1000/1000
Hot
Most Recent

Notwithstanding the progress regarding wound-healing management, the treatment of the majority of skin lesions still represents a serious challenge for biomedical and pharmaceutical industries. Thus, the attention of the researchers has turned to the development of novel materials based on cellulose derivatives. Cellulose derivatives are semi-synthetic biopolymers, which exhibit high solubility in water and represent an advantageous alternative to water-insoluble cellulose. These biopolymers possess excellent properties, such as biocompatibility, biodegradability, sustainability, non-toxicity, non-immunogenicity, thermo-gelling behavior, mechanical strength, abundance, low costs, antibacterial effect, and high hydrophilicity. They have an efficient ability to absorb and retain a large quantity of wound exudates in the interstitial sites of their networks and can maintain optimal local moisture. Cellulose derivatives also represent a proper scaffold to incorporate various bioactive agents with beneficial therapeutic effects on skin tissue restoration. Due to these suitable and versatile characteristics, cellulose derivatives are attractive and captivating materials for the development of multiple biomedical and pharmaceutical applications, such as wound dressings, drug delivery devices, and tissue engineering.
In past years, due to the technology's noteworthy progress, various wound dressings were formulated worldwide to cure all types of tissue lesions. Dressings play a fundamental role in wound healing management, because these protect tissue lesions from external invasion (wound dressings are permeable for oxygen and moisture and function as physical barriers) [26], preventing the infection on the wound site [27]. Moreover, dressings contribute to the regeneration and restoration of epidermis and dermis layers [28][29].
For the development of dressings, which allow rapid healing, with minimal scars on the body surface, it is necessary to develop new biopolymeric materials that accomplish some properties to create the ideal wound dressing that are reviewed in Figure 1.
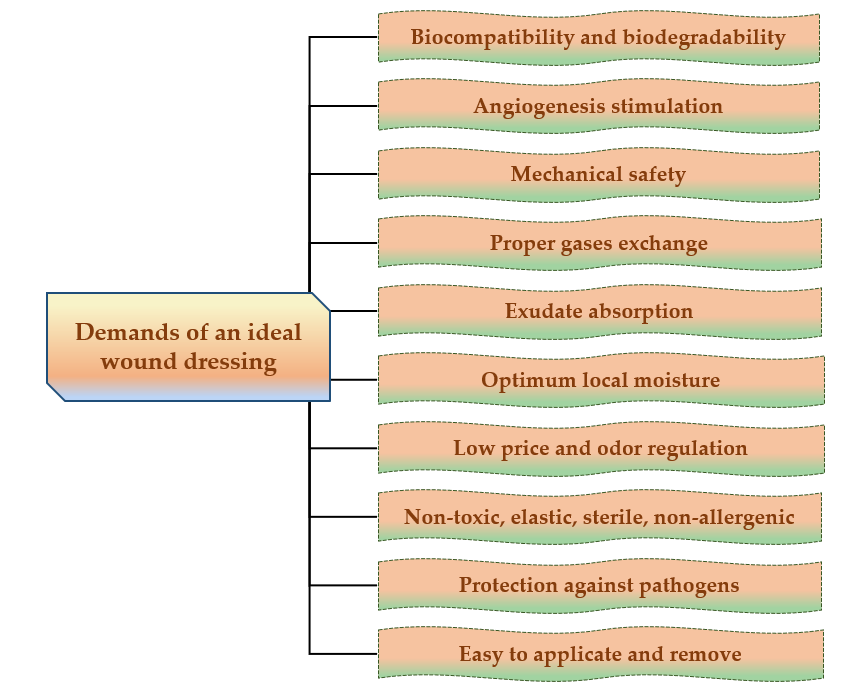
Figure 1. Major demands of an ideal wound dressing.
The ideal wound dressing preferably presents the following features: biocompatibility, biodegradability, non-toxicity, chemical inertness [30], to be applied effortlessly, to have the capacity to keep local moisture, to ensure a suitable exchange of gases (O2 and CO2), to absorb exudates that form on the lesion site [31], to stimulate the angiogenesis, to protect against extraneous pathogens, to clear the injured tissue, to eliminate nonviable tissues, to reduce the exposed area [32], to be able to be removed and replaced without difficulty [33], to adjust the odor, to sustain an adequate temperature to the lesion bed, to promote the blood circulation, and to stimulate cell expansion, to ensure mechanical safety [34]. Also, wound dressings materials must be elastic, sterile, non-adherent, non-allergenic [35], to have an acceptable price and to provide thermal insulation [36].
A potential classification of wound dressings comprises passive dressings and active dressings, depending on the presence or absence of one or more pharmacologically active substances or natural substances [37], which can act to the site of the lesion, with local or systemic action, conditioned by the depth of the wound. Moreover, the progress of manufacturing led to the evolution of wound dressings from traditional dressings to modern (advanced) dressings [38].
Passive dressings can be considered dry traditional dressings, which are fundamental for a faster wound healing process. There is a wide simple range of passive dressings for several types of skin lesions: cotton wool, lint, gauze, natural and synthetic bandages – they work as primary dressing or secondary dressing [34][39]. Active dressings contain a large variety of pharmacologically active substances (antibiotics or other antimicrobials, non-steroidal anti-inflammatory, analgesic, antifungal, and local anesthetics drugs) or natural substances (plant extracts) with anti-inflammatory, astringent, emollient, epithelializing, antioxidant, demulcent, antimicrobial properties [28].
