
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Dominik Brühwiler | + 1260 word(s) | 1260 | 2022-02-10 05:17:19 | | | |
| 2 | Beatrix Zheng | Meta information modification | 1260 | 2022-02-18 02:22:05 | | | | |
| 3 | Beatrix Zheng | Meta information modification | 1260 | 2022-02-21 02:17:42 | | |
Video Upload Options
The biogenic substance E-indigo can form supramolecular, hydrophobic structures using self-organization. These structures show a low coefficient of friction as a gliding layer against polar surfaces. The formation of primary particles with platelet morphology based on hydrogen-bonded E-indigo molecules is ideal to produce the gliding layer. Structures with excellent gliding properties on ice, snow, and water can be achieved by means of directed friction and high pressure, as well as through tempering. The resulting hard, thin gliding layer of E-indigo does not easily absorb dirt and, thus, prevents a rapid increase in friction. Field tests on snow, with cross-country skis, have shown promising results in comparison to fluorinated and non-fluorinated waxes. Based on quantitative structure–activity relationship (QSAR) data for E-indigo, and its isomers and tautomers, it has been demonstrated that both the application and abrasion of the thin indigo layers are harmless to health, and are ecologically benign and, therefore, sustainable.
1. Self-Assembly of Indigo Molecules
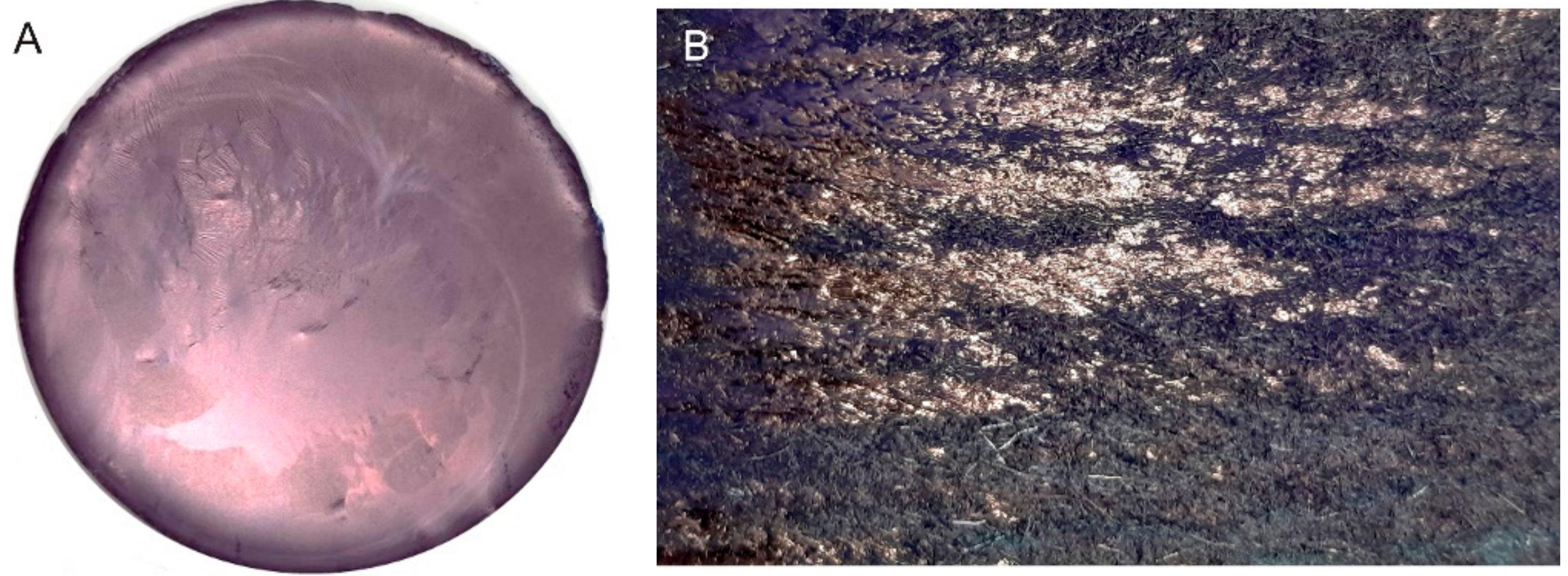 Figure 1. (A): indigo layer formed upon filtration. (B): pure E-indigo on a friction felt. The metallic luster observed on both photographic images indicates that larger indigo structures have formed by self-assembly from suspension or by friction, respectively.[6]
Figure 1. (A): indigo layer formed upon filtration. (B): pure E-indigo on a friction felt. The metallic luster observed on both photographic images indicates that larger indigo structures have formed by self-assembly from suspension or by friction, respectively.