Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Fernando Calzada | + 1465 word(s) | 1465 | 2022-01-14 10:34:03 | | | |
| 2 | Jessie Wu | Meta information modification | 1465 | 2022-01-18 02:33:25 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Calzada, F. Antitumor Potential of Annona muricata Linn. Encyclopedia. Available online: https://encyclopedia.pub/entry/18364 (accessed on 07 February 2026).
Calzada F. Antitumor Potential of Annona muricata Linn. Encyclopedia. Available at: https://encyclopedia.pub/entry/18364. Accessed February 07, 2026.
Calzada, Fernando. "Antitumor Potential of Annona muricata Linn" Encyclopedia, https://encyclopedia.pub/entry/18364 (accessed February 07, 2026).
Calzada, F. (2022, January 17). Antitumor Potential of Annona muricata Linn. In Encyclopedia. https://encyclopedia.pub/entry/18364
Calzada, Fernando. "Antitumor Potential of Annona muricata Linn." Encyclopedia. Web. 17 January, 2022.
Copy Citation
Annona muricata (Am) is a plant used in traditional Mexican medicine to treat cancer. It is widely distributed in tropical and subtropical regions. A. muricata is known as “graviola”, “soursop”, and “guanabana” in Mexico is called “zapote de Viejas,” “catucho,” and “cabeza de negro.”
Annona muricata
cancer
1. Introduction
In Mexico, more than 90% of the population uses medicinal plants to treat their diseases, and between 30 and 70% of cancer patients are willing to use any product obtained from the medicinal plant considering that they perceive these as effective and safe due to their natural origin [1]. Mexico is a country with a great abundance and diversity of natural products that may be a potential source for the discovery of compounds with therapeutic applications [2]. It has been described that about 60% of the drugs approved for cancer have their origin in medicinal plants. There is a large amount of work linked to the search for medicinal plants that have anti-cancer properties, making the natural resources in Mexico an important source for obtaining potential agents for the treatment of cancer [3]. A. muricata (Figure 1) has become one of the best known, commercialized, and widely studied species in recent decades due to its therapeutic potential and traditional use [4][5][6].
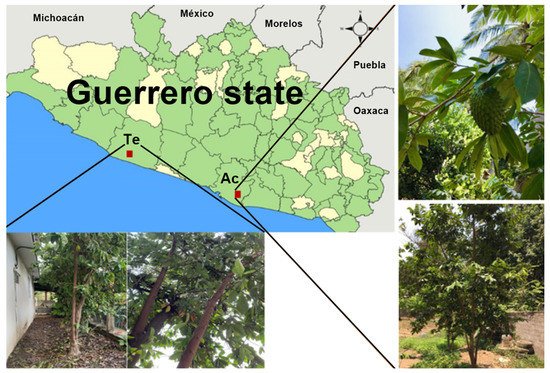
Figure 1. Map of Guerrero state showing Acapulco (Ac) and Tecpan de Galeana (Te) regions where the leaves of A. muricata were collected.
Their leaves, bark, fruit, seeds, and roots are used for various purposes in traditional medicine for the treatment of cancer, inflammation, skin diseases, analgesic, flu, asthma, colds, malaria, diarrhea, hypertension, and diabetes, among others [7]. Several studies of different extracts obtained from A. muricata (fruit, seeds, bark, roots, or leaves) have been reported. In this context, the isolation of roughly 212 secondary active metabolites including acetogenins, alkaloids, polyphenols, and flavonoids have been reported [8][9]. Acetogenins have been identified as one of the major bioactive compounds of A. muricata with activity on several cancer cell lines [10][11]. The ethanol extracts obtained from aerial parts from A. muricata in combination with doxorubicin showed important cytotoxic activity on 4T1 cells and other cancer cell lines [10][11][12][13][14][15][16][17]. Although there is an extensive amount of information about the cytotoxic properties of A. muricata, no information is available regarding its anti-cancer potential in specifically breast cancer using the extracts of plants collected in different periods of the year, and regarding the location of the place of recollection and its influences on their pharmacological and toxic properties. The aim of this study is to evaluate the cytotoxic and antitumor properties using 4T1 cells, BSL, as well as acute oral toxicity of twelve ethanol extracts of Am collected for a year in Acapulco (Ac) and Tecpan de Galeana (Te) in Guerrero state, Mexico.