Modern or advanced dressings were designed to cover tissue lesions and in this category are included the hydrogels, hydrocolloids, semi-permeable films, semi-permeable foams, and alginate dressings [40][41]. The biggest difference between traditional and modern dressings is the local moisture maintenance. Thus, traditional dressings have a lower capacity to maintain the local moisture on the wound site [38], and modern dressings sustain excellent local moisture to enhance wound healing [42].The classification of wound dressings is illustrated in Figure 2.
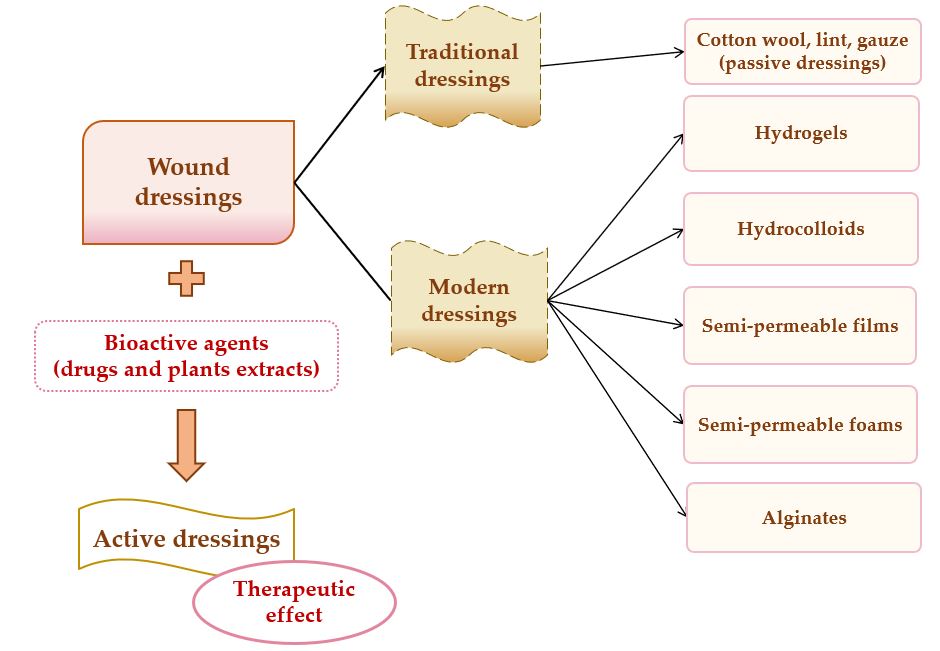
Figure 2. Wound dressings classification.
The main materials underlying the modern wound dressings are polymers, which can be natural (collagen, gelatin, cellulose, hemicellulose, chitin, chitosan, pectins and gums, chondroitin sulfate, alginic acid and alginates, agar, dextran, carrageenan, elastin, hyaluronic acid, silk fibroin, fibrinogen, and fibrin) [43][44], semi-synthetic (cellulose derivatives) [45] or synthetic (poly(α-ester)s, polyanhydrides, polycarbonates, poly(amide), poly(esteramide)s, polyphosphazenes, polyurethanes, pseudo poly(amino acids), polyacetals) [46][47][48].
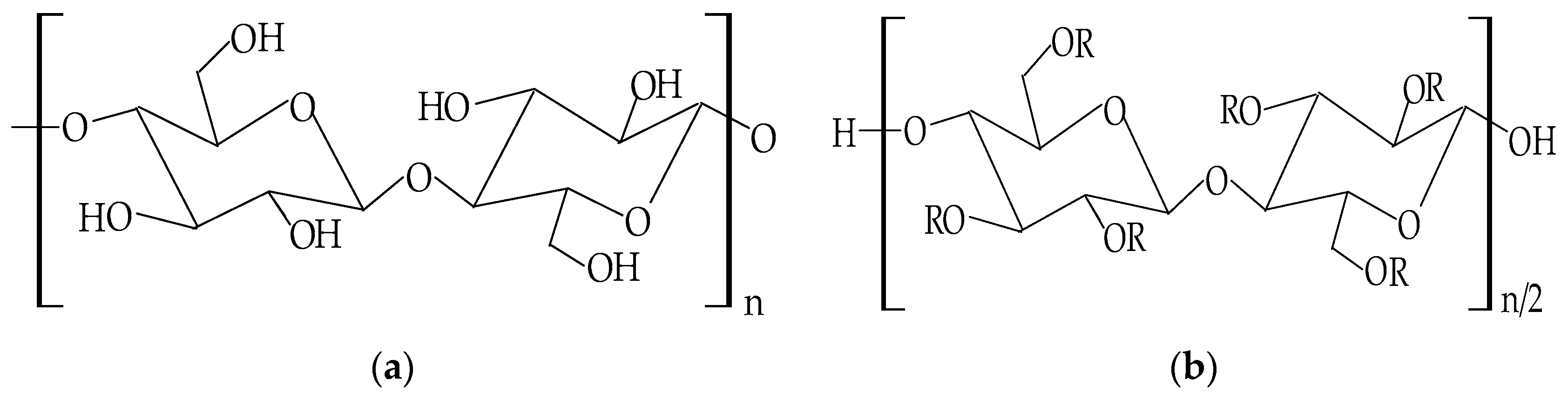
| Cellulose Ethers | R Groups |
|---|---|
| Methylcellulose | H, CH3 |
| Ethylcellulose | H, CH2CH3 |
| Benzylcellulose | H, C6H5CH2 |
| Sodium carboxymethylcellulose | H, CH2COONa |
| Hydroxyethylcellulose | H, [CH2CH2O]nH |
| Hydroxypropylcellulose | H, [CH2CH(CH3)O]nH |
| Hydroxyethylmethylcellulose | H, CH3, [CH2CH2O]nH |
| Hydroxypropylmethylcellulose | H, CH3, [CH2CH(CH3)O]nH |
| Cellulose Esters | R Groups |
|---|---|
| Acetate | H, I |
| Acetate trimelliate | H, I, II |
| Acetate phthalate | I, III |
| Hydroxypropylmthylphthalate | H, CH3, CH2CH(OH)CH3, III, IV |
| Hydroxypropylmthylphthalate acetate succinate |
H, CH3, CH2CH(OH)CH3, III, V |
Due to the general properties of wound dressings presented in Section 2.1., but also the particular properties, such as hydrophilicity, mechanical toughness, pH stability, and rheological characteristics, cellulose and cellulose derivatives have multiple applications in many fields [67]. Areas of the applicability of all these biopolymers involve: biomedical and pharmaceutical industries, where they can act as drug-delivery devices, wound dressings, muco- and bioadhesive drugs, excipients for drug formulations, and support for tissue engineering [68]; also, they can be used for cosmetic and hygienic products, in the textile area, in the food industry and agriculture [64][69]. The representation of cellulose derivatives-based wound dressing on an open wound is illustrated in Figure 4.
