N-Acetyl)-2-epimerase/N-acetylmannosamine kinase (GNE) is one rate-limiting enzyme [13]. GNE participates in the initial two steps of Sia biosynthesis. After catalyzing the conversion from uridine diphosphate N-acetylglucosamine to N-acetyl-D-mannosamine (ManNAc), GNE further phosphorylates ManNAc to form N-acetyl-mannosamine 6-phosphate (ManNAc-6-P). ManNAc-6-P is then condensed and dephosphorylated to generate Neu5Ac by N-acetylneuraminic acid synthase and N-acetylneuraminic acid phosphatase [14]. The Neu5Ac is then conjugated with cytidine 5′-monophosphate (CMP) to form active CMP-Neu5Ac by the enzyme cytidine monophosphate N-acetylneuraminic acid synthetase in the nucleus [15]. Subsequently, sialyltransferases transfer Sias to various molecules terminated with a galactose (Gal), an N-acetylgalactosamine (GalNAc), or another Sia residue. This step leads to the biosynthesis of Sia-containing glycoconjugates and oligosaccharides [16]. There are 20 types of sialyltransferases in humans that conjugate the Sia molecule Neu5Ac to the acceptor sugar (Gal, GalNAc, Neu5Ac) using a defined linkage (α2,3-, α2,6-, α2,8-) between the Sia residue and the acceptor sugar. Moreover, Sias can form homopolymers with different degrees of polymerization via intersialyl linkages. For instance, polysialic acid (polySia) is a homopolymer of α2,8-linked Neu5Ac monomers with a degree of polymerization between 10 and around 200 [17]. Thus, a huge number of glycoconjugates can be generated.
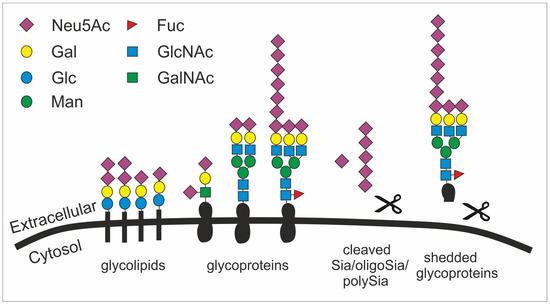
Figure 1. The structural diversity of the glycocalyx is fundament to many cellular processes. Sialic acid is mainly found as terminal saccharide on the glycocalyx that is formed by glycolipids (e.g. gangliosides) as well as sialylated and polysialylated glycoproteins (e.g. polySia-NCAM). Glycoproteins can be shedded (here simplified by showing a scissor) and released as sialylated molecules (e.g., forming the mucus). Sialic acids (Sia), oligosialic acids (oligoSia) and polysialic acids (polySia) can be cleaved by sialidases (scissor) or oxidative processes (scissor) and trapped together with growth factors in the extracellular matrix. Neu5Ac, N-acetylneuraminic acid; Gal, galactose; Glc, glucose; Man, mannose; Fuc, fucose; GlcNAc, N-acetylglucosamine; GalNAc, N-acetylgalactosamine.
Given their abundance and diversity on all vertebrate cell surfaces as the terminal cap, it becomes obvious that Sias fulfill multifarious roles, such as in microdomain formation [18], cell adhesion [19], tissue homeostasis [20], immune cell modulation [21], cell migration [22], chemokine sensing [23], and growth factor retention [24] (figure 1). Particularly, the outermost position of Sias with multiple functional groups allows them to have interactions with other molecules, mainly glycoproteins called lectins. These interactions involve hydrogen bonds, salt bridges, and non-polar interactions. However, the affinity of such interactions between one protein (e.g. a lectin) and the respective glycan is relatively low and often requires multivariant binding between several glycans and protein molecules [25].