[6]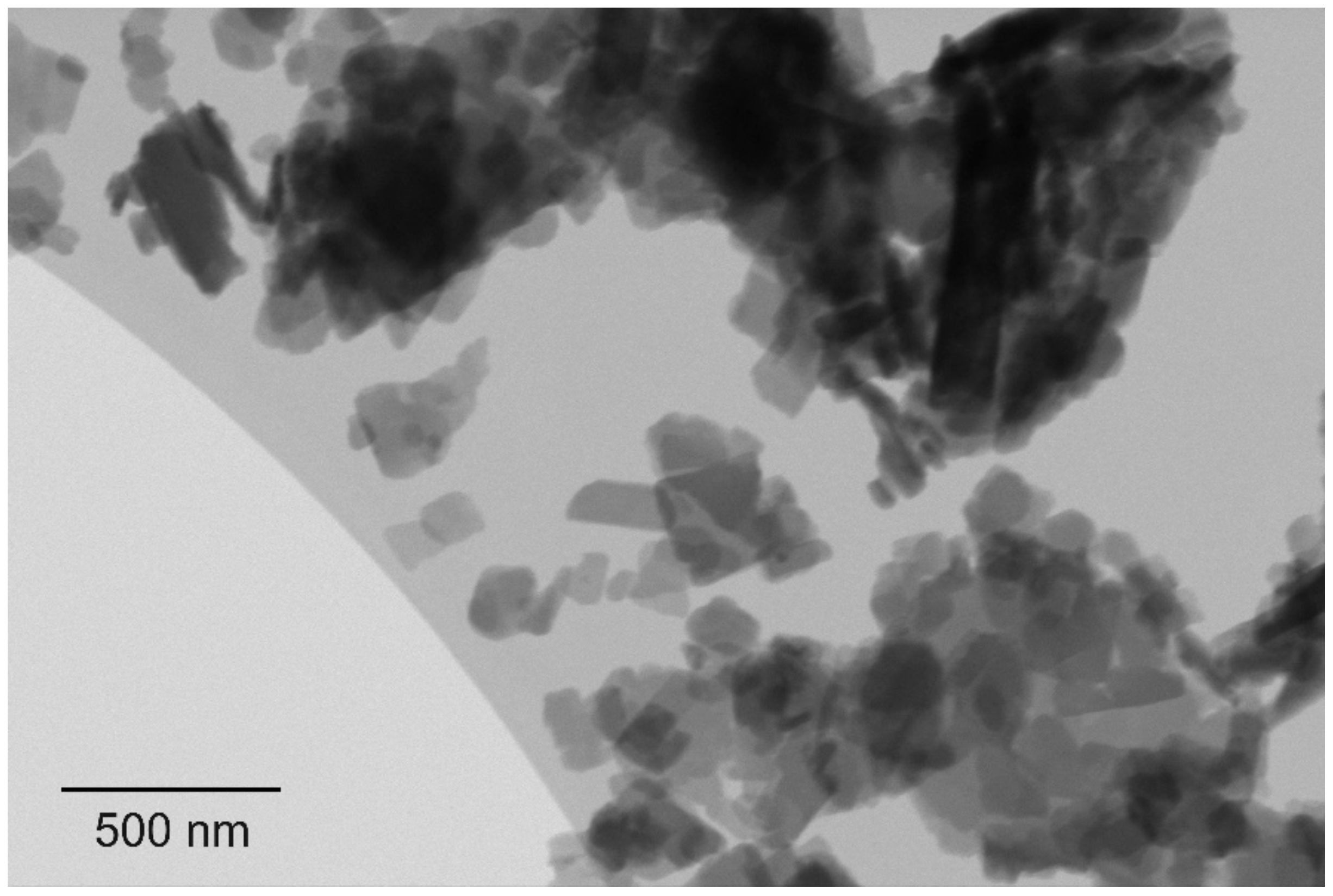 Figure 2. Scanning transmission electron microscopy image, showing primary particles of indigo with platelet morphology.[6]
Figure 2. Scanning transmission electron microscopy image, showing primary particles of indigo with platelet morphology.[6]2. Gliding Properties of Indigo
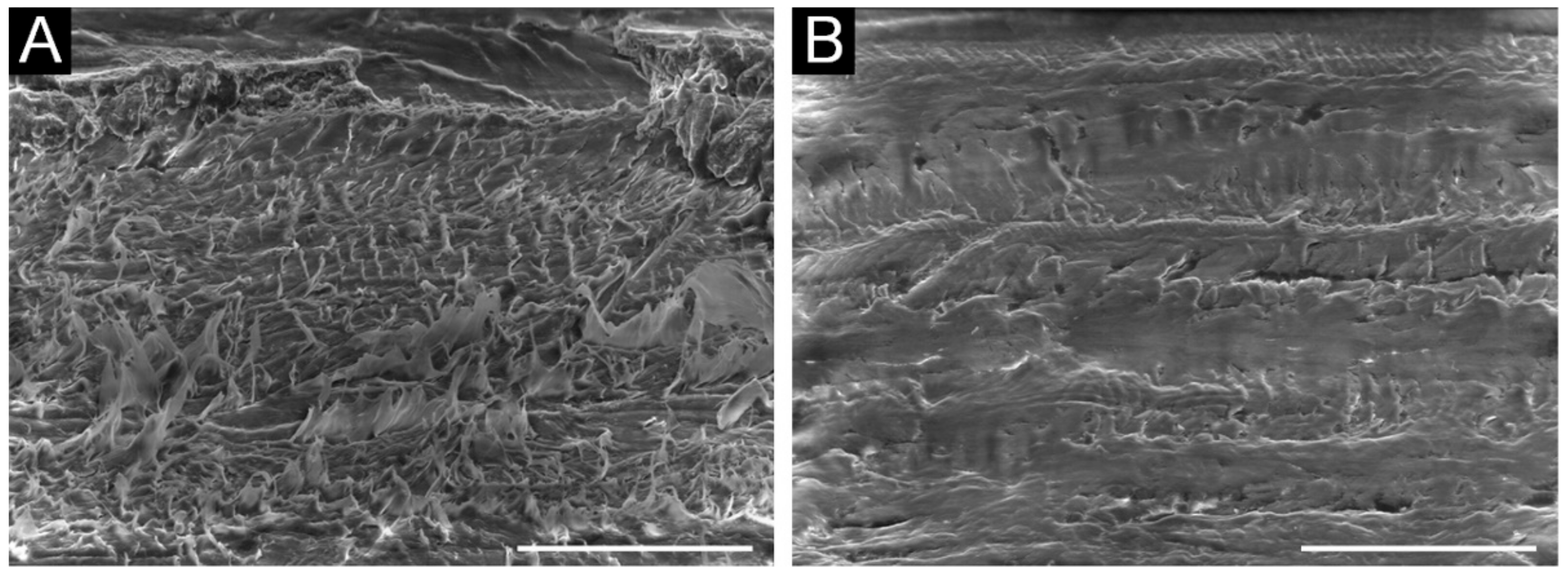 Figure 3. Scanning electron microscopy images showing the roughened surface of the ultra-high-molecular-weight polyethylene (UHMWPE) base before (A) and after coating with E-indigo (B). The scale bar is 100 µm (52° tilt view).[17]
Figure 3. Scanning electron microscopy images showing the roughened surface of the ultra-high-molecular-weight polyethylene (UHMWPE) base before (A) and after coating with E-indigo (B). The scale bar is 100 µm (52° tilt view).