
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Frank Czerwinski | + 3938 word(s) | 3938 | 2020-08-05 05:29:43 | | | |
| 2 | Frank Czerwinski | -1 word(s) | 3937 | 2020-08-12 13:50:01 | | | | |
| 3 | Catherine Yang | Meta information modification | 3937 | 2020-08-20 05:51:48 | | |
Video Upload Options
Thermal stability determines the material ability of retaining its properties at required temperatures over extended service time. In addition to temperature and time, thermal stability is affected by load conditions and environmental conditions.
1. Introduction
Thermal stability is the key design feature that determines a suitability of materials for specific applications and has a particular meaning for aluminum alloys. As documented throughout the decades, practically all aluminum alloys are thermally unstable with their properties being affected, to some extent, by service temperature and time. This includes grades essentially used at room temperatures, as is the case with aircraft components that may become warm due to exposure to sun, due to aerodynamic heating, or heat transferred from engines, which can deteriorate their properties over years of service [1]. The key engineering interest is, however, in the high temperature range and increasing the upper service limit of high-temperature grades [2].
At present, extending thermal stability to higher temperatures is the technology and knowledge barrier that prevents the substantial expansion of application scope of aluminum alloys, especially in automotive, marine, and aerospace transportation vehicles, designed for long-term service and strategically using aluminum for its lightweighting advantages. It is believed that the future aluminum alloys with improved high-temperature capabilities could compete, in selected applications, with more expensive titanium- and nickel-based grades. Therefore, along with recent refocusing on the strategic importance of aluminum alloys as lightweight structural materials for all forms of transportation vehicles, a substantial research interest is devoted to an improvement in their performance at high temperatures.
Although the thermal stability of aluminum alloys represents the major theme or at least a partial subject of a large number of research papers, differences in its understanding, critical property selection, and testing procedures make it difficult or impossible to combine individual results to draw a unified quantitative conclusion. The objective of this report is to review all elements of thermal stability from fundamentals to applications that refer to structural materials and aluminum alloys. Through identifying its detailed controlling factors, a better understanding of the relationship between mechanical, structural, and thermophysical properties that are critical for performance of alloys at increased temperatures will emerge. The outcome will help in optimizing the service conditions for existing aluminum alloys and development of novel alloys with superior thermal stability.
2. Defining Thermal Stability of Structural Materials and Aluminum
There is no universal definition or single criterion describing thermal stability of structural materials. While being typically seen as the “material ability of retaining its properties at required temperatures over extended service time”, in practice, more major parameters influencing thermal stability are involved including, in addition to (i) temperature and (ii) time, also (iii) load conditions and (iv) environmental conditions. Another definition as a “material resistance to permanent property changes caused by heat” is even less accurate as after cooling to room temperature, some portion of properties frequently recovers, whereas in a design, the properties maintained at the service temperature matter. Thermal stability is also defined as a material “property” characterizing changes after long-time exposure to elevated temperatures [3]. In this case, thermal stability is seen as an “intrinsic property” and, therefore, such an approach has further limitations. The related term “dimensional thermal stability” is also used that describes thermal expansion.
When assessing the thermal stability of a material, a future destination of this description is essential. If thermal stability is assessed for the purpose of comparing different alloys, e.g., during alloy development, the temperature and time are sufficient to characterize the alloy behavior. However, when a design input is required, during a material selection for a specific engineering application, detailed service conditions should be assessed. The design input will require an experimental measurement and/or computer simulation of material performance under conditions of its future application, including the load details and service environment nature.
Due to the low melting point of aluminum, 660.5 °C, the thermal stability of its alloys covers the temperature range, which is substantially lower than that of other materials with much higher melting points that, excluding the corrosive factor, can be used to contain molten aluminum alloys, as schematically marked in Figure 1. In this respect, the term “heat-resistant alloys” also has a relative meaning when applied to aluminum-based grades.