Interestingly, some bacteria can use this self-recognition system as camouflage by generating analogues of human sialoglycans. For example, α2.8-linked polySia is used by several bacteria as a neuro-invasive determinant [26][27] and thus could escape host immune attack via inactivating the complement system and silencing innate immune cells via SIGLEC-11. The polySia capsules of Escherichia coli K1 (E. coli K1) specifically restrict alternative complement pathway activation [28], while those of serogroup B Neisseria meningitides help the bacteria to escape elimination by normal human serum [29]. However, the mechanism of how polySia modulates the complement cascade is not fully understood yet. Furthermore, polySia is used by E. coli K1 for immune evasion by a second receptor-mediated mechanism: the human-specific pathogen E. coli K1 uses its polySia capsule as a protective molecular shield to escape immune defense by engaging SIGLEC-11 [30].
Sialidases/neuraminidases are glycoside hydrolase enzymes. They participate in the cleavage of terminal Sias from glycoproteins and glycolipids, a process named desialylation [31]. In mammalian cells, there are four types of sialidases, sialidase 1–4. Sialidase 1 (a lysosomal sialidase, also called N-acetyl-alpha-neuraminidase 1; NEU1) is encoded by the NEU1 gene. It is present in both plasma membrane and lysosome. Sialidase 2 (a cytosolic sialidase, also called N-acetyl-alpha-neuraminidase 2; NEU2), encoded by the NEU2 gene, can be found in the cytosol. Sialidase 3 (a membrane sialidase, also called N-acetyl-alpha-neuraminidase 3; NEU3) is encoded by the NEU3 gene and localized in the plasma membrane, while sialidase 4 (N-acetyl-alpha-neuraminidase 4; NEU4) is encoded by the NEU4 gene and displays on internal membranes. In the mouse brain, NEU1, NEU3 and NEU4 show similar expression patterns [32][33]. Moreover, these sialidases are active against different but overlapping sialylated glycoconjugates. Both NEU1 and NEU4 are active towards sialylated oligosaccharides, glycoproteins, and the gangliosides as glycolipids. However, NEU1 has negligible activity against gangliosides, which are the main substrates of NEU3 [33]. Growing evidence suggests that mammalian sialidases play an important role in the development and function of the CNS [17][31][33].
References
- James W Dennis; Genetic code asymmetry supports diversity through experimentation with posttranslational modifications. Current Opinion in Chemical Biology 2017, 41, 1-11, 10.1016/j.cbpa.2017.08.012.
- Emmanuele Severi; Derek W. Hood; Gavin H. Thomas; Sialic acid utilization by bacterial pathogens. Microbiology 2007, 153, 2817-2822, 10.1099/mic.0.2007/009480-0.
- Ajit Varki; Since there are PAMPs and DAMPs, there must be SAMPs? Glycan “self-associated molecular patterns” dampen innate immunity, but pathogens can mimic them.. Glycobiology 2011, 21, 1121–1124, https://doi.org/10.1093/glycob/cwr087.
- Daniel Ricklin; George Hajishengallis; Kun Yang; John D. Lambris; Complement: a key system for immune surveillance and homeostasis. Nature Immunology 2010, 11, 785-797, 10.1038/ni.1923.
- Matthew S. Macauley; Paul R. Crocker; James C. Paulson; Siglec-mediated regulation of immune cell function in disease. Nature Reviews Immunology 2014, 14, 653-666, 10.1038/nri3737.
- Ajit Varki; Are humans prone to autoimmunity? Implications from evolutionary changes in hominin sialic acid biology. Journal of Autoimmunity 2017, 83, 134-142, 10.1016/j.jaut.2017.07.011.
- S Inoue; K Kitajima; Y Inoue; Identification of 2-keto-3-deoxy-D-glycero--galactonononic acid (KDN, deaminoneuraminic acid) residues in mammalian tissues and human lung carcinoma cells. Chemical evidence of the occurrence of KDN glycoconjugates in mammals.. Journal of Biological Chemistry 1996, 271, 24341-4, 10.1074/jbc.271.40.24341 .