[17]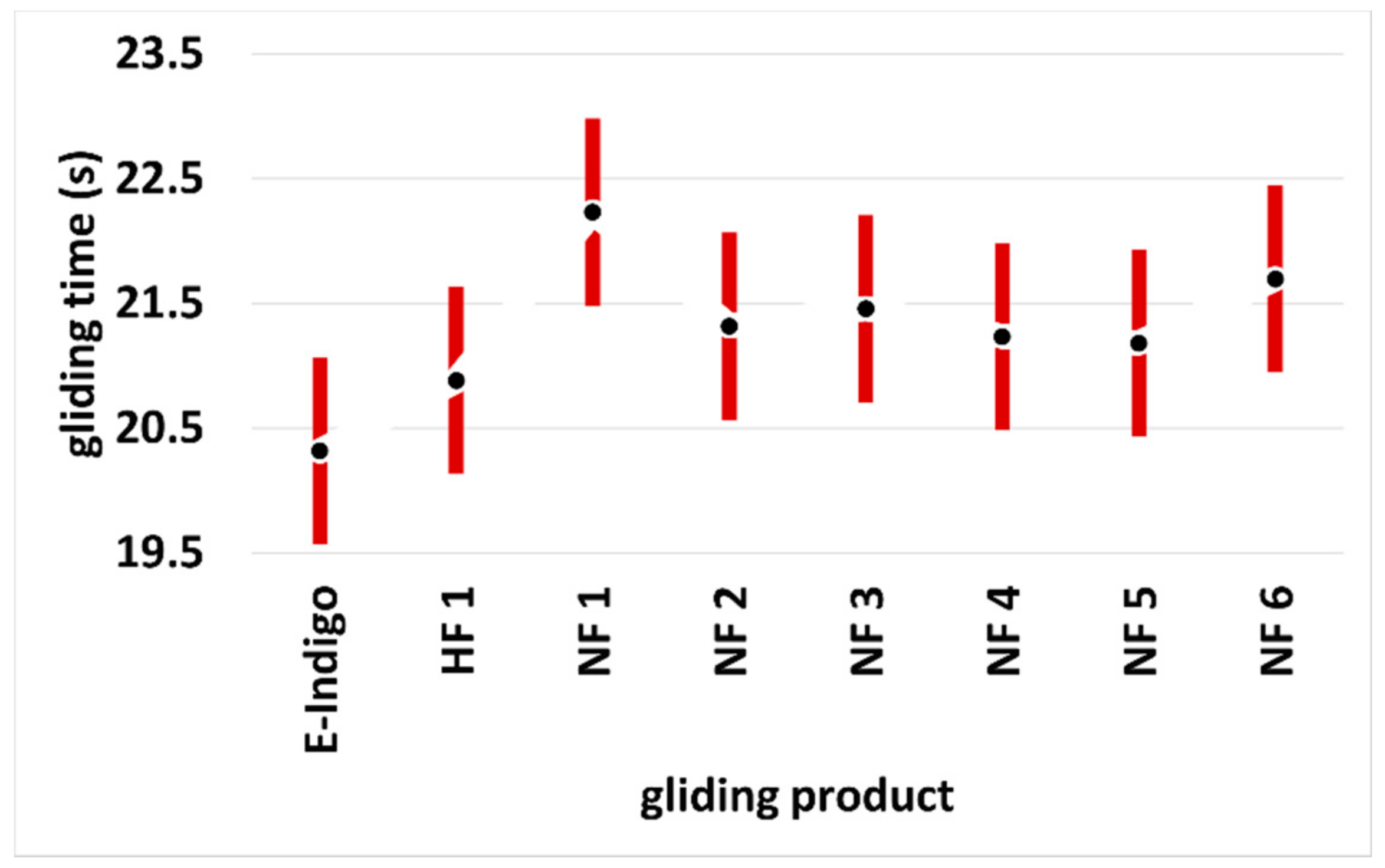 Figure 4. Running times over a measuring section for different ski gliding layers. Tests with calibrated cross-country skis (Lenzerheide, Switzerland, October 2020). The data points show the mean values with the 95% scatter bars. Snow temperature: −8 °C; air humidity: 75%; dry, old snow, grain size 0.5–0.8 mm, star-shaped grains, no dirt (HF: high fluor wax; NF: non-fluorinated wax).[18]
Figure 4. Running times over a measuring section for different ski gliding layers. Tests with calibrated cross-country skis (Lenzerheide, Switzerland, October 2020). The data points show the mean values with the 95% scatter bars. Snow temperature: −8 °C; air humidity: 75%; dry, old snow, grain size 0.5–0.8 mm, star-shaped grains, no dirt (HF: high fluor wax; NF: non-fluorinated wax).[18]3. Conclusions
References
- Ji Hye Lee; Dae-Hyun Cho; Bae Ho Park; Jin Sik Choi; Nanotribology of 2D materials and their macroscopic applications. Journal of Physics D: Applied Physics 2020, 53, 393001, 10.1088/1361-6463/ab9670.