2. Yield of the Extracts Obtained of the Leaves from Annona muricata
The yields of ethanolic extracts obtained of leaves from Annona muricata (Am) are shown in Table 1. The leaves from Annona muricata (Am) were collected in Acapulco and Tecpan de Galeana, in Guerrero state, Mexico, during the months of February (Fe), April (Ap), June (Ju), August (Ag), October (Oc), and December (De) of 2017. The results showed that best yields of the extract were obtained in April from samples collected in Acapulco and Tecpan de Galeana. In general, the best yields of ethanol extracts were obtained in the samples collected in Tecpan de Galeana, consistent with the characteristic subtropical zone. In contrast, Acapulco is a tropical zone with higher temperatures and higher relative air humidity than Tecpan de Galeana.
Table 1. Yield of extracts obtained of leaves from Annona muricata collected in Acapulco and Tecpan de Galeana in Guerrero, Mexico for one year *.
| Zone/Month | Extract ID | Fresh Weight of Leaves (g) | Ethanolic Extract Obtained (g) | Yield (%) |
|---|---|---|---|---|
| Acapulco/February | AcFe | 500 | 52.1 | 10.4 |
| Acapulco/April | AcAp | 500 | 62.4 | 12.5 |
| Acapulco/June | AcJu | 500 | 40.1 | 8.0 |
| Acapulco/August | AcAu | 500 | 42.4 | 8.5 |
| Acapulco/October | AcOc | 500 | 48.7 | 9.7 |
| Acapulco/December | AcDe | 500 | 48.0 | 9.6 |
| Tecpan/February | TeFe | 500 | 50.5 | 10.1 |
| Tecpan/April | TeAp | 500 | 73.1 | 14.6 |
| Tecpan/June | TeJu | 500 | 71.3 | 14.3 |
| Tecpan/August | TeAu | 500 | 42.4 | 8.5 |
| Tecpan/October | TeOc | 500 | 65.0 | 13.0 |
| Tecpan/December | TeDe | 500 | 50.0 | 10.0 |
* All plant material collection was carried out on 2017.
3. Cytotoxicity Assay
The results of cytotoxic activity against 4T1 (Table 2) show that the best effect was observed with the samples collected in Acapulco (AcDe, CC50 of 79.2 µg mL−1) and Tecpan de Galeana (TeDe, CC50 of 75.9 µg mL−1). Their activity was closer than doxorubicin (CC50 of 62.6 µg mL−1), the drug used as a positive control. The remaining extracts showed less activity (CC50 > of 87.5 µg mL−1). All extracts exhibited a dose-dependent cytotoxic effect (Figure 2). In general, a major effect was observable in the samples from Acapulco.
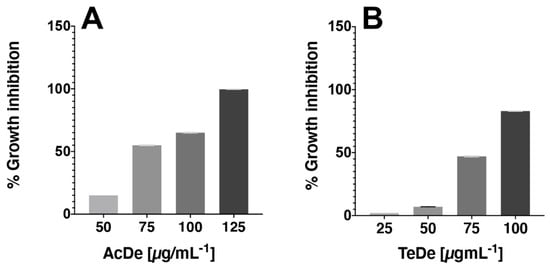
Figure 2. Representative WST-1 assay showing the cytotoxicity activity of AcDe (A) and TeDe (B) in 4T1 type of cancer cell after 24 h of incubation in vitro.