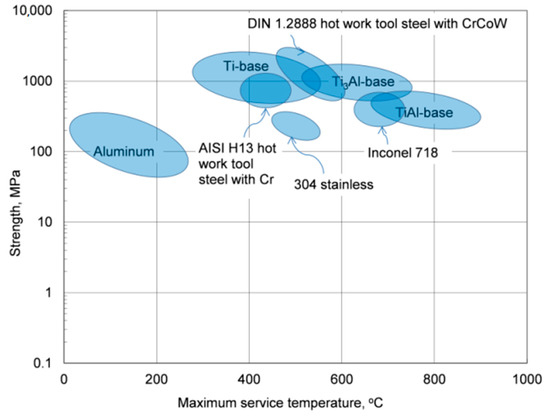
Figure 1. Strength versus maximum service temperature for aluminum alloys and selected structural materials.
The property variation of aluminum alloys at temperatures from cryogenic to over 400 °C is different than that observed in other materials, such as steel. As for other materials, the intensity of the temperature-related property change of aluminum alloys is influenced by their chemical composition and initial microstructure, controlled, in turn, by the manufacturing route and post-manufacturing treatment. An example for the wrought AA6061 alloy in T6/T651 condition is shown in Figure 2a,b. At temperatures above 150 °C, the alloy suffers a loss in strength with deterioration increasing over time. Above 200 °C, the weakening is substantial, and is accompanied by some gain in ductility. Most of the strength reduction induced by exposure to elevated temperatures is permanent, so the loss in strength is not recovered when the material is returned to a lower temperature. In case of AA6061, a major portion of the loss in strength is caused by coarsening of the Mg2Si precipitates. As shown in Figure 2a, aluminum alloys are susceptible to creep and stress relaxation. Creep is a time-dependent, permanent deformation that occurs under sustained load or stress, even at stresses below the yield strength. For the most part, creep is governed by migration of vacant lattice sites, which increases with temperature.
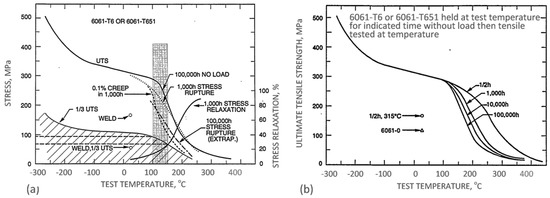
Figure 2. Example of property deterioration of the AA6061 aluminum alloy upon heating in air: (a) effect of temperature on creep and stress relaxation; (b) effect of temperature and time on ultimate strength [4].
2.1. Thermal Stability as a Component Design Criterion: Combined Influence of Temperature and Time
In order to apply a material for a particular design, the certain threshold of properties at service temperature is required. To understand the process of material selection, three hypothetical alloys with different strength vs. temperature/time characteristics are shown schematically in Figure 3a,b.
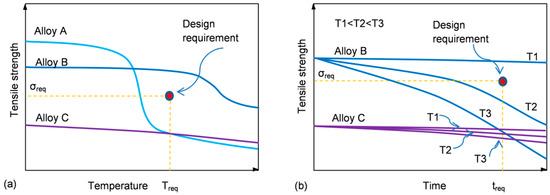
Figure 3. Thermal stability of structural materials and design requirements: (a) schematics of hypothetical changes in tensile strength versus temperature (b) and strength versus time at constant temperature (b), along with the strength required σreq at temperature Treq during service time treq.
Alloy A has an initial strength that substantially exceeds the minimum required but experiences a steep reduction at temperatures much lower than that predicted in its design (Figure 3a). In contrast, alloy C shows high thermal stability with low reduction in strength, taking place within the entire temperature range. However, its overall low strength makes it not suitable for this application. Thus, from a temperature criterion alone, alloy B meets the design specification.
