Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | João Paulo Fabi | + 5905 word(s) | 5905 | 2022-02-15 10:26:17 | | | |
| 2 | Conner Chen | -55 word(s) | 5850 | 2022-02-24 03:38:03 | | | | |
| 3 | Conner Chen | -33 word(s) | 5817 | 2022-02-25 09:45:24 | | | | |
| 4 | Conner Chen | Meta information modification | 5817 | 2022-02-28 01:47:27 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Fabi, J.P. Pectin and Gal-3. Encyclopedia. Available online: https://encyclopedia.pub/entry/19818 (accessed on 08 February 2026).
Fabi JP. Pectin and Gal-3. Encyclopedia. Available at: https://encyclopedia.pub/entry/19818. Accessed February 08, 2026.
Fabi, João Paulo. "Pectin and Gal-3" Encyclopedia, https://encyclopedia.pub/entry/19818 (accessed February 08, 2026).
Fabi, J.P. (2022, February 23). Pectin and Gal-3. In Encyclopedia. https://encyclopedia.pub/entry/19818
Fabi, João Paulo. "Pectin and Gal-3." Encyclopedia. Web. 23 February, 2022.
Copy Citation
Galectin-3 is the only chimeric representative of the galectin family. Although galectin-3 has ubiquitous regulatory and physiological effects, there is a great number of pathological environments where galectin-3 cooperatively participates. Pectin is composed of different chemical structures, such as homogalacturonans, rhamnogalacturonans, and side chains.
galectin-3
pectin
fruit
polysaccharides
1. Introduction
Pectins are complex polysaccharides and versatile hydrocolloids, vastly available in plant cell walls and the middle lamella of higher plants. These polysaccharides are major components for maintaining rigidity and integrity of the plant tissues, positively impacting plant growth and health [1]. Every source of pectin has a variable amount of sub-structures, such as homogalacturonans (HG), rhamnogalacturonans (RG-I and II), and xylogalacturonan [2]. The HG region is primarily composed of a homopolymer of partially esterified 1-4-α-d-galactopyranuronic acid (GalpA). RG-I regions are composed of repeated and intercalated α-d-GalpA with α-l-rhamnopyranose (Rhap) [→2)-α-l-Rhap-(1,4)-α-d-GalpA-(1→]. This region also has variable side chains of galactans (β-1,4-Galp residues with varying degree of polymerization), arabinans (α-1,5-l-Araf with 2- and 3- linked arabinose/arabinan branches), and arabinogalactans (Type I: β-1,4-d-galactans with O-3 linked l-arabinose or arabinan; Type II: β-1,3-linked d-galactans with β-O-6-linked galactans or arabinogalactan), attached to the Rhap residues (side chains are linked at Rhap O-4, as demonstrated in Figure 1 and Figure 2) [2][3][4]. In diverse fruits, these structures undergo a series of chemical and enzymatic alterations during the ripening process, resulting in a wide variety of intramolecular changes inside the pectic chain, as illustrated before for the papaya pulp, a fruit that rapidly undergoes the ripening process [5][6][7][8].
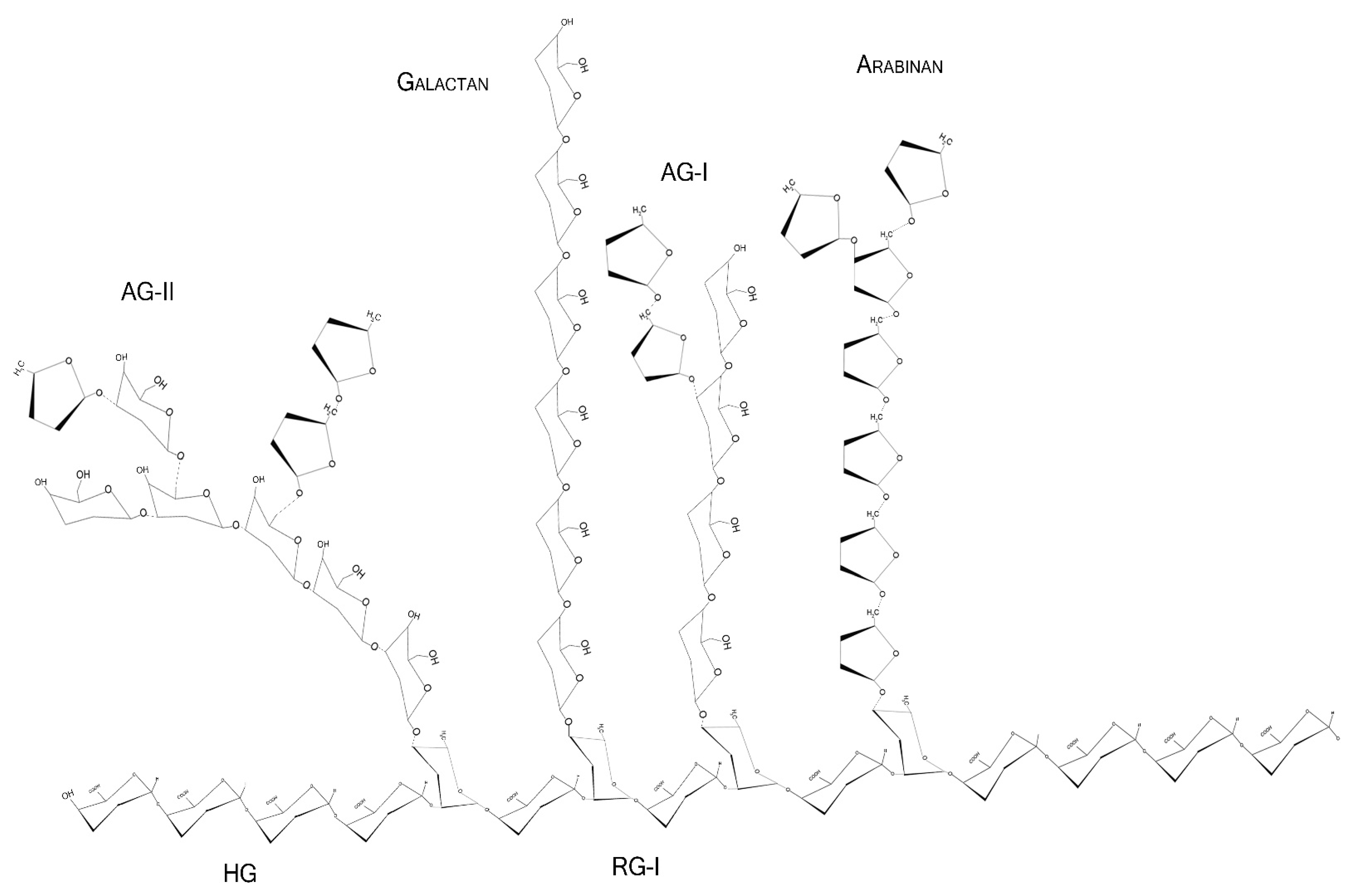
Figure 1. Schematic representation of major pectin components in chair conformation. HG—homogalacturonan, composed of linear α-1,4-d-galactopyranuronic acids; RG-I—intercalated α-d-galactopyranuronic acids and α-l-rhamnopyranose through α-1,4 and 1,2 glycosidic bindings; AG-I—β-1,4-d-galactopyranose with occasional O-3 α-l-arabinofuranose; AG-II—β-1,3-d-galactopyranose with O-6 α-l-arabinofuranose/arabinogalactans. Arabinans and galactans consist of linear α-1,5-l-arabinofuranoses and β-1,4-d-galactopyranoses, respectively.
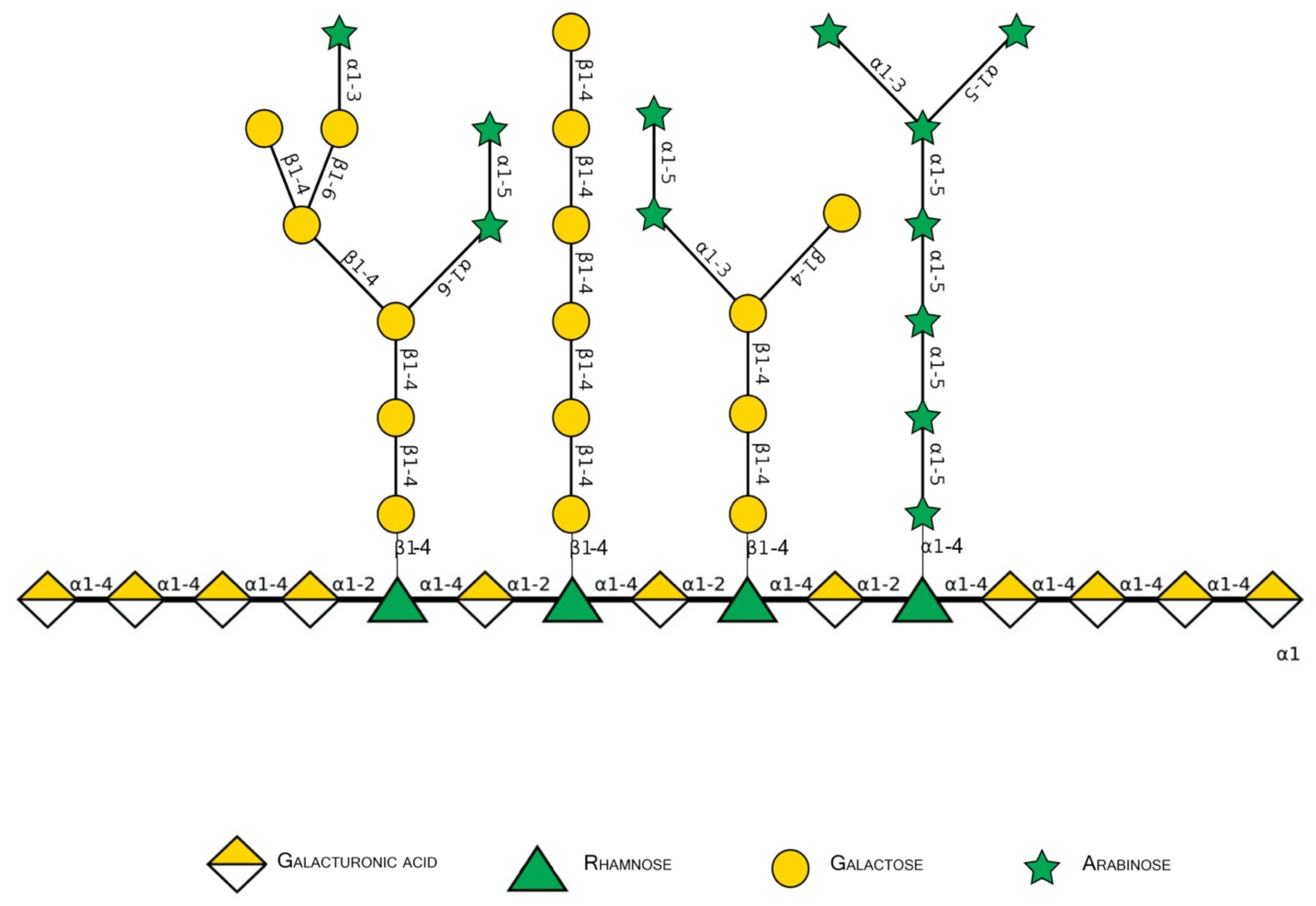
Figure 2. Schematic representation of major pectin components in Symbol Nomenclature for Glycans (SNFG-B) [9] model. Different glycosidic bounds common to pectins are illustrated.