Table 2. Cytotoxic activity of twelve ethanol extracts obtained of the leaves from Annona muricata in CC50 (µg mL−1) against 4T1 cells.
| Treatment | CC50 (µg mL−1) | Treatment | CC50 (µg mL−1) |
|---|---|---|---|
| AcFe | 139.1 ± 2.2 | TeFe | 128.1 ± 0.4 |
| AcAp | 99.9 ± 0.2 | TeAp | 97.5 ± 0.3 |
| AcJu | 87.4 ± 0.5 | TeJu | 98.7 ± 0.1 |
| AcAg | 122.5 ± 0.3 | TeAg | 126. 3 ± 0.2 |
| AcOc | 89.1 ± 0.2 | TeOc | 133.7 ± 0.4 |
| AcDe | 79.2 ± 0.2 | TeDe | 75.9 ± 0.1 |
| Doxorrubicin | 62.6 ± 0.8 | 62.6 ± 0.8 |
Data are expressed as mean ± S. E. M. (n = 3); CC50: cytotoxic concentration 50.
4. Anti-Tumor Activity
The evaluation of the antitumor activity (Table 3) of the ethanol extracts from Am showed that after the oral administration of the samples, the most active extracts were obtained in December from Acapulco (AcDe, ED50 of 10.8 mg kg−1) and Tecpan de Galeana (TeDe, ED50 of 12.1 mg kg−1). The remaining extracts were less active with ED50 > 13.3 mg kg−1. All extracts exhibited a dose-dependent antitumor effect (Figure 3). In general, a major effect was observable in the samples from Acapulco.
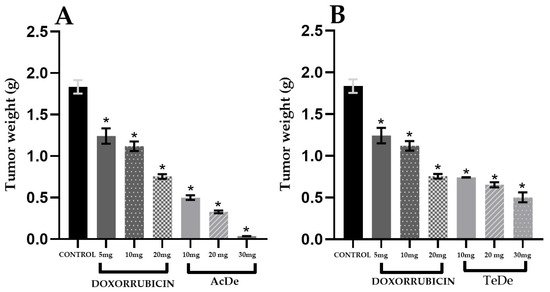
Figure 3. In vivo tumor 4T1 tumor bearing mice growth inhibition experiment (n = 6). Control: tumor weight harvested from untreated group (control), Doxorubicin: pharmacological control. (A) Tumor weight of group treated with different doses of AcDe; (B) Tumor weight of group treated with different doses of TeDe. Each value represents the mean ± standard error of the mean. * p < 0.05 vs. Control group.
Table 3. Results of antitumor activity of the ethanol extracts from A. muricata leaves in a breast cancer model with 4T1 cells.
| Treatment | ED50 (mg kg−1) | Treatment | ED50 (mg kg−1) |
|---|---|---|---|
| AcFe | 13.3 ± 0.06 | TeFe | 16.2 ± 0.42 |
| AcAp | 20.8 ± 0.49 | TeAp | 30.3 ± 2.18 |
| AcJu | 24.8 ± 0.20 | TeJu | 16.9 ± 1.06 |
| AcAg | 40.6 ± 1.9 | TeAg | 20.7 ± 0.22 |
| AcOc | 15.6 ± 0.32 | TeOc | 14.4 ± 0.46 |
| AcDe | 10.8 ± 0.014 | TeDe | 12.1 ± 0.19 |
| Doxorrubicin | 15.57 ± 0.76 | 15.57 ± 0.76 |
Data are expressed as mean ± S. E. M. (n = 6); ED50: effective doses 50.
Results (Table 4) show that the most active ethanol extracts in BSLA were the samples of June and August collected in Acapulco (AcJu, LC50 of 1.8 µg mL−1) and Tecpan (TeAg, LC50 of 2.4 µg mL−1), respectively. In general, the extracts obtained from the leaves of Am collected in Acapulco showed higher lethality than collected in Tecpan. The remaining extracts showed LC50 > 9.7 mg kg−1. In general, a major effect was observable in the samples from Acapulco.