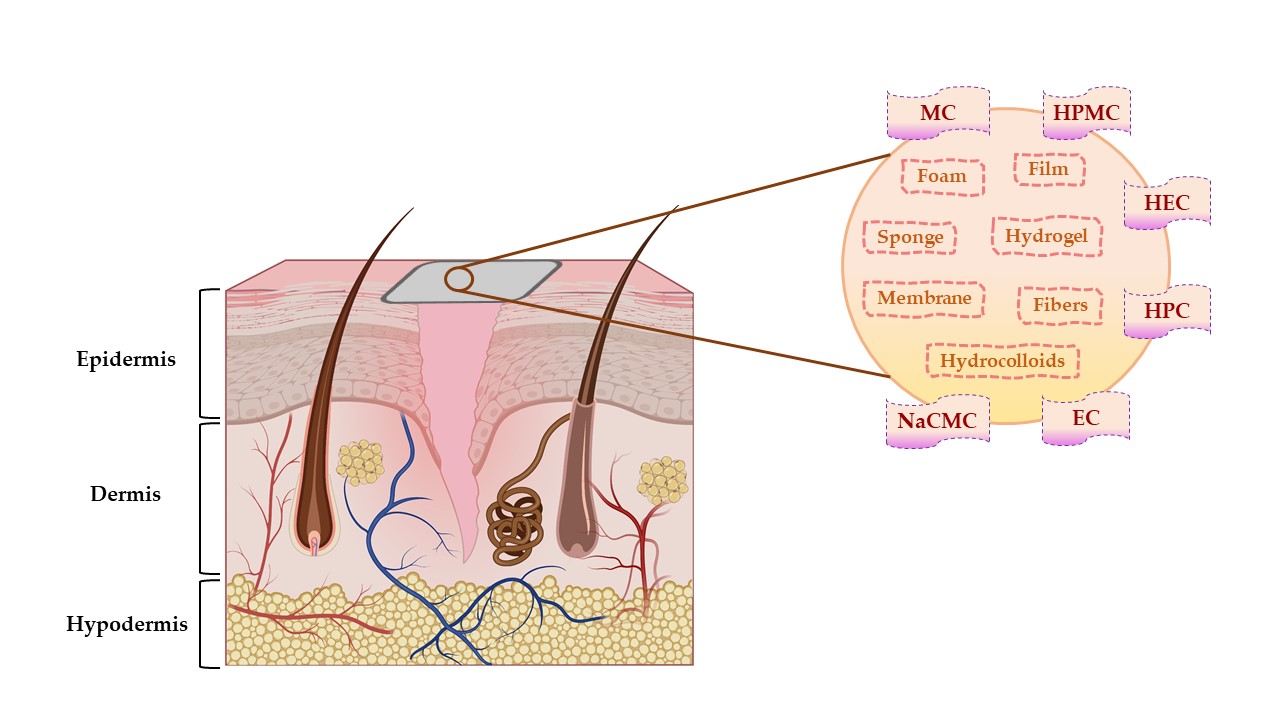
Figure 4. The representation of cellulose derivatives-based wound dressing on an open wound. This illustration has been created with BioRender.com, Inkscape, and PowerPoint.