As part of the dietary fiber group, pectins are not digested by the human tract, although the fermentation process by the human microbiota is greatly important for the maintenance of the colonic and systemic health through local signaling. The fermentation products and molecular fragments can improve metabolic syndrome and attenuate hypercholesterolemia, hypertriglyceridemia, and hyperglycemia, markers related to heart disease risks in mice, rats, and humans [10][11][12][13][14]. The pectic products and fragments after colonic fermentation also possess anti-oncogenic attributions in the colon for mitigating cancer-related risks in the colon and even in other types of neoplasia in humans [15].
Galectin-3 (Gal-3) is the chimeric representative member of the galectin protein family, located ubiquitously in the nucleus, cytoplasm, outer cell surface, and extracellular space in mammals. Gal-3 is classified as a β-galactoside binding protein, although—as it is discussed further ahead—its binding range cannot be restrained only to β-galactoside ligands. Gal-3 is composed of a flexible N-terminal domain, containing up to 150 amino acid residues with sequences rich in proline, tyrosine, glycine, and glutamine. This collagen-like region rich in proline, glycine, and tyrosine ends up in a C-terminal domain with a carbohydrate recognition domain (CRD) containing about 135 amino acids. The CRD region is responsible for the signature pattern of the galectins family [16][17][18]. Although the ubiquitous expression, the main biological source is derived from immunological and collagen-producing cells, where it helps to establish cell recognition and communication through protein–protein or glycan–protein interactions [19][20]. For example, one important and recently elucidated Gal-3 effect in physiological conditions is the recruitment of endosomal sorting complexes required for transport to damaged lysosomes (ESCRTs), so they can be effectively repaired [21]. However, alterations of Gal-3 expression are strongly related to tumor growth, cancer cell proliferation, cell-to-cell adhesion properties, fibrosis stimulation, T-lymphocytes apoptosis, macrophage differentiation into infiltrative forms that stabilize tumor environment, and other features [22][23][24][25][26][27][28]. Those attributions are shown in Figure 3, where the intestinal model was chosen, as it is simple to explain the interface between endogenous and exogenous stimuli that differently activate Gal-3 functions. Moreover, pectin is considered a bioactive compound when ingested as a food component or as a dietary supplement, a topic that is explained in the next sections. It can be noted in Figure 3 the different Gal-3 forms available at biological environments, such as the monomeric unit (intra and extracellular) recently secreted present at initial interactions with natural ligands and pentameric (chimeric appearance) after associations of other monomeric units through their N-terminal domain due to ligand stimulus [18][29].
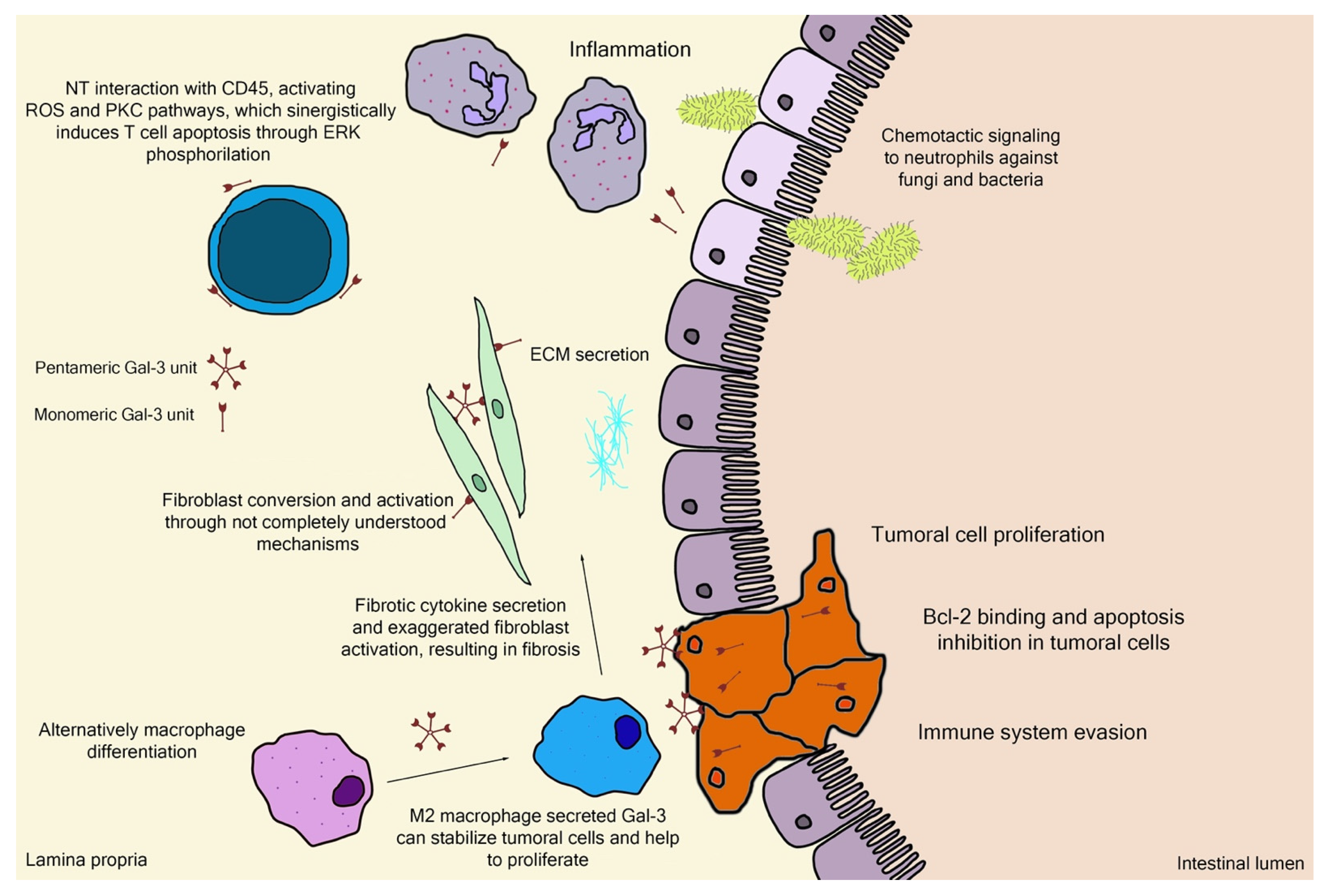
Figure 3. Schematic representation of (patho)physiological effects of galectin-3. As it is demonstrated, the physiology is separated by a thin line from the pathological scenario, such as the extracellular matrix (ECM) secretion stimuli or the chemotactic signaling for infiltrative immune cells. Bcl-2—B-cell lymphoma 2; ERK—extracellular signal-regulated kinases; NT—N-terminal tail/domain; PKC—protein kinase-C; ROS—reactive oxygen species.
While there are extensive high-quality data regarding Gal-3 binding sites modeled to small glycoconjugates and an important level of binding/inhibition by those [30][31], as it is highly controversial due to structural variability and reproducibility of experimental conditions. The gathering of these data is fundamental for a better understanding of the overall perspectives regarding this promising—but not yet fully understood—area to be explored. Likewise, all the structural parameters, such as distinct molecular sizes, a higher number of galactan/arabinan side chains or galacturonic acid contents, methyl esterification, and other properties important to pectin biological effects are also discussed by in vitro and in vivo perspectives [7][32][33].
2. Basic Pectin Molecular Aspects
2.1. Pectin Molecular Weight
Although having extremely variable structures, pectins are composed of large polysaccharide chains translated to solutions with high viscosity, a factor which would likely be an impairing factor for possible absorption through oral administration [34]. There are several approaches for extracting and, at the same time, lowering pectic molecular weight. The most commonly utilized method, especially in industrial extraction, is the chemical modification with mineral acids [35], such as hydrochloric and nitric acids, and organic acids [36][37][38][39], such as citric and malic acids. Enzymatic digestion is a more subtle and precise approach, using different cleavage agents, mostly arabinanases and galactanases, to remove excess side chains and polygalacturonases (exo- and endo-) to break down the larger HG backbones/pectic domain, aiming to obtain pre-planned molecular patterns [33][40]. Another category, thermic modifications, is less specific but far more practical and inexpensive. These modifications can be achieved by different methods, such as high temperature and pressure [41][42], ultrasound-assisted heating [43][44][45], and more underused but promising approaches, such as electromagnetic induction heating [46]. There are described extraction methods capable of conserving more of the native pectin structure, such as the use of low temperatures (40 °C) but with low extraction yields, and the decision which technique should be used depends on the final objective of the work [47], as it is discussed further that high molecular weight polysaccharides are not well suited for potential biological applications. Therefore, breaking down and/or manipulating the large polysaccharide chains into smaller portions while bringing better malleability to the molecule itself, improving viscosity and rheological properties, can set more biologically available binding sites, facilitating the potential interaction with proteins such as lectins or other cell-surface receptors.
2.2. Monosaccharides, Backbone, and Side Chains
The monomeric structure of polysaccharides is also extremely variable and inconsistent depending on the food source, extraction method, and modification strategy. These monomeric sugars are naturally presented as either pyranose (p, saccharides with chemical structure including six-member ring, composed of five carbon and one oxygen atoms) or furanose (f, saccharides with chemical structure including five-member ring, composed of four carbon and one oxygen atoms). This high variability represents a problem regarding standardization of recommended ratios between monosaccharides, such as the Galp/Araf, Araf + Galp/GalpA, associated with the proportions of molecular side chains (e.g., arabinans and galactans) or main structure sequences of α-d-GalpA. For example, important works regarding molecular modeling of Gal-3 inhibitors that have standardized to low molecular weight molecules (<1000 Da) as higher protein-inhibitor ligands [48][49][50] did not consider polysaccharides. Galactan and galactosyl residues were the main focus as contributors to biological effects observed between pectin and Gal-3 interaction and inhibition since this protein has a preference for binding β-d-galactopyranoside [40][51]. However, while still important targets of interest, other monomeric compounds such as the GalpA, Araf, and their association have been described more consistently, as found for polysaccharides with Araf residues in higher quantities, mostly linked in α-(1→3,5)-l-arabinan side chains, demonstrating higher inhibition of Gal-3 through techniques such as Gal-3 hemagglutination assays (G3H) or binding through biolayer interferometry assays (BLI) and surface plasmon resonance assays (SPR) [3][33][52][53]. Therefore, this focus on specific monosaccharides that are not only galactosyl could help to understand the biological action of pectin and the possible Gal-3 interaction and inhibition.
2.3. Esterification Degree
Pectin can also be classified by the esterification degree throughout its molecule, where the common approach is the division in a lower degree of esterification (DE) (<50% DE—low-methylation or LMP) or higher DE (>50%—high-methylation or HMP), as well as how the esterification is distributed on pectin molecule (degree of blockiness). The determination of this parameter as a characterization step is crucial to establish the best application of the referred polysaccharide, such as the gelling property and potential where HMP achieves through hydrophobic interactions and hydrogen bonding, while LMP forms gels through the salt-bridge connecting adjacent or opposite carboxyl groups from divalent ions [54][55], emulsion property for protein complexation or drug delivering [56][57][58], in addition to the direct biologic relationship that is further discussed in the following topics. The RG-I backbone region’s side chains are mostly a mixture of arabinans, galactans, and arabinogalactans attached to the rhamnose residue. RG-II is highly methyl-esterified and much more diverse in sugar composition side chains than RG-I. The main pectin backbone is composed of HG, also known as the “smooth” region. This main portion of pectic molecules can also exhibit varying degrees of methylation or acetylation (DM and DA, respectively) depending on the food source and extraction process [2]. Alkali treatment is the most common chemical method to achieve lower DM for pectic samples through direct saponification but may result in chemical waste or even slight alterations to the main pectin chain by β-elimination. Enzymatic treatment is less practical, requiring up to 25 h, depending on the expected DM. An alternative to this inconvenience is the high hydrostatic pressure-assisted enzymatic process, which is a promising operation to help attenuate this problem and facilitate the industrial application of this method [59].