Table 4. Brine shrimp lethality of the extracts from A. muricata leaves.
| Treatment | LC50 (µg mL−1) | Treatment | LC50 (µg mL−1) |
|---|---|---|---|
| AcFe | 44.7 ± 0.89 | TeFe | 60.5 ± 3.36 |
| AcAp | 47.3 ± 0.06 | TeAp | 40.5 ± 0.29 |
| AcJu | 1.8 ± 0.34 | TeJu | 39.6 ± 1.91 |
| AcAg | 9.7 ± 0.80 | TeAg | 2.4 ± 0.62 |
| AcOc | 23.4 ± 1.50 | TeOc | 36.2 ± 5.39 |
| AcDe | 22.9 ± 1.50 | TeDe | 50.6 ± 0.32 |
| Doxorrubicin | >500 | >500 |
Data are expressed as mean LC50 ± S. E. M. (n = 3).
5. Acute Oral Toxicity and Therapeutic Index
The acute oral toxicity was evaluated in agreement with OECD guidelines 423 for the use of natural products in human consumption. The results (Table 5) show that the most toxic samples were those collected in the months of June and August from Acapulco (AcJu and AcAg) and Tecpan (TeJu and TeAg). In contrast, the samples from AcDe and TeDe showed less toxicity. In agreement with acute toxicity and the therapeutic index the most secure extracts were AcDe and TeDe.
Table 5. Acute oral toxicity and therapeutic index of the extracts from A. muricata leaves.
| Treatment | LD50 (mg kg−1) | TIa | Treatment | LD50 (mg kg−1) | TI a |
|---|---|---|---|---|---|
| AcFe | 1585 | 119.2 | TeFe | 1585 | 97.8 |
| AcAp | 1585 | 76.2 | TeAp | 1585 | 52.5 |
| AcJu | 165 | 6.7 | TeJu | 232 | 13.7 |
| AcAg | 165 | 4.1 | TeAg | 232 | 11.2 |
| AcOc | 1515 | 97.1 | TeOc | 1585 | 110.1 |
| AcDe | 1515 | 140.7 | TeDe | 1585 | 131.0 |
Correlation coefficient > 0.9500; Data are expressed as mean ± S. E. M. (n = 3); LD50: Lethal dose 50. a TI: therapeutic index calculated as LD50 (acute oral toxicity)/ED50 (antitumor activity).
6. HPLC Analysis of the Ethanol Extracts
Analysis of the ethanol extracts, AcDe and TeDe, was performed using high-performance liquid chromatography with diode array detection (HPLC-DAD) and standards of flavonol glycosides, rutin, nicotiflorin, and narcissin. The analysis showed the presence of rutin (32.88 min), nicotiflorin (34.10 min), and narcissin (34.76 min) in AcDe and TeDe (Figure 4). The identification was made by comparing their retention times, UV spectrum (Figure 5), and thin layer chromatography. All extracts showed the presence of rutin, nicotiflorin, and narcissin (Figure 6 and Figure 7).
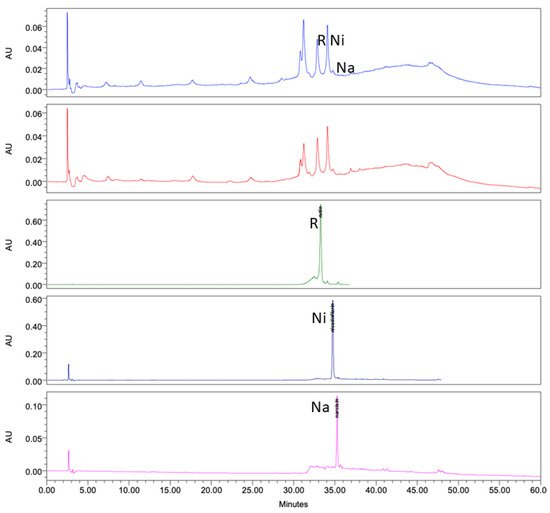
Figure 4. High-performance liquid chromatography with diode-array detection (HPLC-DAD) analysis at 254 nm of ethanol extract from A. muricata leaves. TeDe (blue) and AcDe (red); flavonol glycosides standards, rutin (R, green), nicotiflorin (Ni, gray), and narcissin (Na, magenta).