- Mohammad R. Vazirisereshk; Ashlie Martini; David A. Strubbe; Mehmet Z. Baykara; Solid Lubrication with MoS2: A Review. Lubricants 2019, 7, 57, 10.3390/lubricants7070057.
- Binquan Luan; Ruhong Zhou; Wettability and friction of water on a MoS2 nanosheet. Applied Physics Letters 2016, 108, 131601, 10.1063/1.4944840.
- I. V. Klimovich; L. I. Leshanskaya; S. I. Troyanov; D. V. Anokhin; D. V. Novikov; A. A. Piryazev; D. A. Ivanov; N. N. Dremova; P. A. Troshin; Design of indigo derivatives as environment-friendly organic semiconductors for sustainable organic electronics. Journal of Materials Chemistry C 2014, 2, 7621-7631, 10.1039/c4tc00550c.
- Eric Daniel Głowacki; Gundula Voss; Lucia Leonat; Mihai Irimia-Vladu; Siegfried Bauer; Niyazi Serdar Sariciftci; Indigo and Tyrian Purple - From Ancient Natural Dyes to Modern Organic Semiconductors. Israel Journal of Chemistry 2012, 52, 540-551, 10.1002/ijch.201100130.
- M. Cadalbert; Analyse der Zusammensetzung und der Morphologie von Indigo-Partikeln im Hochleistungsgleitmittel Isantin; Zürich University of Applied Sciences: Wädenswil, Switzerland, 2021.
- Natalia Gospodinova; Elena Tomšík; Hydrogen-bonding versus π–π stacking in the design of organic semiconductors: From dyes to oligomers. Progress in Polymer Science 2015, 43, 33-47, 10.1016/j.progpolymsci.2014.10.010.
- Leonhard Mayrhofer; Gianpietro Moras; Narasimham Mulakaluri; Srinivasan Rajagopalan; Paul A. Stevens; Michael Moseler; Fluorine-Terminated Diamond Surfaces as Dense Dipole Lattices: The Electrostatic Origin of Polar Hydrophobicity. Journal of the American Chemical Society 2016, 138, 4018-4028, 10.1021/jacs.5b04073.
- Priscila Maria Dellamatrice; Maria Estela Silva-Stenico; Luiz Alberto Moraes; Marli Fiore; Regina Teresa Rosim Monteiro; Degradation of textile dyes by cyanobacteria. Brazilian Journal of Microbiology 2016, 48, 25-31, 10.1016/j.bjm.2016.09.012.
- M. Faraday; I. Note on regelation. Proceedings of the Royal Society of London 1860, 10, 440-450, 10.1098/rspl.1859.0082.
- F. P. Bowden; T. P. Hughes; The mechanism of sliding on ice and snow. Proceedings of the Royal Society of London. A. Mathematical and Physical Sciences 1939, 172, 280-298, 10.1098/rspa.1939.0104.
- Angelos Michaelides; Ben Slater; Melting the ice one layer at a time. Proceedings of the National Academy of Sciences 2017, 114, 195-197, 10.1073/pnas.1619259114.
- L. Canale; J. Comtet; A. Niguès; C. Cohen; C. Clanet; A. Siria; L. Bocquet; Nanorheology of Interfacial Water during Ice Gliding. Physical Review X 2019, 9, 041025, 10.1103/physrevx.9.041025.
- Lei Chen; Linmao Qian; Role of interfacial water in adhesion, friction, and wear—A critical review. Friction 2020, 9, 1-28, 10.1007/s40544-020-0425-4.
- Mikael Swarén; Lars Karlöf; Hans-Christer Holmberg; Anders Eriksson; Validation of test setup to evaluate glide performance in skis. Sports Technology 2014, 7, 89-97, 10.1080/19346182.2014.968164.
- Felix Breitschädel; A New Approach for the Grinding of Nordic Skis. Procedia Engineering 2015, 112, 385-390, 10.1016/j.proeng.2015.07.212.
- N. Al-Godari; Charakterisierung indigoider Gleitschichten auf UHMWPE; Zürich University of Applied Sciences: Wädenswil, Switzerland, 2020.
- B. Bruhin; A. Neff; Internal Test Report on Isantin; Swiss-Ski: Muri bei Bern, Switzerland, 2021.