At a given temperature, the material properties are affected by the exposure time. The influence of time on the properties of aluminum alloys depends on temperature. At high temperatures, a reduction of strength is the dominant observation for all classes of alloys. In contrast, at room or slightly elevated temperatures the opposite behavior may be observed, where the alloy strength may increase at the cost of plasticity, so an alloy may become prone to brittle cracking. The alloy selection depends, therefore, on the kinetics of the strength variation, and for alloy B, the strength reduction at temperatures T2 and T3 makes it not suitable for that design.
As portrayed in Figure 3a,b, a definition of thermal stability as “strength (property) retention at service temperature/time” can lead to confusion during a material selection. Therefore, the highly thermally stable alloy C does not meet design requirements due to its overall low strength. Thus, the thermal stability criterion that is viable during a material selection has two factors: (i) an alloy should achieve at room temperature the strength required and (ii) the strength should be retained within the temperature and time space to meet the level required at service temperature and to remain stable for the predicted service time.
Thermal stability is often expressed through graphs of the alloy strength vs. maximum service temperature. While being very educational, the above examples show that without specifying detailed conditions (time, load, environment, etc.), such characteristics are very approximate.
2.2. Understanding the Temperature—Load Factors
The influence of heat on material properties depends not only on temperature but also on temperature changes with time, especially in the case of frequent (periodic) changes, a presence of load applied to a material in structural applications and its nature.
2.2.1. Thermal Exposure—Stable Temperature
In general considerations of thermal stability, it is assumed that a material is exposed to constant (or near constant) temperature. As thermal stability refers to all temperature ranges that also include room environment the term “high thermal stability” may often be misleading, when temperature is not specified. For example, an aluminum alloy may be described as having very high thermal stability just at 100 °C. Examples of applications of aluminum alloys that require thermal stability at essentially different temperatures are shown in Figure 4a–c.
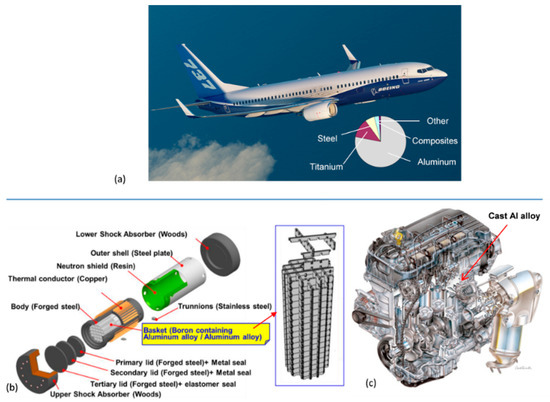
Figure 4. Applications of aluminum alloys requiring thermal stability at different temperatures: (a) commercial airplane with aluminum surface components exposed to room and slightly elevated temperatures [5]; (b) packaging and transportation of radioactive materials: long-term storage/transportation casks for spent fuels in nuclear power plants, requiring exposure to temperature 100–200 °C for over 60 years [6]; and (c) automotive combustion engine with new designs requiring temperature over 200 °C [7].
Stability at room and slightly elevated temperatures refers to temperatures typically below 100 °C. Such a scenario occurs for aircraft components that may become warm during service due to exposure to sun, aerodynamic heating, or heat transferred from engines. Temperatures from 70 °C to 85 °C are typically used to simulate the environment to which the wings and fuselage structures of a commercial aircraft are exposed [8].
The medium temperature range, requiring thermal stability, covers temperatures below 200 °C. They include impellers used in generators/compressors, vacuum pump rotors, and turbocharger impellers of various sizes with service range of 150 to 180 °C. An example of alloy used in this environment is the Al−Cu−Mg−Fe−Ni AA2618 alloy [9]. The medium temperature range will also cover alloys in metastable state, cold-deformed, nanocrystalline, and amorphous ones. However, the most challenging aspect of thermal stability is maximizing the upper service limit of high-temperature grades with the ultimate goal for aluminum alloys being to exceed 400 °C.