2.4. Rheological Properties
When studying pectin rheology parameters, they are especially useful to determine potential applications of the characterized samples towards large-scale applications, both at food and non-food products, also helping at the definition of a better biologic destination [60]. The previously mentioned structural parameters directly impact the food product incorporation, such as viscoelasticity and thickness, which can be interpreted as gelling capacity, stabilizing potential in acidified milk beverages or fruit juices, emulsion capability with protein-rich solutions, and many others [61][62][63][64]. Therefore, the stratification generated from this type of analysis can lead to the best type of use for each pectin sample [65].
2.5. Food Source
The food source—exclusive from plants—together with the pectin extraction methods, and in some cases, the ripening parameters of fruits, are important factors in obtaining functional pectic molecules, as already mentioned. Usually, there is an additional ecologically sustainable status, as many of the possible sources are residues and byproducts from the juice and food processing industries. Some residues of apple [66], Prunus domestica and Prunus mume [67][68], jaboticaba [69], citrus [70][71], and papaya [7][8][72][73] have been explored for pectin extraction and biological activity studies.
3. Gal-3 Binding Sites and Pectin Interactions
Structurally, Gal-3 N-terminal tail (NT) transiently interacts with its CRD F-face and is linked to the glycine/proline-rich sequence, conferring its uniqueness in the galectin family, allowing self-oligomerization [74][75][76]. Gal-3 CRD is composed of 11 β-sheets, whereas five belong to the F-face and six belong to the conventional β-galactosides binding S-face, one opposed to the other, forming a β-sheet-sandwich (Figure 4A–C). In the S-face CRD, there is the NWGR conserved motif (Asparagine–Tryptophan–Glycine–Arginine), which is similarly encountered in the BH1 domain from B-cell Lymphoma-2 (Bcl-2) anti-apoptotic molecule, and suggested as the possible interaction that enables apoptosis evasion in tumoral cells (Figure 4B) [19][77][78]. Within the CRD, specifically the S-face, there are some subsites that can be named for easier comprehension of binding interactions. There are two conserved subsites (C and D), two non-conserved (A and B), and one not well-defined subsite (E) [30], where their interfaces are mainly constituted of hydrogen bonds. The CD-associated subsites are a target of natural ligands such as the N-acetyllactosamine (LacNAc) Gal-3 inhibitor or the β-d-galactopyranoside residue located in the Lactose molecule, where it remains tightly bonded mainly to C subsite amino acids (Figure 4D) [79].
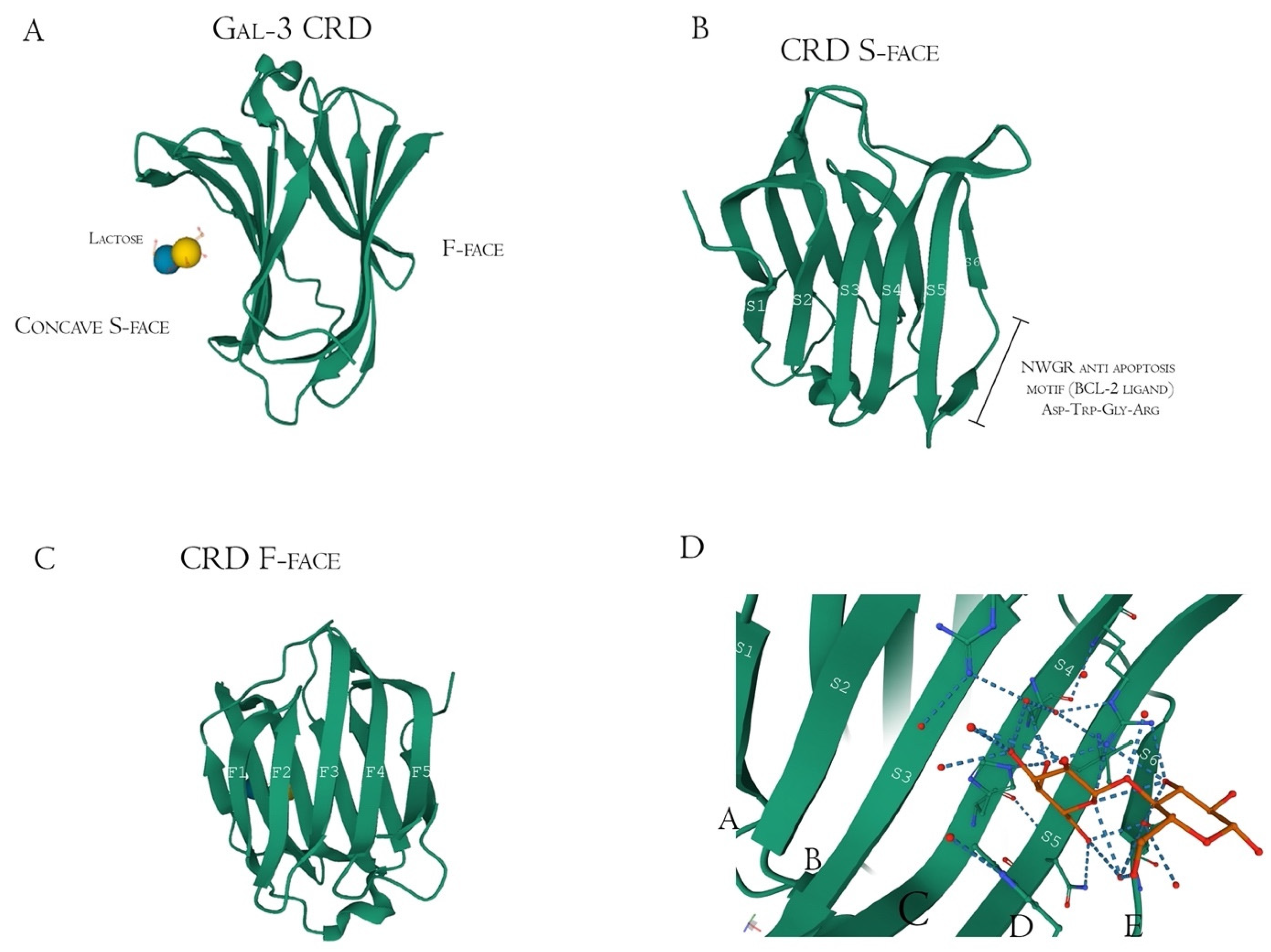
Figure 4. Galectin-3 crystalized tertiary structure X-ray diffraction, PDB ID 49RB [75][80][81]. (A) Gal-3 complete CRD, with the anti-parallel β-sheet sandwich; (B) CRD S-face β-sheets (S1–S6), which holds the ABCDE subsites. The NWGR motif is highlighted because of their biochemical importance; (C) CRD F-face β-sheets (F1–F5, also numbered as B-9, -8, -7, -2, and -11, respectively); (D) Schematic representation of β-d-galactopyranosyl-1,4- β-D glucopyranose (β-Lactose) binding at the canonical S-face. The hydrogens atoms are colored as red, the hydrogen bonds as blue dotted lines, and the lactose chain as orange. The binding is stronger at the C subsite (between S4 and S5) amino acids and the β-d-galactopyranosyl residue.
However, there is extensive literature regarding other synthetic and sugar-derived molecules (especially tetrasaccharides and thiosaccharides) that utilize the whole ABCD region (also mentioned as Gal-3 binding groove, as its morphological 3D structure conformation due to the interactions between the groups AB/CD and each one of them individually) for a better affinity performance and inhibition potential [30][49][74][82]. In addition to this, the interaction of pectic poly- and oligosaccharides with Gal-3 is suggested through N-tail epitope recognition by pectin side-chain residues (e.g., galactans). The F-face interacts with β-galactosides (but also other portions), disrupting the CRD F-face binding with NT. Additionally, the same pectic structure could show multiple interactions involving both the S face region (disturbance of amino acids residues from the canonical binding site, e.g., 154–176 sequence observed with Heteronuclear single quantum coherence spectroscopy (HSQC spectra)) and F-face region (amino acids residues of β-sheets 7, 8, and 9 mostly, e.g., 210–225 sequence) (Figure 5) [76][83][84].
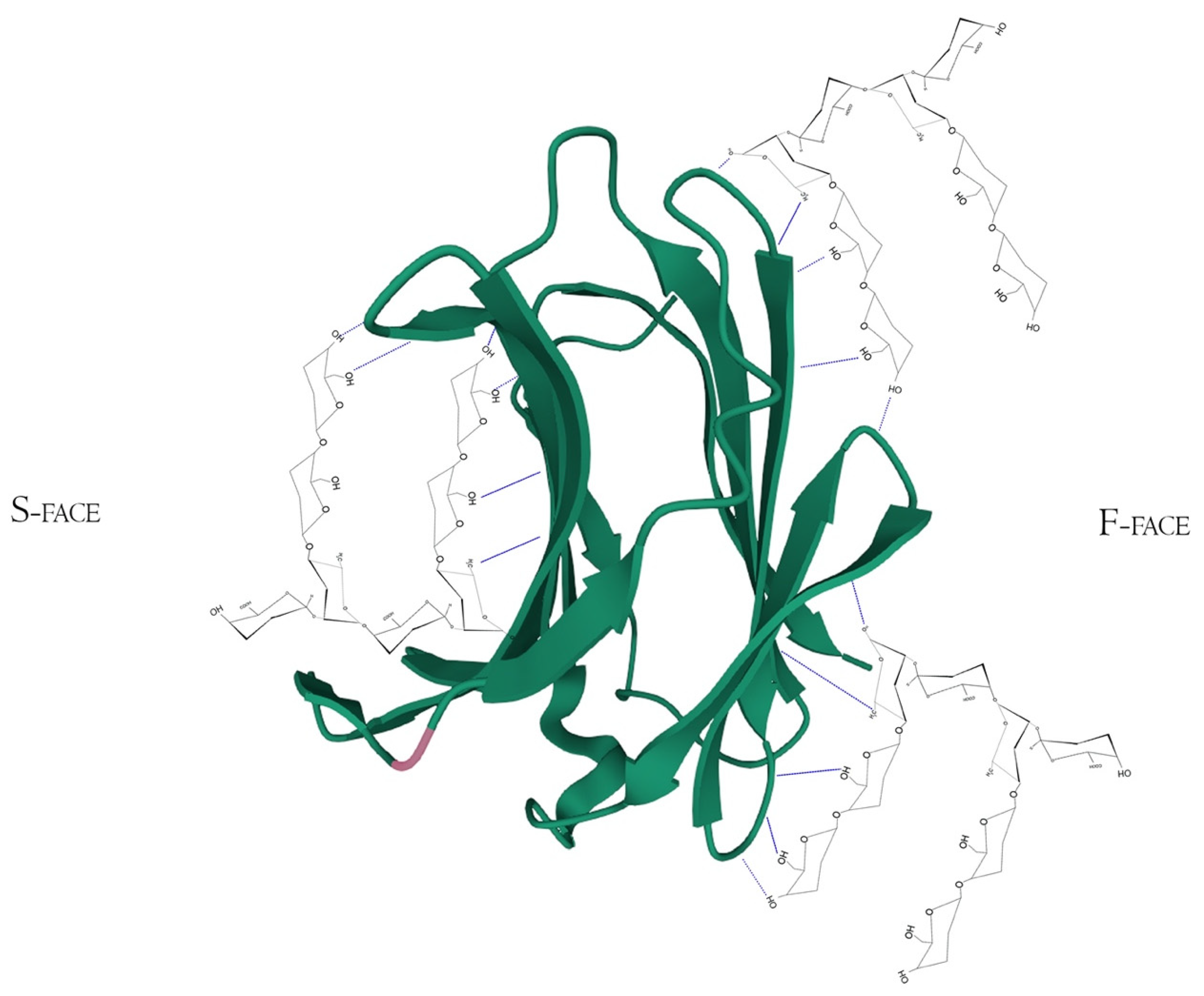
Figure 5. Hypothetical interaction of RG-I fragments with both F and S-face of Galectin-3 simultaneously. Here, the main protagonists would be the β-d-galactopyranose and α-d-galactopyranuronic acid residues and would not act like specific pharmacological inhibitors, but maybe as potential Gal-3-ligand blockers through multivalency or allosteric occupation. Hydrogen bonds are represented by the blue dotted lines. PDB ID 49RB [75][80][81].