- Shuuji Hara; Yasuyo Takemori; Masatoshi Yamaguchi; Masaru Nakamura; Yosuke Ohkura; Fluorometric high-performance liquid chromatography of N-acetyl- and N-glycolylneuraminic acids and its application to their microdetermination in human and animal sera, glycoproteins, and glycolipids. Analytical Biochemistry 1987, 164, 138-145, 10.1016/0003-2697(87)90377-0.
- Muriel Bardor; Dzung H. Nguyen; Sandra Díaz; Ajit Varki; Mechanism of Uptake and Incorporation of the Non-human Sialic AcidN-Glycolylneuraminic Acid into Human Cells. Journal of Biological Chemistry 2004, 280, 4228-4237, 10.1074/jbc.m412040200.
- Pam Tangvoranuntakul; Pascal Gagneux; Sandra Diaz; Muriel Bardor; Nissi Varki; Ajit Varki; Elaine Muchmore; Human uptake and incorporation of an immunogenic nonhuman dietary sialic acid. Proceedings of the National Academy of Sciences 2003, 100, 12045-12050, 10.1073/pnas.2131556100.
- Takashi Angata; Ajit Varki; Chemical Diversity in the Sialic Acids and Related α-Keto Acids: An Evolutionary Perspective. Chemical Reviews 2002, 102, 439-470, 10.1021/cr000407m.
- Brian E. Collins; O. Blixt; Alexis R. DeSieno; Nicolai Bovin; Jamey D. Marth; James C. Paulson; Masking of CD22 by cis ligands does not prevent redistribution of CD22 to sites of cell contact. Proceedings of the National Academy of Sciences 2004, 101, 6104-6109, 10.1073/pnas.0400851101.
- O. T. Keppler; UDP-GlcNAc 2-Epimerase: A Regulator of Cell Surface Sialylation. Science 1999, 284, 1372-1376, 10.1126/science.284.5418.1372.
- Yanhong Li; Xi Chen; Sialic acid metabolism and sialyltransferases: natural functions and applications. Applied Microbiology and Biotechnology 2012, 94, 887-905, 10.1007/s00253-012-4040-1.
- Anja K. Münster-Kühnel; Joe Tiralongo; Stephan Krapp; Birgit Weinhold; Valentina Ritz-Sedlacek; Uwe Jacob; Rita Gerardy-Schahn; Structure and function of vertebrate CMP-sialic acid synthetases. Glycobiology 2004, 14, 43R-51R, 10.1093/glycob/cwh113.
- Xi Chen; Ajit Varki; Advances in the Biology and Chemistry of Sialic Acids. ACS Chemical Biology 2010, 5, 163-176, 10.1021/cb900266r.
- Ronald L Schnaar; Rita Gerardy-Schahn; Herbert Hildebrandt; Sialic acids in the brain: gangliosides and polysialic acid in nervous system development, stability, disease, and regeneration.. Physiological Reviews 2014, 94, 461-518, 10.1152/physrev.00033.2013.
- Leonhard Möckl; Andrea K. Horst; Katharina Kolbe; Thisbe K. Lindhorst; Christoph Bräuchle; Microdomain Formation Controls Spatiotemporal Dynamics of Cell-Surface Glycoproteins. ChemBioChem 2015, 16, 2023-2028, 10.1002/cbic.201500361.
- Sørge Kelm; Roland Schauer; Jean-Claude Manuguerra; Hans-Jürgen Gross; Paul R Crocker; Modifications of cell surface sialic acids modulate cell adhesion mediated by sialoadhesin and CD22. Glycoconjugate Journal 1994, 11, 576-585, 10.1007/bf00731309.
- Ajit Varki; Pascal Gagneux; Multifarious roles of sialic acids in immunity.. Annals of the New York Academy of Sciences 2012, 1253, 16-36, 10.1111/j.1749-6632.2012.06517.x.