Thermal stability is uniquely tested in a case of accidental fire, when aluminum alloys may be exposed to temperatures, exceeding those predicted for regular service that exert damaging effect on their properties. There is a concern of fire safety with using aluminum for load-bearing applications such as lightweight structures, light rail, bridge decks, marine crafts, and off-shore platforms, due to potential dangerous reduction in mechanical properties during exposure to heating [10]. Alloys used in these applications are typically not designed for high temperatures. A related concern is regarding the integrity and stability of an aluminum structure following a fire exposure.
2.2.2. Constant versus Variable (Cyclic) Temperature—Thermal Fatigue
Due to thermal exposures, materials expand during heating and contract during cooling. When a material is geometrically constrained, this leads to generation of tensile and compressive stresses. Stresses may also arise within unconstrained materials due to a spatial temperature gradient. As a result of cyclic expansion and contractions the material experiences thermal fatigue. This phenomenon, also called heat checking, is common when a metal surface is repeatedly heated and cooled. Thus, thermal fatigue may occur without mechanical loads. If both thermal and mechanical strain is involved, the degradation mode is termed as thermomechanical fatigue.
2.2.3. Role of Load in Thermal Stability—Thermal versus Thermomechanical Response
As the structural materials are subjected to a load at service temperature, the effect of heat on their performance depends on the load level and its nature, with a special impact being exerted by heavy and cyclic loads, in particular with high-speed load alterations. To describe a material performance under particular service conditions the term durability is often used, understood as the ability of a material to sustain mechanical or thermomechanical loads over a predicted service time. Although the durability meaning may vary, depending on an application, for structural materials it is seen as the critical design consideration.
2.3. Environmental Effect on Thermal Stability
The ability of retaining the properties by an alloy is strongly affected by reactive environments leading, for example, to oxidation, erosion, molten metal or salt corrosion, or irradiation damage.
2.3.1. Surface Deterioration
For room temperature service, surface corrosion, leading to localized reaction and a material loss, forming pitting and stress risers, is of concern. For service at high temperatures, a process of selective oxidation resulting in localized surface degradation should be considered.
The oxidation of aluminum in air can be described as occurring in four distinct stages [11]. At room and lower temperatures, the amorphous alumina layer covers the metallic surface, protecting it against further oxidation, which results in very slow film thickening up to 550 °C. At this stage, the oxide growth is controlled by outward diffusion of Al ions with a reaction taking place at the oxide−gas interface. The amorphous oxide remains stable, due to the energy of the oxide–metal interface, only up to a critical thickness of ~5 nm, then transforms to γ-alumina, when crystallites are no longer able to form a continuous layer that would cover the aluminum surface. This leads to stage II, above 550 °C, with higher oxidation rate and polycrystalline layer of γ-alumina covering the entire aluminum surface. At the stage III, which starts at 650 °C, very close to melting, the growth of γ-alumina continues at a rate controlled by the inward diffusion of oxygen anions along oxide grain boundaries, acting as fast diffusion paths.
In the case of aluminum alloys, the process of high-temperature oxidation may have a preferential nature, leading to localized oxide patches, formed on specific alloy phases, potentially forming stress risers. The role of aluminum surface reactivity is better understood when compared with another light metal, magnesium. Due to the high affinity of magnesium with oxygen, at high temperatures, its surface degradation and formation of MgO is of higher concern than a reduction in its mechanical properties [12][13]. In this regards, aluminum shows an advantage over magnesium. In contrast to magnesium, there is no concern of ignition or flammability with aluminum and its oxidation rate is substantially slower due to a formation of the protective Al2O3 alumina film. However, for Al−4−5Mg (wt.%), only MgO is formed with a reaction following the linear law up to 500 °C and parabolic law above 550 °C [14][15]. Then, an environment may essentially change the oxidation kinetics. For example, in a presence of traces of sulfur, spallation of otherwise protective alumina occurs, which is the chronic problem in some aerospace applications.