Other Gal-3 inhibitors that have been thoroughly and increasingly studied are pectin and its fragments. Some of the possible Gal-3 inhibition effects by pectin and fragments might include protecting pancreatic β-cells against oxidative and inflammatory stress [85]. Xu et al. [86] observed modified citrus pectin (MCP) downregulated pathways involved in myocardial fibrosis, but the authors did not study the Gal-3 inhibition by MCP in vivo, although Gal-3 was downregulated in the treated group [86]. Some other biological effects from Gal-3 inhibition are related to cancer proliferation control [7][32], with a strong positive relationship between overexpression of Gal-3 and carcinogenic processes, such as apoptosis evasion, higher cell invasion, and metastatic progression, which are key signatures in tumor and metastatic types of cells [87]. The structural relationship between the pectic chain and Gal-3 binding sites is also very important for the expected positive functional effects [33][88]. Extracellular Gal-3 biological functions are exerted mostly by interacting with glycoconjugates in cell surfaces, such as laminin and adhesin, signaling and activating specific pathways. Some studies regarding binding interactions between pectin and its fragments with Gal-3 are listed below (Table 1).
Table 1. Polysaccharide-Gal-3 binding articles summary.
| Authors | Polysaccharide Residue | Analysis Method | Binding Evaluation |
|---|---|---|---|
| Wu et al., 2020 [33] | RG-I from citrus canning process water | Surface plasmon resonance | Smooth binding curve through SPR with decreased affinity with galactan side-chain removal |
| Zhang et al., 2016 [34] | MCP, RG-I-4, and p-galactan | Gal-3 hemagglutination, bio-layer interferometry, and surface plasmon resonance | RG-I-4 demonstrated higher Gal-3 avidity in comparison to the other two polysaccharides, with a KD at sub-micromolar range (RG-I-4 and p-galactan), but no significant result when testing competitive assays with known S-face inhibitors such as lactose |
| Gao et al., 2013 [40] | Ginseng RG-I-4 domain | Gal-3 hemagglutination and surface plasmon resonance | RG-I-4 inhibited G3H and was bound specifically to CRD with high affinity with Ara residue location in the RG-I, changing the activity detected at the G3H assay |
| Gunning, Bongaerts, Morris et al., 2009 [51] | RG-I, PG, and galactans | Atomic force microscopy, fluorescence microscopy, nuclear magnetic resonance, and flow cytometry | Galactan binding to Gal-3 is lectin-saccharide highly specific, while RG-I has low specificity, and PG was not specific. The data suggest that the lesser “sterical crowding” of the galactans alongside its beta-1,4 linear chain could be the reason for the better performance observed |
| Shi et al., 2017 [52] | Ginseng RG-I-3A domain | Bio-layer interferometry, Gal-3 hemagglutination | Binding kinetics of RG-I-3A showed a high binding affinity with a KD of 28 nM through and also presented notable G3H inhibition |
| Zhang et al., 2017 [83] | MCP-derived RG-I and HG portions | Gal-3 hemagglutination, bio-layer interferometry, ELISA, and nuclear magnetic resonance | Gal-3 bound to both portions separately but with a much more notable avidity when a combination of them (RG + HG) is performed, suggesting that this interaction exposes more binding sites at the lectin |
| Miller et al., 2015 [84] | Galactomannans (GM) and polymannan | Nuclear magnetic resonance | The primary binding surface of the GM’s located mainly at F-face beta-sheets (7,8 and 9) |
| Zheng et al., 2020 [89] | MCP-derived HGs of varying molecular weights | Nuclear magnetic resonance heteronuclear single quantum coherence spectroscopy and crystallography | Higher molecular weight HGs demonstrated more perturbances at F-face resonances and involved more S-face beta-sheets at the binding footprint. A possible binding of Gal-3 to the non-terminal HG sites is suggested, and it is shown a different S-face binding pattern of HG’s compared to lactose |
| Miller et al., 2019 [90] | Galactan oligosaccharides of varying chain lengths | Nuclear magnetic resonance heteronuclear single quantum coherence spectroscopy | Binding affinity at the terminal non-reducing end of the galactans in the CRD S-face (beta-sheets 4, 5, and 6 chemical shifts mostly) increases with the increase in chain length |
| Zhao et al., 2017 [91] | Pumpkin RG-I-containing pectin | Surface plasmon resonance | Moderate binding affinity towards Gal-3 through SPR, with a fast association between protein and polysaccharide (KA) and slow dissociation (KD) |
| Miller et al., 2017 [92] | Ginseng RG-I-4 domain | Nuclear magnetic resonance heteronuclear single quantum coherence spectroscopy | Epitopes from RG-I-4 bind to three different labeled Gal-3 sites, two at the CRD and another one at NT. At lower concentrations, the F-face site is more activated, turning to S-face at higher ones |
The Gal-3 binding range is considerably wide, including oligolactosamines, sulfated and sialylated glycans, and α-binding glycans, such as Fucα1-2, Galα1-3, and Galα1-4 added to the core of LacNAc molecule (as terminations) [89][90][93][94], which could indicate why non-β-Gal pectic fragments and other sources can still perform positively in some binding experiments. The β-1-4-galactan side chains inside the pectic RG-I domains have been highly attributed as the molecular factor involved in direct inhibition of Gal-3 (recombinant proteins and native proteins from cells), mainly discussed as a motif varying reaction, such as galactose residues in the middle or at terminal parts of those linear molecules. The longer galactan side chains in RG-I molecules identified in MCP and ginseng pectin were also associated with a stronger interaction with Gal-3 in vitro [40][95]. Similar positive binding affinity results with Gal-3, higher than observed with potato galactan, were obtained when isolating a 22.6 kDa RG-I polysaccharide from pumpkin [91]. Meanwhile, the RG-II enriched fractions extracted from Panax ginseng flower buds, with a high methyl-esterification and backbone substitution, as well as with lower content of galactose residues and low molecular weight, were associated with an absence of Gal-3 binding [3]. Highly esterified HG samples also did not inhibit Gal-3 [3]. However, other ginseng samples with an equivalent ratio between HG and RG-II regions had a positive binding affinity to Gal-3 in a similar way to the synergistic effect of HG and RG-I of citrus pectin [34][83]. In the latter case, the authors also suggested that the unesterified characteristic of pectin, alongside non-substituted GalA segments, were crucial structural elements for the observed biological effects [83]. Following this trend, commercial lemon pectin samples (from CP Kelco) with a low degree of methyl-esterification and low molecular weight were consistently and extremely potent in preventing negative outcomes in human islets with β-cell apoptosis (diabetes model) induced stress in vitro [85], in a dose–response manner. Gal-3 inflammatory stress induction was analyzed by evaluating oxygen consumption rate (OCR) in the presence and absence of a Gal-3 known inhibitor, α-lactose. After the previous incubation with α-lactose, the pectin sample effects that reversed OCR reduction induced by streptozocin and minimized inflammation induced by cytokine incubation were greatly compromised. The author’s suggestions were based on the observed effects derived from pectin binding to Gal-3 [85]. However, specific data regarding the connection between the in vitro effects and Gal-3 inhibition by pectins were not proposed.
As already mentioned before in this topic, Gal-3 does not have only one direct binding site toward polysaccharides, known as the canonical S-face (sugar-binding) region inside the CRD. This can help to explain the different results in distinct binding experiments based upon competitive inhibition and chain length [88]. Detailed data suggest that the Gal-3 N-terminal binds to a 60 kDa RG-I-rich pectin portion of ginseng through galactans located in RG-I ramifications [92]. This same molecule with removed ramifications (Rhap and GalpA intercalated residues only) did not bind with Gal-3 [92]. Another molecule rich in galactose—but not derived from pectin—is a galactomannan isolated from Cyamopsis tetragonoloba guar gum flour (1-4-β-d-mannopyranose backbone with 1-6-α-d-galactose ramifications) also did not bind with Gal-3 [92]. However, experiments performed with potato galactans oligosaccharides derived from pectin had similar results as observed for the ginseng RG-I regarding Gal-3 binding [92], demonstrating the probable uniqueness structural features of pectin-derived molecules that can result in Gal-3 binding.
Gal-3 F-face has shown strong binding signals with pectin molecules, mainly at lower polysaccharide concentrations, opening more space to conventional S-face binding in higher concentrations. Like S-face, the F-face non-orthodox site is enriched by hydrophilic and charged amino acids residues. In addition to not having the Trp key-residue, other hydrophobic side chains could have similar functionality, as well as similar concave shape conformation [84][92] (Figure 4A–D). It is specified that resonance broadening was attributed as the primary capability of binding between Gal-3 and the RG-I-4 (a ginseng-derived rhamnogalacturonan), but also that broadening would be directly correlated to higher noise and lower sensitivity in analytical techniques [84][92], a factor which has to be taken into account. The presence of those non-conventional sites was suggested when lactose (CRD S-face inhibitor) did not interfere in RG-I binding to Gal-3 [40]. Furthermore, the binding of a determined ligand to the F-face of Gal-3 CRD could allosterically modify S-face residues. These shifts influence the conformation of the opposing face affecting the affinity between ligand (peptide, glycoconjugate, or polysaccharide) and the Gal-3, improving or attenuating the inhibitory/activity effects [76]. Moreover, Gal-3 N-terminal tail phosphorylation, although having little impact in CRD F-face, may be related to allosterically influencing carbohydrate-binding to the canonical CRD S-face site [76]. This structure-related information and respective identification method improvement contributed to more profoundly describing how the synergistic effects between pectin fragments work, as will be thoroughly discussed below.
Unusual non-galactoside poly-oligomeric ligands could act through multivalence interaction with Gal-3. The synthesis or identification of molecules that represent potential inhibitors of multimeric (chimeric) Gal-3 to cell-membrane glycoconjugates could be critical for minimizing known observed effects, such as the glycoclustering of receptors resulting in apoptosis induction of T cells [96]. Chelation, subsite binding, and reassociation of the binding site towards different monomeric structures within a molecule are also candidates for multivalent interactions with Gal-3 [97]. Although pectins and other natural ligands are harder to validate in comparison to synthetic compounds, parameters such as molecular flexibility and occupation of binding sites can be less manipulated/predicted [98]; the study of monosaccharide and consequently substructures ratios influencing Gal-3 binding is a promising area of investigation.