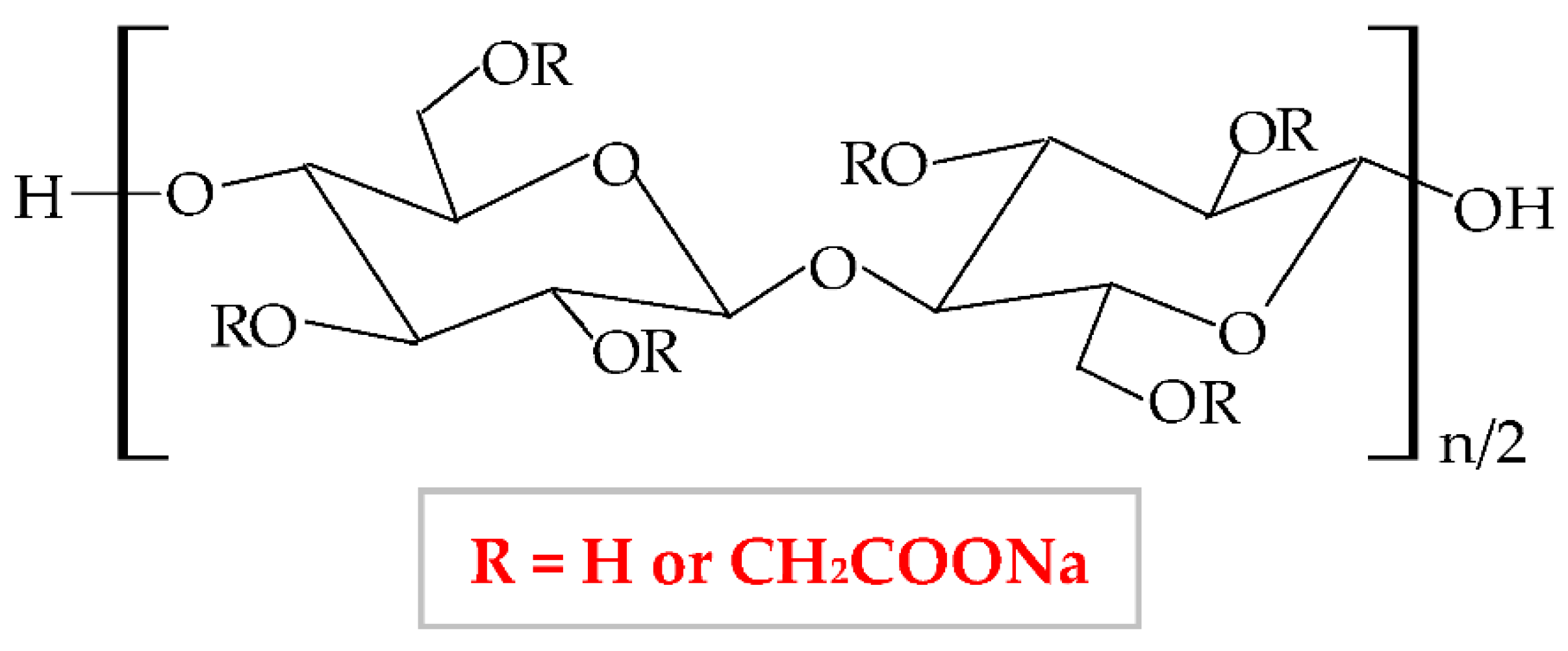
Figure 5. Chemical structure of sodium carboxymethylcellulose (NaCMC).
The NaCMC network illustrates a thixotropic behavior to generate 3D structures through intermolecular attraction. Its thixotropy is influenced by concentration and degree of substitution [76]. NaCMC presents excellent physicochemical and mechanical properties [77], optimal biocompatibility and biodegradability, proper capacity to absorb the water and to swell, high gelation behavior, non-toxicity, and low-immunogenicity [78]. It is the most used cellulose derivative in the pharmaceutical industry, mainly for the development of new wound dressings because it has the capacity to absorb heavy exudates [79][80], to ensure excellent moisture at the lesion site, and to avoid skin tissues water loss and tissues necrosis. Moreover, an optimal local humidity can impede dehydration, facilitate the synergy between target cells and growth factors, promote angiogenesis advancement, the mitigation of the ache, and the disruption of the fibrin network [81]. NaCMC is also used as a drug-delivery device and excipient for drug formulations (used as an emulsifier, thickener, stabilizer, and film-maker) [82]. Besides its applicability in the pharmaceutical area, this biopolymer possesses different usefulness in the food (E466 food additive) industry [83], in paper, textile and cosmetics domains [73][84], for tissue culture and dental medicine field [85].NaCMC can be combined with other polymers to enhance its properties and to develop its applicability. Thus, it is more advantageous to blend two or more polymers for the development of a new material comparative to the chemical industrial development of that material. Moreover, the new material obtained by mixing other well-known polymers presents all the properties or is more favorable than the component polymers [30]. Furthermore, the blend of polymers can be realized to compensate for their drawbacks. Hence, Liu et al., combined NaCMC with HEC by electrostatic complexing and obtained a sponge and a membrane with a porous network, enhanced viscoelastic properties, and high swelling behavior [86]. Hu et al., mixed NaCMC with PVA and quaternized chitosan and designed a new composite with enhanced flexibility, water absorption rate, mechanical strength, swelling ratio, and humidity permeability [87]. A novel NaCMC/PVA-based composite was formulated, with higher properties than two polymers: improved swelling capacity, elasticity, water solubility, porosity, water vapor transmission rate, bioavailability, and biodegradability for the tissue repair process; this formulation also presented an extension of its applicability, such as agriculture, biomedical field as drug delivery systems and food packaging [88][89]. NaCMC was blended with PEG through a photo-click reaction based on thiol-norbornene. It formed a pH-sensitive hydrogel with an augmented swelling ratio [85]. Zhang et al., designed a novel hydrogel based on NaCMC and sodium alginate. In a ratio of 1:4, the hydrogel exhibited high biocompatibility, mechanical characteristics, degradation rate, and local humidity [90]. Shin et al., blended NaCMC with PVA and PEG 400 through cyclic freezing/thawing method and obtained a hydrogel with improved properties: the swelling rate, the compressive strength, and cytocompatibility [91].