2.3.2. Irradiation Damage
An important environmental factor, necessary to consider during analysis of thermal stability of aluminum, is the influence of radiation. Aluminum alloys are used in applications subjected to irradiation, e.g., as the primary structural material for the reactor reflector vessel of Advanced Neutron Source and for most of the components housed within the vessel [4] or in casks for transportation of nuclear fuels [16]. The deciding factors for the use of an alloy are good combination of low neutron absorption cross section and high thermal conductivity, good resistance to aqueous corrosion, and good performance in high flux reactors. Therefore, changes in mechanical properties expected to occur in alloys under irradiation during their intended lifetimes are of key importance.
The effects of irradiation exposures result in an increase in the metal’s volume, caused by development of voids, bubbles, and low-density phases, termed as swelling. Although generally metals undergo hardening during irradiation, softening is also possible, particularly for cold work-hardened or precipitation-hardened alloys, subjected to irradiation. That softening may take place during irradiation at temperatures below the normal temperature for thermal recovery, as a result of radiation enhanced diffusion processes or cascade dissolution of precipitates.
An example for the AA6061 aluminum alloy target holder from the High Flux Isotope Reactor, originally in a precipitation-hardened condition, after exposure to a maximum fast neutron fluence of 9.2 × 1022 neutrons/cm2 (E > 0.1 MeV) and a thermal fluence of 1.38 × 1023 neutrons/cm2 (E < 0.414 eV) at ~60 °C, is shown in Figure 5a,b [17]. At temperatures in the range from 25 to 200 °C, significant strength increases were observed that are attributed to the silicon precipitates and to irradiation-induced dislocations. There was a corresponding loss of ductility, particularly severe at 200 °C for slow strain rate testing conditions. The alloy, tested at temperatures between 200 and 500 °C, at which the microstructure was unstable, showed substantial loss in strength, accompanied by a gain in ductility. For the entire temperature range from 25 to 500 °C, however, the strength of irradiated alloy was higher than that for the alloy without irradiation. The same nature of changes was reported for the AA4043 alloy welds.
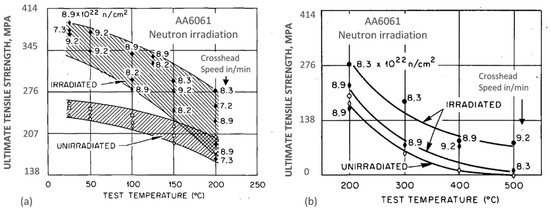
Figure 5. Effect of neutron irradiation on thermal stability of the AA6061 aluminum alloy at temperatures below 200 °C (a) and above 200 °C up to 500 °C (b) [17].
In another example, a microwave radiation influenced the thermal stability of aluminum nanosize powder. After microwave radiation with a power flux density of 80 W/cm2 and carrier frequency of 9.4 GHz, the chemical activity of aluminum powder increased and the temperature for the beginning of its oxidation decreased by 40 °C, while the thermal effect of oxidation decreased by 13.5% [18].
3. Thermal Stability and Exposure to Accidental Fire
In case of accidental fire hazards a metal might be exposed to enormously high temperatures. Due to relatively low melting temperature of aluminum alloys as compared to steel, often coexisting in a design, the outcome may be catastrophic for the former (Figure 6). For aluminum, however, much higher heat input is necessary to bring the same mass of metal to a given temperature, compared with steel. This is caused by high thermal conductivity of aluminum, being about four times that of steel and its specific heat twice that of steel, resulting in higher heat transfer away from the source.