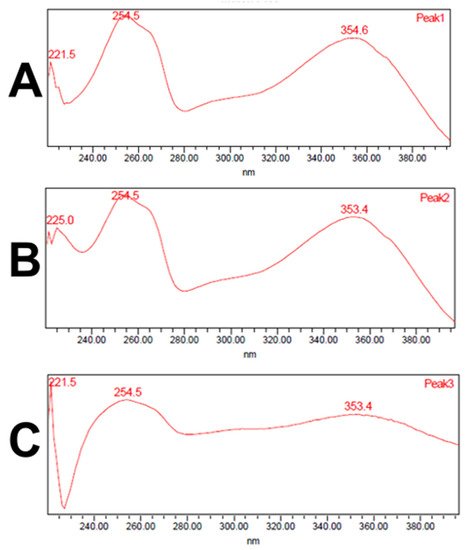
Figure 5. UV spectrum obtained from HPLC-DAD analysis of rutin (A), nicotiflorin (B), and narcissin (C).
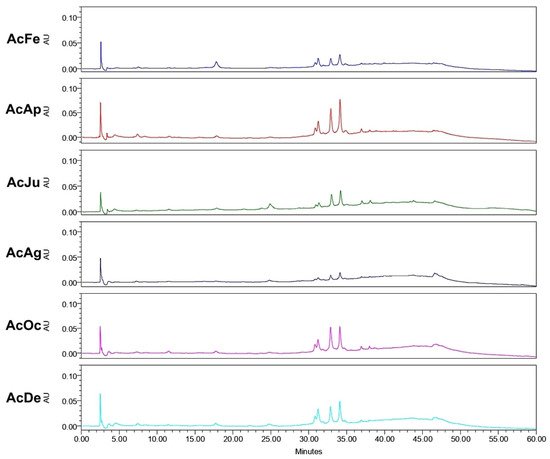
Figure 6. High-performance liquid chromatography with diode-array detection (HPLC-DAD) analysis of ethanol extracts from A. muricata leaves collected in Acapulco, Mexico.
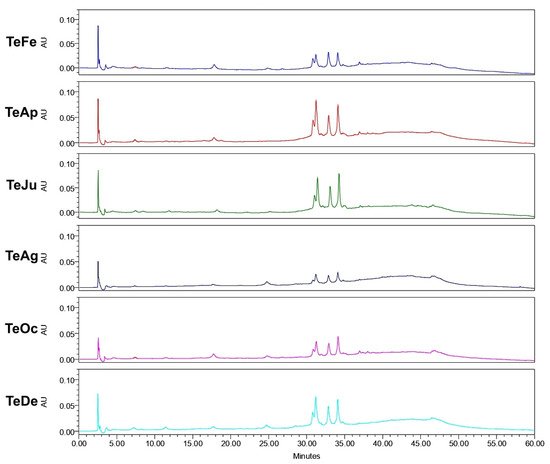
Figure 7. High-performance liquid chromatography with diode-array detection (HPLC-DAD) analysis of ethanol extracts from A. muricata leaves collected in Tecpan de Galeana, Mexico.
References
- Alonso-Castro, A.J.; Villarreal, M.L.; Salazar-Olivo, L.A.; Gomez-Sanchez, M.; Dominguez, F.; Garcia-Carranca, A. Mexican medicinal plants used for cancer treatment: Pharmacological, phytochemical and ethnobotanical studies. J. Ethnopharmacol. 2011, 133, 945–972.
- World Health Organization. WHO Traditional Medicine Strategy: 2014–2023. Available online: https://www.who.int/publications/i/item/9789241506096 (accessed on 6 November 2021).
- Loraine, S.; Mendoza-Espinoza, J.A. Las plantas medicinales en la lucha contra el cáncer, relevancia para México. Rev. Mex. de Cienc. Farm. 2010, 41, 18–27.
- Qazi, A.K.; Siddiqui, J.A.; Jahan, R.; Chaudhary, S.; Walker, L.A.; Sayed, Z.; Jones, D.T.; Batra, S.K.; Macha, M.A. Emerging therapeutic potential of graviola and its constituents in cancers. Carcinogenesis 2018, 39, 522–533.