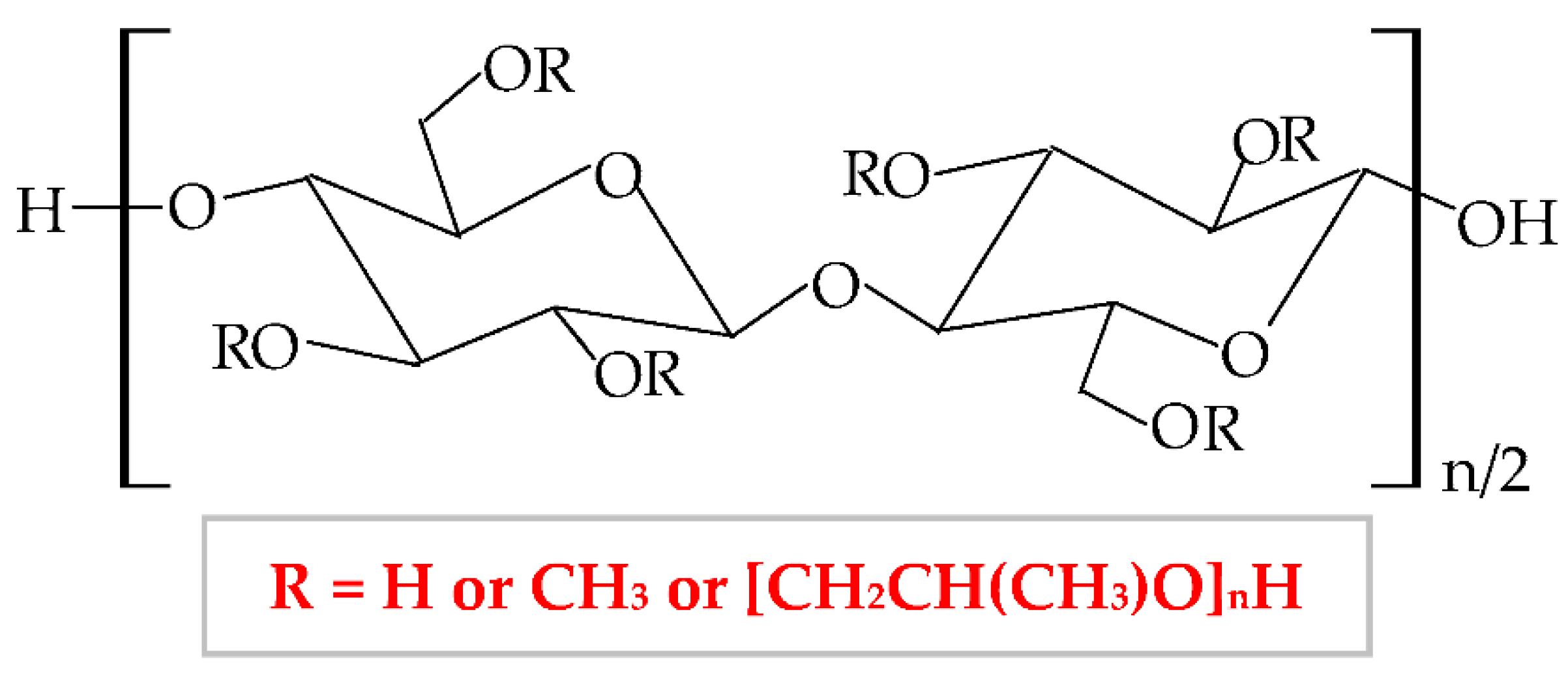
Therefore, HPMC presents many degrees of substitution, that give to this biopolymer different molecular weight and physicochemical features (rheological properties and crystalline nature) [95][96]. The hydrophilic or hydrophobic nature is related to the values of the degree of substitution (DS) and the molar substitution (MS). Thus, the HPMC molecule with decreased values of DS and MS is more hydrophilic and the HPMC molecule with increased values of DS and MS is more hydrophobic [97]. Following this chemical substitution, HPMC gets both polar (hydroxypropyl) and non-polar (methyl) character; consequently, it can form hydrophobic, intermolecular, and intramolecular linkages with many other materials [95]. The non-ionic character leads to a limited adhesive capacity [98]. At high temperature, the biopolymer can suffer a thermoreversible phase transition from sol to gel, with a temperature of gelation over 60°C, superior to the temperature of the body (37°C) [99]. HPMC-based hydrogels are temperature-responsive [100].
According to United States Pharmacopeia (USP), there are four distinct forms of HPMC, which are categorized by the content of methoxy, respectively hydroxypropoxy groups in: HPMC 1828, HPMC 2208, HPMC 2906, and HPMC 2910 [101]. This biopolymer has been approved as a food additive, E464 [102], by the American Institute, Food and Drug Administration (FDA), by the European Institution, European Parliament, and Council Directive, and by the Joint Expert Committee on Food Additives [103].
HPMC has a proper solubility in water, and it is one of the most used cellulose derivatives in many industries. It is widely used in the biotechnological field (construction, food, cosmetics, biomedical, and pharmaceutical industry), due to its excellent characteristics, such as biocompatibility, biodegradability, superior stability, large availability, excellent swelling, high surface activity, and mechanical properties [104], remarkable ability to form films and poor toxicity [105]. Regarding the applicability of HPMC in biomedical and pharmaceutical domains, it is used as a drug-delivery device, with a large practice for wound dressings development and it can also have remarkable applicability in tissue engineering [106]. HPMC can also be used as an excipient because it possesses proper abilities of emulsification, stabilization, suspension, and thickening [107][108].