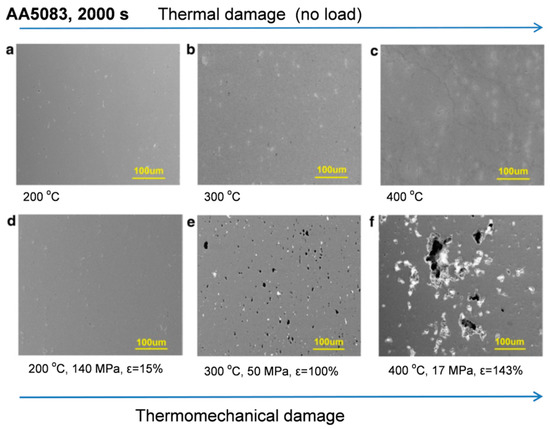
Figure 6. Comparison of (a–c) thermally and (d–f) thermomechanically damaged 5083−H116 alloy exposed for 2000 s. The shown conditions are (a) 200 °C, (b) 300 °C, (c) 400 °C, (d) 200 °C, 140 MPa, ε = 15%, (e) 300 °C, 50 MPa, ε = 100% and (f) 400 °C, 17 MPa, ε = 143%. The rolling/loading direction for all micrographs is along the long axis of the page [10].
3.1. Fire Initiation—Thermic Sparking
Aluminum is non-sparking in all environments if struck against aluminum, stainless steel, or any other material. The purpose in using non-sparking metals is to prevent ignition of combustible or explosive materials from an impact-generated spark. There is, however, one known exception: when unpainted or uncoated aluminum is struck by or strikes rusty ferrous metals, sparks may result [19]. Therefore, to avoid any possibility of sparking, where it is likely that aluminum may be struck by rusty ferrous metals, protective coatings such as paint are recommended.
Even if pure, non-ferrous aluminum is used, sparks can occur during an aluminothermic reaction, also called a thermic reaction. Such a reaction occurs when an aluminum particle and a metal oxide, such as rust, are ignited by a heat source and chemically burn as a “Class D” fire (i.e., combustible metal).
3.2. Exposure to Temperatures Exceeding the Melting Range
The solid bulk aluminum alloys exposed in air to temperatures exceeding the liquidus convert to molten state but are not subjected to burning, generating smoke or hazardous fumes. After fire is extinguished the metal remains as a re-solidified pool. Similarly, temperatures leading to semisolid state will result in an integrity lost by a design. The resistance of aluminum to burning is controlled by a number of national standards including ASTM. This is in contrast to fine powders or flakes of aluminum, which are highly flammable and oxidize exothermically. The ignition behavior of aluminum powder is similar to other finely divided materials including iron and titanium, which also readily oxidize exothermically while in the powder form.
3.3. Deterioration of Alloy Mechanical Properties during Fire
When structural collapse does not occur as a result of a fire, there is a need to evaluate the residual properties of overheated material to assess whether the structures should be dismantled, repaired, or directly reused. The influence of fire on mechanical properties depends on temperature and exposure time. The key difference from thermal stability issues discussed in this report is that alloys exposed to fire may not be designed at all for high temperature service. Therefore, even relatively low temperature such as 150 °C may lead to very poor performance during fire and the permanent property deterioration after fire.
There are a number of studies where the heat exposure on aluminum alloys that are not designed for high-temperature service, were evaluated. The objective was to accurately assess the post-fire performances of aluminum alloy structures. The models with predictive equations were developed, combining an influence of hardening factors and temperature on the material stress−strain relationship [20].
An example of research where an existing constitutive model for creep was modified in order to be used for fire-exposed alloys involved AA5083−O/H111 and AA6060−T66 grades [21]. For temperature range of 170 to 380 °C the model predicted properties for the 5xxx series alloys. The same AA5083−H116 and AA6061−T651 marine-grade aluminum alloys were subject of extensive mechanical testing to determine the residual mechanical behavior after fire exposure [22] (Figure 7). The constitutive models were developed as a series of sub-models to predict (i) microstructural evolution, (ii) residual yield strength, and (iii) strain hardening after fire exposure. The properties of AA5000 series following a simulated fire exposure were evaluated in [23]. The 5xxx series alloys with different tempers resulted in residual strengths between 85 and 157 MPa following the fire exposure. Most alloys exhibited structural recovery between 100 to 280 °C followed by recrystallization between 300 to 340 °C. However, the AA5456−H116 alloy, which has the highest magnesium content, maintained 60% of room temperature yield strength. This alloy underwent recovery but did not have a clear recrystallization, preserving strength. Another study [24] found that the mechanical properties of AA6061−T6 were drastically reduced after exposure to temperatures exceeding 300 °C. For AA7075−T73, reduction in properties took place at lower temperature of 200 °C. An additional factor affecting post−fire mechanical properties of these two grades was a cooling rate from a relatively high fire temperature.