A recent in vitro study analyzed Gal-3 inhibition and MCF cell viability after specific enzymatic modification of citrus water-soluble fraction (WSF) rich in pectin. It was demonstrated that although β-1,4-galactan side chains in RG-I are still considered the most accountable molecular part for the observed effects, the partial removal of the side chains composed of other monosaccharide residues, such as α-1,5-arabinan, also contributed to inferior results both at cancer cell proliferation and direct gal-3 binding [33]. Other complex structures, such as the pectic acidic fractions extracted from Camellia japonica pollen, described as an RG-I-like polysaccharide, had their branched α-1-3,5 arabinan and type II arabinogalactans attributed to their strong Gal-3 inhibition effects [53]. It is suggested that, despite not having a significant relationship to the ratio of RG-I/HG, pectin-rich WSF bioactivity was dependent on cooperation between RG-I and HG regions [33]. In addition to this pectin fragment ratio, the monosaccharide residue composition ratio had an impactful performance. The Galp amount is necessary for the best Gal-3 inhibitory results since pectic fragments with lower Galp/Araf ratio resulted in lower Gal-3 inhibition even with similar molecular sizes than other studies (50 to 60 kDa) [52].
There are also in vitro data for anti-proliferative characteristics for sugar-beet pectin, in which both the RGI/HG backbone and the galactans/arabinan side chains exhibited those positive effects in HT-29 (human colorectal adenocarcinoma) cell populations [99]. The alkali treatment not only increased the RG-I/HG ratio (which translates to more neutral sugar side chains) but also enhanced the anti-proliferative effects. The removal of almost all side chains in the sample did not completely abolish the effects, denoting the importance of the cooperative effects between different pectic structures [99].
Zhang and colleagues [83] indicated that a combination of RG and HG polysaccharides enhances the Gal-3 binding in vitro through HG interaction with RG, opening more Gal-3 binding epitopes in the RG molecule. At a particular RG/HG molar ratio, the interaction between polysaccharide and the F-face of Gal-3 could establish the new activated binding epitopes due to the higher prevalence of galactose residues, facilitating the S-face CRD interaction. This interaction between HG and RG could perform alterations in its conformation and increase or enhance the synergistic functional effects [83]. Each 130 kDa molecule of the isolated pectin fraction could bind up to 16 Gal-3 molecules. Overall, the combination of both structures showed biologically better activity than separated molecules [83]. Additional ginseng HG-rich fractions were responsible for inducing apoptotic process in vitro at higher doses and cell cycle arrest at lower doses in HT-29 cells, and these biological effects were increased after heat treatment of the polysaccharide fractions [100].
Another ginseng polysaccharide fraction had 91 kDa, an Araf/Rhap ratio of 2:1, and a Galp/Xylp ratio of 1:1 and was characterized as xylo/rhamnogalacturonan I with arabinan/galactan side chains. This fraction was evaluated for in vitro Gal-3 inhibition, anticancer effect, and in vivo gut microbiota modulation [101]. The authors indicated that the xylans were mostly responsible for the microbiota’s healthy recovery and protection. Meanwhile, arabinogalactan side chains can interact with Gal-3 down-regulating tyrosinase through its N-glycan binding site [101]. Overall, the polysaccharide was effective in restoring normal levels of the important interleukin for tumor rejection and T cell activation, such as IL-10. The polysaccharide also modulated IL-17, a mediating molecule over-produced by the tumor cell microenvironment, revealing a multi-targeted functionality of the ginseng polysaccharide [101]. Although studied for many years, this pectic polysaccharide immunomodulatory property is still a trending topic, especially when the structural differences influence the interaction between the pectin and immune receptors, such as the toll-like receptor family (TLR) and interleukins at the macrophage cell surface [73][102], and will be more profoundly discussed later.
After determining the direct inhibition potential by competing with Gal-3 ligands in ECM, it was demonstrated that MCP can also downregulate Gal-3 expression. The in vitro induction of cell cycle arrest at the G2/M phase, through Cyclin B1 decrease and cyclin-dependent kinase 1 (Cdc2) phosphorylation, disrupts the Gal-3 pathological function of maintaining cell cycle arrest at the late G1 phase that leads to evasion from apoptosis induction [103]. Gal-3 can also induce phosphorylation in the signal transducer and activator of transcription 3 (STAT-3) in ovarian cancer cell spheroids. Although demonstrating slight decreases in cell viability after treating with paclitaxel, a strong synergistic effect between this drug and MCP was observed. The IC50 values decreased when both compounds were together, alongside a 70% increase in caspase-3 activity and a 75% Cyclin D1 expression level decrease against the values obtained using only paclitaxel [104].
Regarding fibrosis induction, MCP alleviated liver fibrosis and stress-induced secretions, such as decreased malondialdehyde (MDA), TIMP metallopeptidase inhibitor 1 (TIMP-1), collagen-1 α-1 (Col1A1), and Gal-3 expressions, improving HSC apoptosis rate and the upregulation of glutathione and superoxide dismutase in vivo [105]. Renal fibrosis-related biomarkers in an adult male Wistar murine model were also attenuated by treatment with MCP in drinking water. Albuminuria, proinflammatory cytokines, such as small inducible cytokine A2, osteopontin, epithelial transforming growth factor-β1, and other epithelial to mesenchymal transition factors, were all controlled or restored to normal levels after treatment in normotensive experimental models of renal damage [106].
Gal-3 is up-regulated by aldosterone, mediating inflammatory and fibrotic response in vascular muscle cells in vitro and in vivo [107]. The mechanism was demonstrated as inducing Gal-3 secretion by macrophages via phosphatidylinositol 3-kinase inhibitor/AKT and nuclear factor κB transcription signaling pathways in vitro and in vivo [108]. Furthermore, in aldosterone-induced cardiac and renal injuries, MCP subcutaneous injection in mice—different from the major in vivo experiments when MCP was diluted in drinking water—downregulated Gal-3 at protein and mRNA levels while also minimizing cardiac adverse effects induced by aldosterone salt [109]. In addition, for the renal injuries, MCP inhibited Gal-3 at the tubular level but not in the glomeruli, which is highlighted by the authors since Gal-3 is not expressed at the glomerular level [109]. Nephrotoxicity is a major side-effect of cisplatin chemotherapy, which contributes to the number of acute kidney injury (AKI) hospitalizations. Mice that received 1% MCP in drinking water during the same period after cisplatin injection had better morphometric preservation and prognostic regarding renal fibrosis, such as lower serum creatinine levels, Gal-3, fibronectin, and collagen-1 expression, which could point to a protective effect of MCP in this treatment [110]. Specific mechanisms and routes of Gal-3 downregulation, however, were not successfully addressed by those studies. Therefore, those points should be considered for better knowledge of the possible link between treatment and the biological effect.
AKI-induced mice through the ischemia/reperfusion (IR) model led to Gal-3 expression, cardiac injury, and systemic inflammation. The authors indicate that the induction of inflammation alongside cardiac fibrosis was Gal-3 dependent, demonstrated through significant reduced deleterious outcomes in genetically Gal-3-KO mice that received orally MCP (100 mg/kg/day), which was also seen at WT-MCP-treated mice group [111], but the expression of other markers such as MCP-1 and ICAM-1 mRNA were also decreased by MCP treatment. Rats submitted to myocardial IR also had better prognostics when treated with MCP (in drinking water) one day before and eight days after the procedure, such as improved perfusion, serum brain natriuretic peptide normalization, IL-1b and C-reactive protein (CRP) reduction, lower Gal-3 expression levels at the ischemic tissue, and other parameters. The Gal-3 specific blockade suggested was measured by the authors through the expression of two proteins that are down-regulated by Gal-3, fumarase, and reticulocalbin-3, which were restored after MCP treatment [112]. Perindopril and MCP (in drinking water) were similarly effective as treatments for ischemic heart failures in rabbit models, lowering Collagen-I, III, and Gal-3 mRNA and protein expression, alongside slight reversion of histological remodeling (an important level of fibrosis was still maintained, visible by Masson staining of myocardial tissues). However, the exact mechanisms by which MCP or perindopril could exert their observed effects are still unclear and were not directly addressed [113]. Both the effects of Gal-3 and isoproterenol-induced left ventricular systolic dysfunction in the mice model with selective hyperaldosteronism, alongside myocardial fibrosis installation. Combined therapies with MCP in drinking water and canrenoate as an aldosterone blocker enhanced the anti-inflammatory and anti-fibrotic effects [114]. Rats in a pressure overload model that were treated with MCP in drinking water had lower Gal-3 mRNA expression and Gal-3 immunostaining-confirmed presence than control. Other fibrosis-related proteins such as α-smooth muscle actin (α-SMA), connective tissue growth factor (CTGF), transforming growth factor (TGF)β-1 and fibronectin, and also inflammation factors such as IL-6, IL-1β, and TNF-α were at lower levels than control [115]. The studies described herein regarding oral MCP supplementation, either isolated or in combination with other molecules: (i) did not only show effects influencing Gal-3, but also other pathways, receptors, and protein expressions; (ii) authors did not explain, or at least brought up for the discussion, if orally consumed MCP could reach the target organs, such as kidney, heart or liver. These factors should be considered whenever reading these interesting but overly biased results, denoting not-so exclusive observations that are also promising but opening many questions regarding the systemic distribution of pectin’s pre- or post- colonic fermentation.
As mentioned before, there are physiological activation pathways that could involve Gal-3 action, important data to account for whenever testing new possible therapeutic agents that could selectively induce activation/apoptosis depending on the target [116]. This in vitro work has demonstrated that recombinant human Gal-3 initiates three distinct pathways, one for T cell activation (PIK3) and two hybrids (reactive oxygen species and protein kinase C—ROS and PKC, respectively). Furthermore, MCP and acidic fraction from ginseng roots inhibited T cell apoptosis in vitro by caspase-3 cleavage, and ginseng-derived fractions did not interfere in IL-2 secretion [116]. One of the Gal-3 connections established by the authors to the observed effects is proposed as upregulation of PI3K/Akt phosphorylation, in which the presence of an inhibitor for each molecule also inhibited IL-2 secretion. However, no similar effects regarding the effects of MCP in cardiac protection, fibrosis regulation, and normalized hypertension were observed in a recently published clinical trial from Lau et al. [117] in patients with high Gal-3 levels and established hypertension. The results raise many doubts and counterpoints regarding the MCP effects in in vitro and in vivo studies to be replicated in humans, which is discussed in the next chapter.
Recently, Gal-3 has been treated as a treatment target and prognostic marker for patients with severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2). Higher serum levels of Gal-3 were related to a tendency of severe acute respiratory distress syndrome (ARDS) development, and alongside IL-6 and CRP, Gal-3 was demonstrated to be the best predictive power for mortality outcome [118]. There are also positive correlations between Gal-3 and other inflammatory markers such as PTX-3, ferritin, and the marker of endothelial dysfunction, sFlt-1 [119], which could be utilized for intensive care unit (ICU) admission biomarker. A phase 2a study, the first clinical trial using an inhalator treatment targeting Gal-3-GB0139—associated with the standard of care procedure (dexamethasone)—identified lower Gal-3 serum levels, higher mean downward of CRP levels (although higher CRP was identified at first in the treatment group) and other inflammatory agents and better fibrosis marker levels than the control group. However, there was no statistical difference between patient mortality rates between groups [120]. This enhances and augments the discussion to further explore alternative treatments regarding Gal-3 inhibition.
References
- Gawkowska, D.; Cybulska, J.; Zdunek, A. Structure-related gelling of pectins and linking with other natural compounds: A review. Polymers 2018, 10, 762.
- Maxwell, E.G.; Belshaw, N.J.; Waldron, K.W.; Morris, V.J. Pectin—An emerging new bioactive food polysaccharide. Trends Food Sci. Technol. 2012, 24, 64–73.
- Cui, L.; Wang, J.; Huang, R.; Tan, Y.; Zhang, F.; Zhou, Y.; Sun, L. Analysis of pectin from Panax ginseng flower buds and their binding activities to galectin-3. Int. J. Biol. Macromol. 2019, 128, 459–467.
- Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277.
- Fabi, J.P.; Seymour, G.B.; Graham, N.S.; Broadley, M.R.; May, S.T.; Lajolo, F.M.; Cordenunsi, B.R.; Oliveira do Nascimento, J.R. Analysis of ripening-related gene expression in papaya using an Arabidopsis-based microarray. BMC Plant Biol. 2012, 12, 242.
- Fabi, J.P.; Broetto, S.G.; da Silva, S.L.G.L.; Zhong, S.; Lajolo, F.M.; do Nascimento, J.R.O. Analysis of papaya cell wall-related genes during fruit ripening indicates a central role of polygalacturonases during pulp softening. PLoS ONE 2014, 9, e105685.
- De Freitas Pedrosa, L.; Lopes, R.G.; Fabi, J.P. The acid and neutral fractions of pectins isolated from ripe and overripe papayas differentially affect galectin-3 inhibition and colon cancer cell growth. Int. J. Biol. Macromol. 2020, 164, 2681–2690.
- Do Prado, S.B.R.; Ferreira, G.F.; Harazono, Y.; Shiga, T.M.; Raz, A.; Carpita, N.C.; Fabi, J.P. Ripening-induced chemical modifications of papaya pectin inhibit cancer cell proliferation. Sci. Rep. 2017, 7, 16564.
- Varki, A.; Cummings, R.D.; Aebi, M.; Packer, N.H.; Seeberger, P.H.; Esko, J.D.; Stanley, P.; Hart, G.; Darvill, A.; Kinoshita, T.; et al. Symbol nomenclature for graphical representations of glycans. Glycobiology 2015, 25, 1323–1324.
- Chen, Y.; Xu, C.; Huang, R.; Song, J.; Li, D.; Xia, M. Butyrate from pectin fermentation inhibits intestinal cholesterol absorption and attenuates atherosclerosis in apolipoprotein E-deficient mice. J. Nutr. Biochem. 2018, 56, 175–182.
- Li, W.; Zhang, K.; Yang, H. Pectin Alleviates High Fat (Lard) Diet-Induced Nonalcoholic Fatty Liver Disease in Mice: Possible Role of Short-Chain Fatty Acids and Gut Microbiota Regulated by Pectin. J. Agric. Food Chem. 2018, 66, 8015–8025.
- Brouns, F.; Theuwissen, E.; Adam, A.; Bell, M.; Berger, A.; Mensink, R.P. Cholesterol-lowering properties of different pectin types in mildly hyper-cholesterolemic men and women. Eur. J. Clin. Nutr. 2012, 66, 591–599.
- Liu, Y.; Dong, M.; Yang, Z.; Pan, S. Anti-diabetic effect of citrus pectin in diabetic rats and potential mechanism via PI3K/Akt signaling pathway. Int. J. Biol. Macromol. 2016, 89, 484–488.
- Fotschki, B.; Jurgoński, A.; Juśkiewicz, J.; Kołodziejczyk, K.; Sójka, M. Effects of dietary addition of a low-pectin apple fibre preparation on rats. Pol. J. Food Nutr. Sci. 2014, 64, 193–199.
- Kunzmann, A.T.; Coleman, H.G.; Huang, W.-Y.; Kitahara, C.M.; Cantwell, M.M.; Berndt, S.I. Dietary fiber intake and risk of colorectal cancer and incident and recurrent adenoma in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Am. J. Clin. Nutr. 2015, 102, 881–890.
- Song, L.; Tang, J.-W.; Owusu, L.; Sun, M.-Z.; Wu, J.; Zhang, J. Galectin-3 in cancer. Clin. Chim. Acta 2014, 431, 185–191.
- Sciacchitano, S.; Lavra, L.; Morgante, A.; Ulivieri, A.; Magi, F.; De Francesco, G.P.; Bellotti, C.; Salehi, L.B.; Ricci, A. Galectin-3: One molecule for an alphabet of diseases, from A to Z. Int. J. Mol. Sci. 2018, 19, 379.
- Fortuna-Costa, A.; Gomes, A.M.; Kozlowski, E.O.; Stelling, M.P.; Pavão, M.S.G. Extracellular galectin-3 in tumor progression and metastasis. Front. Oncol. 2014, 4.
- Suthahar, N.; Meijers, W.C.; Silljé, H.H.W.; Ho, J.E.; Liu, F.-T.; de Boer, R.A. Galectin-3 activation and inhibition in heart failure and cardiovascular disease: An update. Theranostics 2018, 8, 593–609.
- Filipová, M.; Bojarová, P.; Rodrigues Tavares, M.; Bumba, L.; Elling, L.; Chytil, P.; Gunár, K.; Křen, V.; Etrych, T.; Janoušková, O. Glycopolymers for Efficient Inhibition of Galectin-3: In Vitro Proof of Efficacy Using Suppression of T Lymphocyte Apoptosis and Tumor Cell Migration. Biomacromolecules 2020, 21, 3122–3133.
- Jia, J.; Claude-Taupin, A.; Gu, Y.; Choi, S.W.; Peters, R.; Bissa, B.; Mudd, M.H.; Allers, L.; Pallikkuth, S.; Lidke, K.A.; et al. Galectin-3 Coordinates a Cellular System for Lysosomal Repair and Removal. Dev. Cell 2020, 52, 69–87.e8.
- Maxwell, E.G.; Colquhoun, I.J.; Chau, H.K.; Hotchkiss, A.T.; Waldron, K.W.; Morris, V.J.; Belshaw, N.J. Rhamnogalacturonan i containing homogalacturonan inhibits colon cancer cell proliferation by decreasing ICAM1 expression. Carbohydr. Polym. 2015, 132, 546–553.
- Wu, K.-L.; Huang, E.-Y.; Jhu, E.-W.; Huang, Y.-H.; Su, W.-H.; Chuang, P.-C.; Yang, K.D. Overexpression of galectin-3 enhances migration of colon cancer cells related to activation of the K-Ras-Raf-Erk1/2 pathway. J. Gastroenterol. 2013, 48, 350–359.
- Song, S.; Ji, B.; Ramachandran, V.; Wang, H.; Hafley, M.; Logsdon, C.; Bresalier, R.S. Overexpressed galectin-3 in pancreatic cancer induces cell proliferation and invasion by binding ras and activating ras signaling. PLoS ONE 2012, 7, e42699.
- Margadant, C.; Van Den Bout, I.; Van Boxtel, A.L.; Thijssen, V.L.; Sonnenberg, A. Epigenetic regulation of galectin-3 expression by β1 integrins promotes cell adhesion and migration. J. Biol. Chem. 2012, 287, 44684–44693.
- Wang, W.; Guo, H.; Geng, J.; Zheng, X.; Wei, H.; Sun, R.; Tian, Z. Tumor-released galectin-3, a soluble inhibitory ligand of human NKp30, plays an important role in tumor escape from NK cell attack. J. Biol. Chem. 2014, 289, 33311–33319.
- Voss, J.J.L.P.; Ford, C.A.; Petrova, S.; Melville, L.; Paterson, M.; Pound, J.D.; Holland, P.; Giotti, B.; Freeman, T.C.; Gregory, C.D. Modulation of macrophage antitumor potential by apoptotic lymphoma cells. Cell Death Differ. 2017, 24, 971–983.
- Xue, H.; Liu, L.; Zhao, Z.; Zhang, Z.; Guan, Y.; Cheng, H.; Zhou, Y.; Tai, G. The N-terminal tail coordinates with carbohydrate recognition domain to mediate galectin-3 induced apoptosis in T cells. Oncotarget 2017, 8, 49824–49838.
- Freichel, T.; Heine, V.; Laaf, D.; Mackintosh, E.E.; Sarafova, S.; Elling, L.; Snyder, N.L.; Hartmann, L. Sequence-Defined Heteromultivalent Precision Glycomacromolecules Bearing Sulfonated/Sulfated Nonglycosidic Moieties Preferentially Bind Galectin-3 and Delay Wound Healing of a Galectin-3 Positive Tumor Cell Line in an In Vitro Wound Scratch Assay. Macromol. Biosci. 2020, 20, 2000163.
- Laaf, D.; Bojarová, P.; Elling, L.; Křen, V. Galectin—Carbohydrate Interactions in Biomedicine and Biotechnology. Trends Biotechnol. 2019, 37, 402–415.
- Rajput, V.K.; MacKinnon, A.; Mandal, S.; Collins, P.; Blanchard, H.; Leffler, H.; Sethi, T.; Schambye, H.; Mukhopadhyay, B.; Nilsson, U.J. A Selective Galactose-Coumarin-Derived Galectin-3 Inhibitor Demonstrates Involvement of Galectin-3-glycan Interactions in a Pulmonary Fibrosis Model. J. Med. Chem. 2016, 59, 8141–8147.
- Do Prado, S.B.R.; Santos, G.R.C.; Mourão, P.A.S.; Fabi, J.P. Chelate-soluble pectin fraction from papaya pulp interacts with galectin-3 and inhibits colon cancer cell proliferation. Int. J. Biol. Macromol. 2019, 126, 170–178.
- Wu, D.; Zheng, J.; Hu, W.; Zheng, X.; He, Q.; Linhardt, R.J.; Ye, X.; Chen, S. Structure-activity relationship of Citrus segment membrane RG-I pectin against Galectin-3: The galactan is not the only important factor. Carbohydr. Polym. 2020, 245, 116526.
- Zhang, T.; Lan, Y.; Zheng, Y.; Liu, F.; Zhao, D.; Mayo, K.H.; Zhou, Y.; Tai, G. Identification of the bioactive components from pH-modified citrus pectin and their inhibitory effects on galectin-3 function. Food Hydrocoll. 2016, 58, 113–119.
- Do Nascimento Oliveira, A.; de Almeida Paula, D.; de Oliveira, E.B.; Saraiva, S.H.; Stringheta, P.C.; Ramos, A.M. Optimization of pectin extraction from Ubá mango peel through surface response methodology. Int. J. Biol. Macromol. 2018, 113, 395–402.
- Chan, S.-Y.; Choo, W.-S. Effect of extraction conditions on the yield and chemical properties of pectin from cocoa husks. Food Chem. 2013, 141, 3752–3758.
- Pereira, P.H.F.; Oliveira, T.Í.S.; Rosa, M.F.; Cavalcante, F.L.; Moates, G.K.; Wellner, N.; Waldron, K.W.; Azeredo, H.M.C. Pectin extraction from pomegranate peels with citric acid. Int. J. Biol. Macromol. 2016, 88, 373–379.
- Oliveira, T.Í.S.; Rosa, M.F.; Cavalcante, F.L.; Pereira, P.H.F.; Moates, G.K.; Wellner, N.; Mazzetto, S.E.; Waldron, K.W.; Azeredo, H.M.C. Optimization of pectin extraction from banana peels with citric acid by using response surface methodology. Food Chem. 2016, 198, 113–118.
- Srivastava, P.; Malviya, R. Sources of pectin, extraction and its applications in pharmaceutical industry—An overview. Indian J. Nat. Prod. Resour. 2011, 2, 10–18.
- Gao, X.; Zhi, Y.; Sun, L.; Peng, X.; Zhang, T.; Xue, H.; Tai, G.; Zhou, Y. The inhibitory effects of a rhamnogalacturonan I (RG-I) domain from ginseng Pectin on galectin-3 and its structure-activity relationship. J. Biol. Chem. 2013, 288, 33953–33965.
- Leclere, L.; Van Cutsem, P.; Michiels, C. Anti-cancer activities of pH- or heat-modified pectin. Front. Pharmacol. 2013, 4.