HPMC can be combined with other polymers to enhance its properties and to develop its applicability [109]. To improve the physicochemical properties of a new composite, HPMC has been blended with several natural, semi-synthetic, or synthetic polymers [110]. In this way, to improve the thermal stability, HPMC has been blended with collagen [111][112], gelatin [109], chitosan [113], chitosan, and xanthan gum [114]; to improve the mechanical properties (tensile strength and ultimate elongation), HPMC has been mixed with chitosan [115], collagen [112], poloxamer 407 [116], silk fibroin [117], PVA and PVP [118], chitosan and xanthan gum [114]; to increase the swelling rate, HPMC has been combined with methylcellulose [119], κ-carrageenan [120], chitosan and hyaluronic acid [121], chitosan and xanthan gum [114].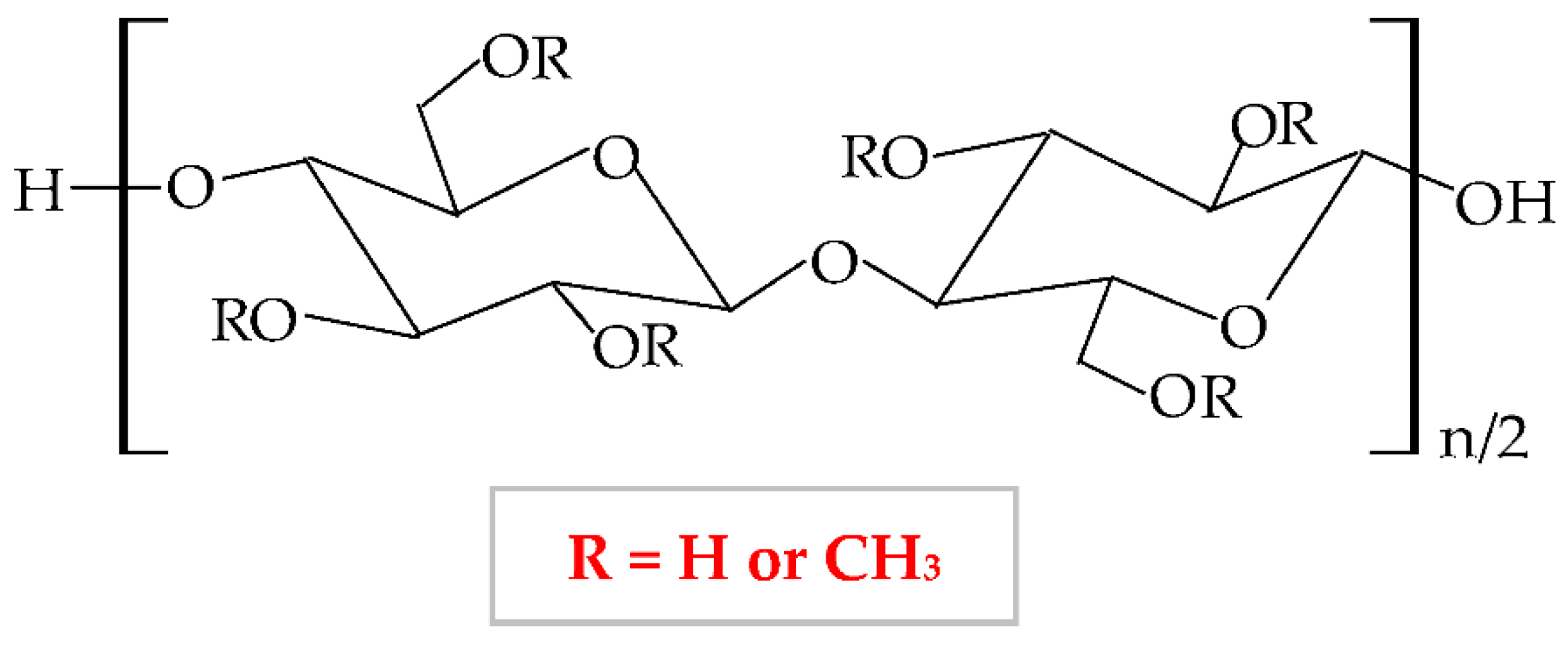
At a variation of temperature, MC has a thermo-sensitive behavior with a reversible sol-gel transition in an aqueous solution [125]. At a lower temperature than lower critical solution temperature, it realizes the hydration of the MC network in solution, with the formation of hydrogen bonds. At a higher temperature than lower critical solution temperature, the MC aqueous solution takes in the heat, with the disintegration of hydrogen bonds [100]. Thus, MC presents increased viscosity at higher temperatures, and at lower temperatures it exhibits a reduced viscosity [126].
The degree of substitution for commercial MC varies from 1.7 to 2.2 when it results in a semiflexible biopolymer because the inter-and intra- hydrogen bonds from cellulose molecule break off [127]. There are many substances, which influence the gelation behavior of MC, such as inorganics salts, ethanol, propylene glycol, polyethylene glycol 400, sucrose, glycerin, sorbitol, and different surfactants (sodium dodecyl sulfate and cetyltrimethylammonium bromide) [128]. MC is extensively used in biomedical, pharmaceutical, cosmetic, and food industries as a thickening, binding, and film-forming agent because it possesses excellent biocompatibility, biodegradability, and reduced toxicity [129][130][131].