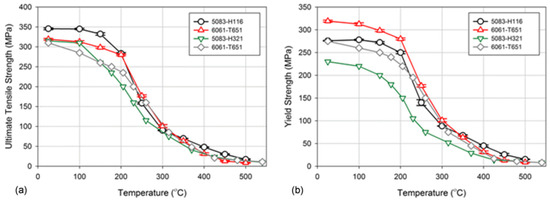
Figure 7. Degradation of tensile properties of 5083−H116 and 6061−T651 alloys during exposures to heat, expressed through ultimate tensile strength (a) and yield stress (b) [10].
4. Concluding Remarks
Thermal stability is becoming the next frontier for aluminum alloys; understanding and overcoming limitations in this area would lead to substantial expansion in their structural applications, especially in aerospace and automotive sectors.
As practically all aluminum alloys are thermally unstable, with their properties being affected, to some extent, by service temperature and time, thermal stability is of concern throughout the entire temperature range of their applications. This includes grades essentially used at room temperatures, as is the case with aircraft components that may become warm due to exposure to sun, due to aerodynamic heating or heat transferred from engines, which can deteriorate alloys properties over years of service.
The major challenge in thermal stability of aluminum is, however, in increasing the upper service limit of high-temperature grades. There are efforts to develop new alloys with thermally stable microstructure through alloying aluminum with a variety of elements, in particular, transition, and rare earth metals. In parallel, there are efforts to generate more stable microstructure through novel processing routes, such as rapid solidification, powder metallurgy, mechanical alloying and additive manufacturing, engineering alloys in a liquid state prior to casting, and post-casting treatments.
The ultimate goal is to overcome the present knowledge and manufacturing barriers and develop aluminum alloys with superior properties that remain stable across the temperature and time space, required by modern designs.
References
- Starke, E.; Staley, J. Application of modern aluminum alloys to aircraft. Prog. Aerosp. Sci. 1996, 32, 131–172.
- Czerwinski, F.; Kasprzak, W.; Sediako, D.; Emadi, D.; Shaha, S.; Friedman, J.; Chen, D. High-temperature aluminum alloys for automotive powertrains. Adv. Mater. Process. 2016, 174, 16–20.
- Matthews, S. Thermal Stability of Solid Solution Strengthened High. Performance Alloys; Cabot Corporation, Technology Division: Kokomo, Indiana, 1974; pp. 215–226.
- Farrell, K. Assessment of Aluminum Structural Materials for Service within the ANS Reflector Vessel ORNL/TM-13049; ORNL/US Department of Energy: Oak Ridge, TN, USA, 1995.
- Boeing Commercial Airplanes. Boeing. Available online: https://www.boeing.com/commercial/737ng/ (accessed on 18 June 2020).
- Ishiko, D.; Kawahara, Y.; Maeguchi, T.; Yamamoto, R.; Kishimoto, J. Mechanical Properties of Aluminum Alloys for Transport and Storage Casks. Mitsubishi Heavy Industry Ltd. Available online: https://www-pub.iaea.org/iaeameetings/cn226p/Session4/ID78Kishimoto.pdf (accessed on 15 June 2020).