- Leclere, L.; Fransolet, M.; Cote, F.; Cambier, P.; Arnould, T.; Van Cutsem, P.; Michiels, C. Heat-modified citrus pectin induces apoptosis-like cell death and autophagy in HepG2 and A549 cancer cells. PLoS ONE 2015, 10, e0115831.
- Xu, Y.; Zhang, L.; Bailina, Y.; Ge, Z.; Ding, T.; Ye, X.; Liu, D. Effects of ultrasound and/or heating on the extraction of pectin from grapefruit peel. J. Food Eng. 2014, 126, 72–81.
- Wang, W.; Ma, X.; Xu, Y.; Cao, Y.; Jiang, Z.; Ding, T.; Ye, X.; Liu, D. Ultrasound-assisted heating extraction of pectin from grapefruit peel: Optimization and comparison with the conventional method. Food Chem. 2015, 178, 106–114.
- Wang, W.; Chen, W.; Zou, M.; Lv, R.; Wang, D.; Hou, F.; Feng, H.; Ma, X.; Zhong, J.; Ding, T.; et al. Applications of power ultrasound in oriented modification and degradation of pectin: A review. J. Food Eng. 2018, 234, 98–107.
- Zouambia, Y.; Ettoumi, K.Y.; Krea, M.; Moulai-Mostefa, N. A new approach for pectin extraction: Electromagnetic induction heating. Arab. J. Chem. 2017, 10, 480–487.
- Chen, J.; Cheng, H.; Zhi, Z.; Zhang, H.; Linhardt, R.J.; Zhang, F.; Chen, S.; Ye, X. Extraction temperature is a decisive factor for the properties of pectin. Food Hydrocoll. 2021, 112, 106160.
- Blanchard, H.; Bum-Erdene, K.; Bohari, M.H.; Yu, X. Galectin-1 inhibitors and their potential therapeutic applications: A patent review. Expert Opin. Ther. Pat. 2016, 26, 537–554.
- Laaf, D.; Bojarová, P.; Pelantová, H.; Křen, V.; Elling, L. Tailored Multivalent Neo-Glycoproteins: Synthesis, Evaluation, and Application of a Library of Galectin-3-Binding Glycan Ligands. Bioconjugate Chem. 2017, 28, 2832–2840.
- Stegmayr, J.; Lepur, A.; Kahl-Knutson, B.; Aguilar-Moncayo, M.; Klyosov, A.A.; Field, R.A.; Oredsson, S.; Nilsson, U.J.; Leffler, H. Low or No Inhibitory Potency of the Canonical Galectin Carbohydrate-binding Site by Pectins and Galactomannans. J. Biol. Chem. 2016, 291, 13318–13334.
- Gunning, A.P.; Bongaerts, R.J.M.; Morris, V.J. Recognition of galactan components of pectin by galectin-3. FASEB J. 2009, 23, 415–424.
- Shi, H.; Yu, L.; Shi, Y.; Lu, J.; Teng, H.; Zhou, Y.; Sun, L. Structural characterization of a rhamnogalacturonan I domain from ginseng and its inhibitory effect on galectin-3. Molecules 2017, 22, 1016.
- Zhou, L.; Ma, P.; Shuai, M.; Huang, J.; Sun, C.; Yao, X.; Chen, Z.; Min, X.; Zhang, T. Analysis of the water-soluble polysaccharides from Camellia japonica pollen and their inhibitory effects on galectin-3 function. Int. J. Biol. Macromol. 2020, 159, 455–460.
- Shao, P.; Wang, P.; Niu, B.; Kang, J. Environmental stress stability of pectin-stabilized resveratrol liposomes with different degree of esterification. Int. J. Biol. Macromol. 2018, 119, 53–59.
- Do Nascimento, G.E.; Simas-Tosin, F.F.; Iacomini, M.; Gorin, P.A.J.; Cordeiro, L.M.C. Rheological behavior of high methoxyl pectin from the pulp of tamarillo fruit (Solanum betaceum). Carbohydr. Polym. 2016, 139, 125–130.
- Schmidt, U.S.; Pietsch, V.L.; Rentschler, C.; Kurz, T.; Endreß, H.U.; Schuchmann, H.P. Influence of the degree of esterification on the emulsifying performance of conjugates formed between whey protein isolate and citrus pectin. Food Hydrocoll. 2016, 56, 1–8.
- Schmidt, U.S.; Schütz, L.; Schuchmann, H.P. Interfacial and emulsifying properties of citrus pectin: Interaction of pH, ionic strength and degree of esterification. Food Hydrocoll. 2017, 62, 288–298.
- Jacob, E.M.; Borah, A.; Jindal, A.; Pillai, S.C.; Yamamoto, Y.; Maekawa, T.; Kumar, D.N.S. Synthesis and characterization of citrus-derived pectin nanoparticles based on their degree of esterification. J. Mater. Res. 2020, 35, 1514–1522.
- Wan, L.; Chen, Q.; Huang, M.; Liu, F.; Pan, S. Physiochemical, rheological and emulsifying properties of low methoxyl pectin prepared by high hydrostatic pressure-assisted enzymatic, conventional enzymatic, and alkaline de-esterification: A comparison study. Food Hydrocoll. 2019, 93, 146–155.
- Dranca, F.; Oroian, M. Extraction, purification and characterization of pectin from alternative sources with potential technological applications. Food Res. Int. 2018, 113, 327–350.
- Begum, R.; Yusof, Y.A.; Aziz, M.G.; Uddin, M.B. Structural and functional properties of pectin extracted from jackfruit (Artocarpus heterophyllus) waste: Effects of drying. Int. J. Food Prop. 2017, 20, S190–S201.
- Karnik, D.; Wicker, L. Emulsion stability of sugar beet pectin fractions obtained by isopropanol fractionation. Food Hydrocoll. 2018, 74, 249–254.
- Juttulapa, M.; Piriyaprasarth, S.; Takeuchi, H.; Sriamornsak, P. Effect of high-pressure homogenization on stability of emulsions containing zein and pectin. Asian J. Pharm. Sci. 2017, 12, 21–27.
- Petkowicz, C.L.O.; Vriesmann, L.C.; Williams, P.A. Pectins from food waste: Extraction, characterization and properties of watermelon rind pectin. Food Hydrocoll. 2017, 65, 57–67.
- Picot-Allain, M.C.N.; Ramasawmy, B.; Emmambux, M.N. Extraction, Characterisation, and Application of Pectin from Tropical and Sub-Tropical Fruits: A Review. Food Rev. Int. 2020, 38, 282–312.
- Zhang, L.; Ye, X.; Ding, T.; Sun, X.; Xu, Y.; Liu, D. Ultrasound effects on the degradation kinetics, structure and rheological properties of apple pectin. Ultrason. Sonochemistry 2013, 20, 222–231.
- Basanta, M.F.; Ponce, N.M.A.; Rojas, A.M.; Stortz, C.A. Effect of extraction time and temperature on the characteristics of loosely bound pectins from Japanese plum. Carbohydr. Polym. 2012, 89, 230–235.
- Kosmala, M.; Milala, J.; Kołodziejczyk, K.; Markowski, J.; Zbrzeźniak, M.; Renard, C.M.G.C. Dietary fiber and cell wall polysaccharides from plum (Prunus domestica L.) fruit, juice and pomace: Comparison of composition and functional properties for three plum varieties. Food Res. Int. 2013, 54, 1787–1794.
- Moreno, L.; Nascimento, R.F.; Zielinski, A.A.F.; Wosiacki, G.; Canteri, M.H.G. Extraction and characterization of pectic substances in Myrciaria cauliflora (Jaboticaba sabará) fruit. Rev. Strict. Sensu 2016, 1, 1–11.
- Sayah, M.Y.; Chabir, R.; Benyahia, H.; Kandri, Y.R.; Chahdi, F.O.; Touzani, H.; Errachidi, F. Yield, esterification degree and molecular weight evaluation of pectins isolated from orange and grapefruit peels under different conditions. PLoS ONE 2016, 11, e0161751.
- Hao, M.; Yuan, X.; Cheng, H.; Xue, H.; Zhang, T.; Zhou, Y.; Tai, G. Comparative studies on the anti-tumor activities of high temperature- and pH-modified citrus pectins. Food Funct. 2013, 4, 960–971.
- Do Prado, S.B.R.; Melfi, P.R.; Castro-Alves, V.C.; Broetto, S.G.; Araújo, E.S.; Do Nascimento, J.R.O.; Fabi, J.P. Physiological degradation of pectin in papaya cell walls: Release of long chains galacturonans derived from insoluble fractions during postharvest fruit ripening. Front. Plant Sci. 2016, 7, 1–11.
- Prado, S.B.R.; Beukema, M.; Jermendi, E.; Schols, H.A.; de Vos, P.; Fabi, J.P. Pectin Interaction with Immune Receptors is Modulated by Ripening Process in Papayas. Sci. Rep. 2020, 10, 1–11.
- Flores-Ibarra, A.; Vértesy, S.; Medrano, F.J.; Gabius, H.J.; Romero, A. Crystallization of a human galectin-3 variant with two ordered segments in the shortened N-terminal tail. Sci. Rep. 2018, 8, 1–11.
- Su, J.; Zhang, T.; Wang, P.; Liu, F.; Tai, G.; Zhou, Y. The water network in galectin-3 ligand binding site guides inhibitor design. Acta Biochim. Biophys. Sin. 2015, 47, 192–198.
- Ippel, H.; Miller, M.C.; Vértesy, S.; Zheng, Y.; Cañada, F.J.; Suylen, D.; Umemoto, K.; Romanò, C.; Hackeng, T.; Tai, G.; et al. Intra- and intermolecular interactions of human galectin-3: Assessment by full-assignment-based NMR. Glycobiology 2016, 26, 888–903.
- Kim, S.-J.; Chun, K.-H. Non-classical role of Galectin-3 in cancer progression: Translocation to nucleus by carbohydrate-recognition independent manner. BMB Rep. 2020, 53, 173–180.
- Ruvolo, P.P. Galectin 3 as a guardian of the tumor microenvironment. Biochim. Biophys. Acta—Mol. Cell Res. 2016, 1863, 427–437.
- Chan, Y.-C.; Lin, H.-Y.; Tu, Z.; Kuo, Y.-H.; Hsu, S.-T.D.; Lin, C.-H. Dissecting the structure–Activity relationship of galectin—Ligand interactions. Int. J. Mol. Sci. 2018, 19, 392.
- Sehnal, D.; Bittrich, S.; Deshpande, M.; Svobodová, R.; Berka, K.; Bazgier, V.; Velankar, S.; Burley, S.K.; Koča, J.; Rose, A.S. Mol* Viewer: Modern web app for 3D visualization and analysis of large biomolecular structures. Nucleic Acids Res. 2021, 49, W431–W437.
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242.
- Johannes, L.; Jacob, R.; Leffler, H. Galectins at a glance. J. Cell Sci. 2018, 131, 1–9.
- Zhang, T.; Miller, M.C.; Zheng, Y.; Zhang, Z.; Xue, H.; Zhao, D.; Su, J.; Mayo, K.H.; Zhou, Y.; Tai, G. Macromolecular assemblies of complex polysaccharides with galectin-3 and their synergistic effects on function. Biochem. J. 2017, 474, 3849–3868.
- Miller, M.C.; Ippel, H.; Suylen, D.; Klyosov, A.A.; Traber, P.G.; Hackeng, T.; Mayo, K.H. Binding of polysaccharides to human galectin-3 at a noncanonical site in its carbohydrate recognition domain. Glycobiology 2015, 26, 88–99.