To improve the characteristics of MC, it can be blended with other polymers in different ratios to enhance the physicochemical, morphological, and structural properties of both polymers and of the resulting composite [131]. Abu et al., illustrated that a higher concentration of MC led to increased hydrophilicity and porosity of the MC-chitosan scaffold due to the hydroxyl groups from the MC molecule, which can attract water molecules. The higher wettability has been described by the suitable results of the water uptake capacity [132]. Another combination of MC and chitosan was studied by Tan et al., They illustrated that an augmented concentration of MC led to improved tensile strength, moisture content, whitish index, and elongation at break [133]. El-Naggar et al., mixed MC with PVA and doxycycline hyclate (drug model) to develop a new drug delivery device, which showed a proper swelling capacity and a high drug release at basic medium [129]. The combination between MC and poly(acrylic acid) presented optimal mechanical properties and thermal stability [134]. The novel composite resulting by blending MC and tragacanth gum exhibited a higher capacity to form a gel and adequate mechanical and rheological properties [135].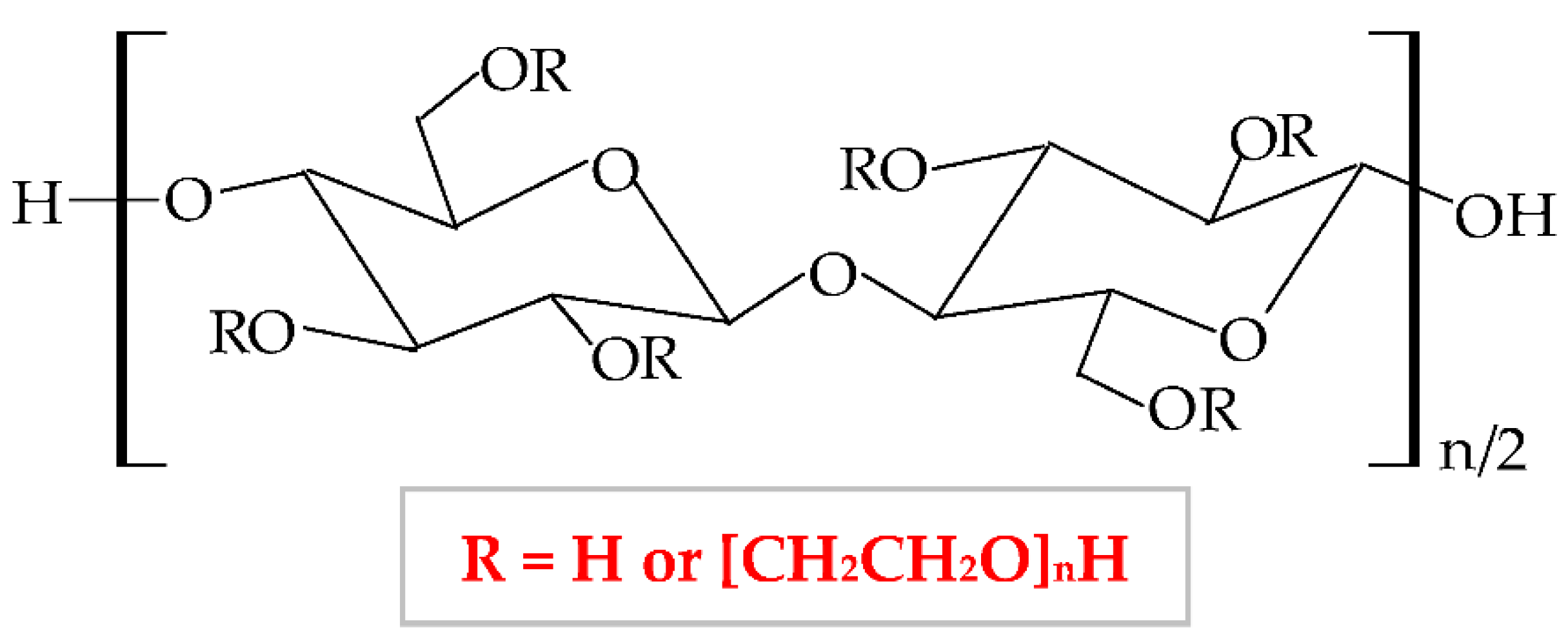
It has a low price, without taste and smell, with no color to light yellowish [138]; presents optimal stability at pH values between 2 and 12 [139]. HEC exhibits a proper capacity to scavenge free radicals and to form hydrogen and electrostatic bonds [140]. HEC is regarded as a hydrogel-like material, with two important characteristics: liquid-like and solid-like. Due to its polysaccharide structure, this hydrophilic biopolymer exhibits a high capacity to absorb and hold a large quantity of water or wound exudates. The elastic strength of its structure leads to an expansion of the molecule dimensions, without the modification of the structural stability and the gel form [141]. HEC possesses excellent physicochemical properties: rheological, hydrodynamic, and thermodynamic [142]. HEC also presents adequate biocompatibility, biodegradability, insignificant toxicity, immunogenicity, and cementing properties [143]. Due to its nonionic behavior, HEC exhibits the ability to coexist with a large field of other polymers, which have an appropriate solubility in water, salts, or surfactants. Therefore, HEC presents optimal toughness in a dielectric solution with a large concentration [144]. This biopolymer presents the largest commercial availability from all cellulose derivatives [145]; therefore, HEC is a noticeable biopolymer, which can be used successfully as an emulsifier, film-coating, stabilizer, suspender, and thickener agent in biomedical, pharmaceutical (wound dressing development) [146], cosmetic, food, adhesive, and textile industries [138][147][148]. The most predictive method for hydrogels synthesis is the crosslinking of free radicals generated by irradiation (electron beam and gamma-radiation) [149].