- Czerwinski, F.; Birsan, G.; Benkel, F.; Kasprzak, W.; Walker, M.; Smith, J.; Trinowski, D.; Mousalem, I. Developing casting core technology for high pressure die casting. Adv. Mater. Process. 2017, 175, 18–20.
- Polmear, I.; Pons, G.; Barbaux, Y.; Octor, H.; Sanchez, C.; Morton, A.; Borbidge, W.; Rogers, S.A. After Concorde: Evaluation of creep resistant Al-Cu-Mg-Ag alloys. J. Mater. Sci. Technol. 2013, 15, 861–868.
- Tanaka, T.; Kamitakahara, Y. Highly Heat-Resistant Aluminum Alloy “KS2000”. KOBELCO Technol. Rev. 2017, 35, 28–33.
- Summers, P.; Chen, Y.; Rippe, C.; Allen, B.; Mouritz, A.; Case, S.; Lattimer, B. Overview of aluminum alloy mechanical properties during and after fires. Fire Sci. Rev. 2015, 4, 3.
- Coker, E. The Oxidation of Aluminum at High. In Temperature Studied by Thermogravimetric Analysis and Differential Scanning Calorimetry; SAND2013-8424; Sandia National Laboratories: Albuquerque, NM, USA, 2013.
- Czerwinski, F. Controlling the ignition and flammability of magnesium for aerospace applications. Corros. Sci. 2014, 86, 1–16.
- Czerwinski, F. The reactive element effect on high temperature oxidation of magnesium. Int. Mater. Rev. 2015, 59, 264–296.
- Smeltzer, W.W. Oxidation of an aluminum 3pct magnesium alloy in the temperature range 200–250 °C. J. Electrochem. Soc. 1958, 105, 67–71.
- Tenorio, J.; Espinosa, D. High-temperature oxidation of Al–Mg alloys. Oxid. Met. 2000, 53, 361–373.
- Maeguchi, T.; Kawahara, Y.; Yamamoto, R.; Hase, T. Study for evaluation method of design strength of aluminum alloy for basket material. In Proceedings of the 19-th International Symposium on the Packaging and Transportation of Radioactive Materials PATRAM 2019, New Orleans, LA, USA, 4–9 August 2019.
- Farrell, K.; King, R.; Jostons, A. Examination of Irradiated 6061 Alumuminum HFIR Target. Holder ORNL-TM-4139; US Atomic Energy Commissiony: Oak Ridge, TN, USA, 1973.
- Mostovshchikov, A.; Ilyin, A.; Chumerin, P. The influence of microwave radiation on the thermal stability of aluminum nanopowder. Tech. Phys. Lett. 2016, 42, 344–346.
- Kaufman, J. Fire Resistance of Aluminum and Aluminum Alloys and Measuring the Effects of Fire Exposure on the Properties of Aluminum Alloys; ASM International: Materials Park, OH, USA, 2016.
- Faggiano, B.; De Matteis, G.; Landolfo, R.; Mazzolani, F. Behaviour of aluminum alloy structures under fire. J. Civil Eng. Manag. 2004, 10, 183–190.
- Maljaars, J.; Soetens, E.; Katgerman, L. Constitutive model for aluminum alloys exposed to fire conditions. Metall. Mater. Trans. A 2008, 39, 778–789.
- Summers, P. Microstructure-based Constitutive Models for Residual Mechanical Behavior of Aluminum. Ph.D. Thesis, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA, 2014.
- Free, J.; Summers, P.; Lattimer, B.; Case, S. Mechanical properties of 5000 series aluminum alloys following fire exposure. In TMS 2016 145th Annual Meeting & Exhibition; Springer: Cham, Switzerland, 2016; pp. 657–664.
- Chen, Z.; Lu, J.; Liu, H.; Liao, X. Experimental investigation on the post-fire mechanical properties of structural aluminum alloys 6061-T6 and 7075-T73. Thin Walled Struct. 2016, 106, 187–200.