- Joyce Lübbers; Ernesto Rodríguez; Yvette Van Kooyk; Modulation of Immune Tolerance via Siglec-Sialic Acid Interactions. Frontiers in Immunology 2018, 9, 2807, 10.3389/fimmu.2018.02807.
- Sònia Bassagañas; Marta Pérez-Garay; Rosa Peracaula; Cell Surface Sialic Acid Modulates Extracellular Matrix Adhesion and Migration in Pancreatic Adenocarcinoma Cells. Pancreas 2014, 43, 109-117, 10.1097/mpa.0b013e31829d9090.
- Eva Kiermaier; Christine Moussion; Christopher T. Veldkamp; Rita Gerardy-Schahn; Ingrid De Vries; Larry G. Williams; Gary R. Chaffee; Andrew J. Phillips; Friedrich Freiberger; Richard Imre; et al.Deni TaleskiRichard J. PayneAsolina BraunReinhold FörsterKarl MechtlerMartina MühlenhoffBrian F. VolkmanMichael Sixt Polysialylation controls dendritic cell trafficking by regulating chemokine recognition. Science 2015, 351, 186-190, 10.1126/science.aad0512.
- Chihiro Sato, Ken Kitajima. Chapter One - Sialic Acids in Neurology; David C. Baker, Eds.; ELSEVIER: Advances in Carbohydrate Chemistry and Biochemistry, 2019; pp. 1-64.
- Ursula Neu; Johannes Bauer; Thilo Stehle; Viruses and sialic acids: rules of engagement. Current Opinion in Structural Biology 2011, 21, 610-618, 10.1016/j.sbi.2011.08.009.
- John B. Robbins; George H. McCracken; Emil C. Gotschlich; Frits Ørskov; Ida Ørskov; Lars Å. Hanson; Escherichia coliK1 Capsular Polysaccharide Associated with Neonatal Meningitis. New England Journal of Medicine 1974, 290, 1216-1220, 10.1056/nejm197405302902202.
- F A Troy; The Chemistry and Biosynthesis of Selected Bacterial Capsular Polymers. Annual Review of Microbiology 1979, 33, 519-560, 10.1146/annurev.mi.33.100179.002511.
- G Pluschke; J Mayden; M Achtman; R P Levine; Role of the capsule and the O antigen in resistance of O18:K1 Escherichia coli to complement-mediated killing.. Infection and Immunity 1983, 42, 907-913, https://iai.asm.org/content/42/3/907.
- C. M. Kahler; L. E. Martin; G. C. Shih; M. M. Rahman; R. W. Carlson; D. S. Stephens; The (α2→8)-Linked Polysialic Acid Capsule and Lipooligosaccharide Structure Both Contribute to the Ability of Serogroup B Neisseria meningitidis To Resist the Bactericidal Activity of Normal Human Serum. Infection and Immunity 1998, 66, 5939-5947, 10.1128/IAI.66.12.5939-5947.1998.
- Flavio Schwarz; Corinna S Landig; Shoib S Siddiqui; Ismael Secundino; Joshua Olson; Nissi Varki; Victor Nizet; Ajit Varki; Paired Siglec receptors generate opposite inflammatory responses to a human‐specific pathogen. The EMBO Journal 2017, 36, 751-760, 10.15252/embj.201695581.
- Mohui Wei, Peng George Wang. Chapter Two - Desialylation in physiological and pathological processes: New target for diagnostic and therapeutic development; Lijuan Zhang, Eds.; ELSEVIER: Progress in Molecular Biology and Translational Science, 2019; pp. 25-57.
- Taeko Miyagi; K. Yamaguchi; Mammalian sialidases: Physiological and pathological roles in cellular functions. Glycobiology 2012, 22, 880-896, 10.1093/glycob/cws057.
- Alexey V. Pshezhetsky; Mila Ashmarina; Keeping it trim: roles of neuraminidases in CNS function. Glycoconjugate Journal 2018, 35, 375-386, 10.1007/s10719-018-9837-4.