- Quílez, A.M.; Fernández-Arche, M.A.; García-Giménez, M.D.; De la Puerta, R. Potential therapeutic applications of the genus Annona local and traditional uses and pharmacology. J. Ethnopharmacol. 2018, 225, 244–270.
- Errayes, A.; Mohammed, W.; Darwish, M. Review of phytochemical and medical applications of Annona muricata Fruits. J. Chem. Rev. 2020, 2, 70–79.
- Gavamukulya, Y.; Wamunyokoli, F.; El-Shemy, H.A. Annona muricata: Is the natural therapy to most disease conditions including cancer growing in our backyard? A systematic review of its research history and future prospects. Asian Pac. J. Trop. Med. 2017, 10, 835–848.
- Coria-Téllez, A.V.; Montalvo-Gónzalez, E.; Yahia, E.M.; Obledo-Vázquez, E.N. Annona muricata: A comprehensive review on its traditional medicinal uses, phytochemicals, pharmacological activities, mechanisms of action and toxicity. Arab. J. Chem. 2018, 11, 662–691.
- Nayak, A.; Hegde, K. Comprehensive review on the miracle nature of Annona muricata Linn. J. Pharm. Sci. 2021, 11, 1–8.
- Rady, I.; Bloch, M.B.; Chamcheu, R.-C.N.; Banang Mbeumi, S.; Anwar, M.R.; Mohamed, H.; Babatunde, A.S.; Kuiate, J.-R.; Noubissi, F.K.; El Sayed, K.A.; et al. Anticancer properties of graviola (Annona muricata): A comprehensive mechanistic review. Oxid. Med. Cell Longev. 2018, 2018, 1826170.
- Abdul Wahab, S.M.; Jantan, I.; Haque, M.A.; Arshad, L. Exploring the leaves of Annona muricata L. as a source of potential anti-inflammatory and anticancer agents. Front. Pharmacol. 2018, 9, 661.
- Chan, W.-J.J.; McLachlan, A.J.; Hanrahan, J.R.; Harnett, J.E. The safety and tolerability of Annona muricata leaf extract: A systematic review. J. Pharm. Pharmacol. 2019, 72, 1–16.
- Salsabila, I.; Nugraheni, N.; Ahlina, F.; Haryanti, S.; Meiyanto, E. Synergistic cotreatment potential of soursop (Annona muricata L.) leaves extract with Doxorubicin on 4T1 cells with antisenescence and anti-reactive-oxygen-species properties. Iran. J. Pharm. Res. 2021, 20, 57–67.
- González-Pedroza, M.G.; Argueta-Figueroa, L.; García-Contreras, R.; Jiménez-Martínez, Y.; Martínez-Martínez, E.; Navarro-Marchal, S.A.; Marchal, J.A.; Morales-Luckie, R.A.; Boulaiz, H. Silver nanoparticles from Annona muricata peel and leaf extracts as a potential potent, biocompatible and low cost antitumor tool. Nanomaterials 2021, 11, 1273.
- Syed Najmuddin, S.U.F.; Romli, M.F.; Hamid, M.; Alitheen, N.B.; Nik Abd Rahman, N.M.A. Anti-cancer effect of Annona muricata Linn leaves crude extract (AMCE) on breast cancer cell line. BMC Complement. Altern. Med. 2016, 16, 311.
- Alshaeri, H.K.; Alasmari, M.; Natto, Z.; Pino-Figueroa, A. Effects of Annona muricata extract on triple-negative breast cancer cells mediated through EGFR signaling. Cancer Manag. Res. 2020, 12, 12519–12526.
- Akpan, U.M.; Pellegrini, M.; Salifu, A.A.; Obayemi, J.D.; Ezenwafor, T.; Browe, D.; Ani, C.J.; Danyuo, Y.; Dozie-Nwachukwu, S.; Odusanya, O.S.; et al. In vitro studies of Annona muricata L. extract-loaded electrospun scaffolds for localized treatment of breast cancer. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 2041–2056.
More
Information
Subjects:
Others
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
2 times
(View History)
Update Date:
18 Jan 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