- Hu, S.; Kuwabara, R.; Beukema, M.; Ferrari, M.; de Haan, B.J.; Walvoort, M.T.C.; de Vos, P.; Smink, A.M. Low methyl-esterified pectin protects pancreatic β-cells against diabetes-induced oxidative and inflammatory stress via galectin-3. Carbohydr. Polym. 2020, 249, 116863.
- Xu, G.-R.; Zhang, C.; Yang, H.-X.; Sun, J.-H.; Zhang, Y.; Yao, T.-T.; Li, Y.; Ruan, L.; An, R.; Li, A.-Y. Modified citrus pectin ameliorates myocardial fibrosis and inflammation via suppressing galectin-3 and TLR4/MyD88/NF-κB signaling pathway. Biomed. Pharmacother. 2020, 126, 110071.
- Ilmer, M.; Mazurek, N.; Byrd, J.C.; Ramirez, K.; Hafley, M.; Alt, E.; Vykoukal, J.; Bresalier, R.S. Cell surface galectin-3 defines a subset of chemoresistant gastrointestinal tumor-initiating cancer cells with heightened stem cell characteristics. Cell Death Dis. 2016, 7, 1–9.
- Zhang, T.; Zheng, Y.; Zhao, D.; Yan, J.; Sun, C.; Zhou, Y.; Tai, G. Multiple approaches to assess pectin binding to galectin-3. Int. J. Biol. Macromol. 2016, 91, 994–1001.
- Zheng, Y.; Su, J.; Miller, M.C.; Geng, J.; Xu, X.; Zhang, T.; Mayzel, M.; Zhou, Y.; Mayo, K.H.; Tai, G. Topsy-turvy binding of negatively-charged homogalacturonan oligosaccharides to galectin-3. Glycobiology 2020, 31, 341–350.
- Miller, M.C.; Zheng, Y.; Zhou, Y.; Tai, G.; Mayo, K.H. Galectin-3 binds selectively to the terminal, non-reducing end of β(1→4)-galactans, with overall affinity increasing with chain length. Glycobiology 2019, 29, 74–84.
- Zhao, J.; Zhang, F.; Liu, X.; St. Ange, K.; Zhang, A.; Li, Q.; Linhardt, R.J. Isolation of a lectin binding rhamnogalacturonan-I containing pectic polysaccharide from pumpkin. Carbohydr. Polym. 2017, 163, 330–336.
- Miller, M.C.; Zheng, Y.; Yan, J.; Zhou, Y.; Tai, G.; Mayo, K.H. Novel polysaccharide binding to the N-terminal tail of galectin-3 is likely modulated by proline isomerization. Glycobiology 2017, 27, 1038–1051.
- Rapoport, E.M.; Bovin, N.V. Specificity of human galectins on cell surfaces. Biochemistry 2015, 80, 846–856.
- García Caballero, G.; Beckwith, D.; Shilova, N.V.; Gabba, A.; Kutzner, T.J.; Ludwig, A.-K.; Manning, J.C.; Kaltner, H.; Sinowatz, F.; Cudic, M.; et al. Influence of protein (human galectin-3) design on aspects of lectin activity. Histochem. Cell Biol. 2020, 154, 135–153.
- Gao, X.; Zhi, Y.; Zhang, T.; Xue, H.; Wang, X.; Foday, A.D.; Tai, G.; Zhou, Y. Analysis of the neutral polysaccharide fraction of MCP and its inhibitory activity on galectin-3. Glycoconj. J. 2012, 29, 159–165.
- Farhadi, S.A.; Liu, R.; Becker, M.W.; Phelps, E.A.; Hudalla, G.A. Physical tuning of galectin-3 signaling. Proc. Natl. Acad. Sci. USA 2021, 118, 1–10.
- Cecioni, S.; Imberty, A.; Vidal, S. Glycomimetics versus multivalent glycoconjugates for the design of high affinity lectin ligands. Chem. Rev. 2015, 115, 525–561.
- Hevey, R. Strategies for the development of glycomimetic drug candidates. Pharmaceuticals 2019, 12, 55.
- Maxwell, E.G.; Colquhoun, I.J.; Chau, H.K.; Hotchkiss, A.T.; Waldron, K.W.; Morris, V.J.; Belshaw, N.J. Modified sugar beet pectin induces apoptosis of colon cancer cells via an interaction with the neutral sugar side-chains. Carbohydr. Polym. 2016, 136, 923–929.
- Cheng, H.; Li, S.; Fan, Y.; Gao, X.; Hao, M.; Wang, J.; Zhang, X.; Tai, G.; Zhou, Y. Comparative studies of the antiproliferative effects of ginseng polysaccharides on HT-29 human colon cancer cells. Med. Oncol. 2011, 28, 175–181.
- Pynam, H.; Dharmesh, S.M. A xylorhamnoarabinogalactan I from Bael (Aegle marmelos L.) modulates UV/DMBA induced skin cancer via galectin-3 & gut microbiota. J. Funct. Foods 2019, 60, 103425.
- Popov, S.V.; Ovodov, Y.S. Polypotency of the immunomodulatory effect of pectins. Biochemistry 2013, 78, 823–835.
- Fang, T.; Liu, D.-D.; Ning, H.-M.; Liu, D.; Sun, J.-Y.; Huang, X.-J.; Dong, Y.; Geng, M.-Y.; Yun, S.-F.; Yan, J.; et al. Modified citrus pectin inhibited bladder tumor growth through downregulation of galectin-3. Acta Pharmacol. Sin. 2018, 39, 1885–1893.
- Hossein, G.; Halvaei, S.; Heidarian, Y.; Dehghani-Ghobadi, Z.; Hassani, M.; Hosseini, H.; Naderi, N.; Sheikh Hassani, S. Pectasol-C Modified Citrus Pectin targets Galectin-3-induced STAT3 activation and synergize paclitaxel cytotoxic effect on ovarian cancer spheroids. Cancer Med. 2019, 8, 4315–4329.
- Abu-Elsaad, N.M.; Elkashef, W.F. Modified citrus pectin stops progression of liver fibrosis by inhibiting galectin-3 and inducing apoptosis of stellate cells. Can. J. Physiol. Pharmacol. 2016, 94, 554–562.
- Martinez-Martinez, E.; Ibarrola, J.; Calvier, L.; Fernandez-Celis, A.; Leroy, C.; Cachofeiro, V.; Rossignol, P.; Lopez-Andres, N. Galectin-3 blockade reduces renal fibrosis in two normotensive experimental models of renal damage. PLoS ONE 2016, 11, e0166272.
- Calvier, L.; Miana, M.; Reboul, P.; Cachofeiro, V.; Martinez-Martinez, E.; De Boer, R.A.; Poirier, F.; Lacolley, P.; Zannad, F.; Rossignol, P.; et al. Galectin-3 mediates aldosterone-induced vascular fibrosis. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 67–75.
- Lin, Y.-H.; Chou, C.-H.; Wu, X.-M.; Chang, Y.-Y.; Hung, C.-S.; Chen, Y.-H.; Tzeng, Y.-L.; Wu, V.-C.; Ho, Y.-L.; Hsieh, F.-J.; et al. Aldosterone induced galectin-3 secretion in vitro and in vivo: From cells to humans. PLoS ONE 2014, 9, e95254.
- Calvier, L.; Martinez-Martinez, E.; Miana, M.; Cachofeiro, V.; Rousseau, E.; Sádaba, J.R.; Zannad, F.; Rossignol, P.; López-Andrés, N. The impact of galectin-3 inhibition on aldosterone-induced cardiac and renal injuries. JACC Heart Fail. 2015, 3, 59–67.
- Li, H.-Y.; Yang, S.; Li, J.-C.; Feng, J.-X. Galectin 3 inhibition attenuates renal injury progression in cisplatin-induced nephrotoxicity. Biosci. Rep. 2018, 38.
- Prud’homme, M.; Coutrot, M.; Michel, T.; Boutin, L.; Genest, M.; Poirier, F.; Launay, J.M.; Kane, B.; Kinugasa, S.; Prakoura, N.; et al. Acute Kidney Injury Induces Remote Cardiac Damage and Dysfunction through the Galectin-3 Pathway. JACC Basic Transl. Sci. 2019, 4, 717–732.
- Ibarrola, J.; Matilla, L.; Martínez-Martínez, E.; Gueret, A.; Fernández-Celis, A.; Henry, J.P.; Nicol, L.; Jaisser, F.; Mulder, P.; Ouvrard-Pascaud, A.; et al. Myocardial Injury after Ischemia/Reperfusion Is Attenuated by Pharmacological Galectin-3 Inhibition. Sci. Rep. 2019, 9, 1–10.
- Li, S.; Li, S.; Hao, X.; Zhang, Y.; Deng, W. Perindopril and a Galectin-3 inhibitor improve ischemic heart failure in rabbits by reducing gal-3 expression and myocardial fibrosis. Front. Physiol. 2019, 10, 1–8.
- Vergaro, G.; Prud’Homme, M.; Fazal, L.; Merval, R.; Passino, C.; Emdin, M.; Samuel, J.L.; Cohen Solal, A.; Delcayre, C. Inhibition of Galectin-3 Pathway Prevents Isoproterenol-Induced Left Ventricular Dysfunction and Fibrosis in Mice. Hypertension 2016, 67, 606–612.
- Ibarrola, J.; Martínez-Martínez, E.; Sádaba, J.R.; Arrieta, V.; García-Peña, A.; Álvarez, V.; Fernández-Celis, A.; Gainza, A.; Rossignol, P.; Ramos, V.C.; et al. Beneficial effects of galectin-3 blockade in vascular and aortic valve alterations in an experimental pressure overload model. Int. J. Mol. Sci. 2017, 18, 1664.
- Xue, H.; Zhao, Z.; Lin, Z.; Geng, J.; Guan, Y.; Song, C.; Zhou, Y.; Tai, G. Selective effects of ginseng pectins on galectin-3-mediated T cell activation and apoptosis. Carbohydr. Polym. 2019, 219, 121–129.
- Lau, E.S.; Liu, E.; Paniagua, S.M.; Sarma, A.A.; Zampierollo, G.; López, B.; Díez, J.; Wang, T.J.; Ho, J.E. Galectin-3 Inhibition With Modified Citrus Pectin in Hypertension. JACC Basic Transl. Sci. 2021, 6, 12–21.
- Portacci, A.; Diaferia, F.; Santomasi, C.; Dragonieri, S.; Boniello, E.; Di Serio, F.; Carpagnano, G.E. Galectin-3 as prognostic biomarker in patients with COVID-19 acute respiratory failure. Respir. Med. 2021, 187, 106556.
- Kuśnierz-Cabala, B.; Maziarz, B.; Dumnicka, P.; Dembiński, M.; Kapusta, M.; Bociąga-Jasik, M.; Winiarski, M.; Garlicki, A.; Grodzicki, T.; Kukla, M. Diagnostic significance of serum galectin-3 in hospitalized patients with COVID-19—A preliminary study. Biomolecules 2021, 11, 1136.
- Gaughan, A.E.; Sethi, T.; Quinn, T.; Hirani, N.; Mills, A.; Annya, M.; Mackinnon, A.; Aslanis, V.; Li, F.; Connor, R.O.; et al. GB0139, an inhaled small molecule inhibitor of galectin-3, in COVID-19 pneumonitis: A randomised, controlled, open-label, phase 2a experimental medicine trial of safety, pharmacokinetics, and potential therapeutic value. medRxiv 2022.
More
Information
Subjects:
Cell Biology; Food Science & Technology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.6K
Revisions:
4 times
(View History)
Update Date:
28 Feb 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