To enhance its properties, HEC can be blended with other polymers. For example, Zia et al., mixed HEC with poly(lactic acid) and polyurethane. They obtained a new composite with higher thermal stability and mechanical (tensile strength and elongation) properties compared to other polymers [150][151]. Moreover, HEC has been blended with polyvinyl alcohol (PVA), resulting in suitable electrical conductibility, viscoelasticity, stretchability, and thermosensitivity [152]. Guo et al., combined HEC with poly(caprolactone) by trimethylsilyl group technology and the result was the formation of a new copolymer with enhanced thermal properties [153]. HEC was also blended with chitosan to obtain a copolymer with improved physicochemical and mechanical characteristics [154], with gelatin to obtain a superparamagnetic composite [155], with sodium alginate to form a copolymer with enhanced swelling efficacy and drug delivery profile.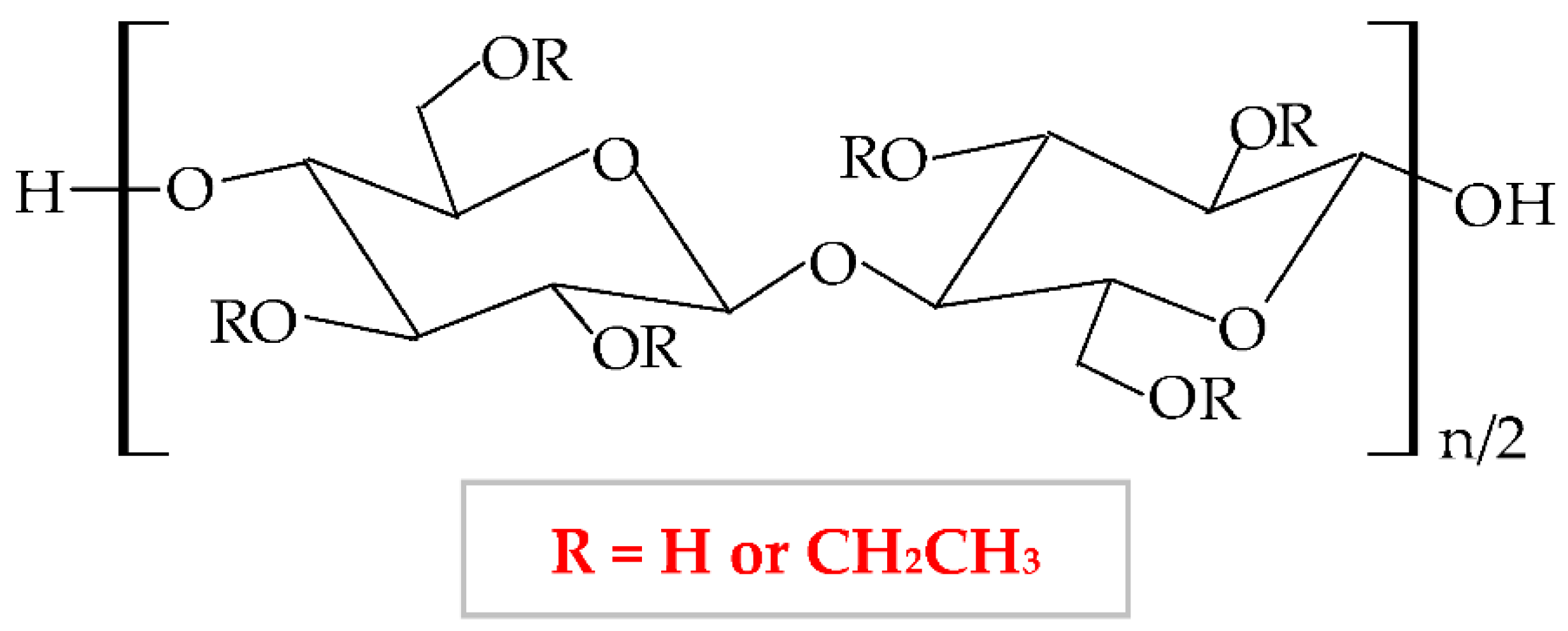
This biopolymer presents numerous advantageous characteristics, such as mechanical properties, biodegradability, flexibility, lowcity, hydrophobicity, gelling capacity [157], light, moisture, oxygen resistance, thermoplasticity [158], and low price, which make EC an excellent material for use in different industries (pharmaceutical, cosmetic and food) [159]. Moreover, this biopolymer has several particular features in addition to the other cellulose derivatives: high film-forming capacity, suitable chemical strength, and optimal mechanical properties [160]. EC represents the most extensively analyzed biopolymer due to its capacity to form film for coating solid pharmaceutical forms (tablets, microcapsules, and microspheres) and formulation of new topical forms [161]. EC is a promising material to be used for encapsulation due to its optimal optical transparency, processing temperature, and electronic insulation [162]. It also presents a good capacity to bind, preserve and dissolve [163], and possesses a proper control of drug delivery [164]. Films based on EC are brittle because of the stiffness of hydrogen linkages from its molecule. This biopolymer has high stability to chemical substances and can be associated with different plasticizers to design heavy and impermeable films [165].
EC can be mixed with various polymers to enhance the physicochemical and mechanical properties and thus, its applicability. To develop a novel drug-delivery device, Li et al., blended EC by electrospinning method with poly(di(ethylene glycol) methyl ether methacrylate), a thermosensitive polymer. The new formulation showed normal morphology, a large porosity, and an increased wettability at a higher temperature, which led to more hydrophobic behavior, causing an extended release of the drug [166]. EC was mixed with poly (ethylene-co-vinyl acetate) and resulted in a new composite with higher mechanical properties[167]. Chen et al., mixed EC and poly(β-hydroxybutyrate) when EC acted as a thickening agent because it increased the viscosity of the new composite. In a concentration of 1%, EC augmented the tensile strength [168]. Li et al., blended EC with konjac glucomannan to formulate a novel composite with higher mechanical properties, moisture resistance, permeability of oxygen, and stability at a high temperature [169]. EC was also associated with another cellulose derivative, HPC, and obtained a scaffold with enhanced mechanical properties and 3D printing capacity [170].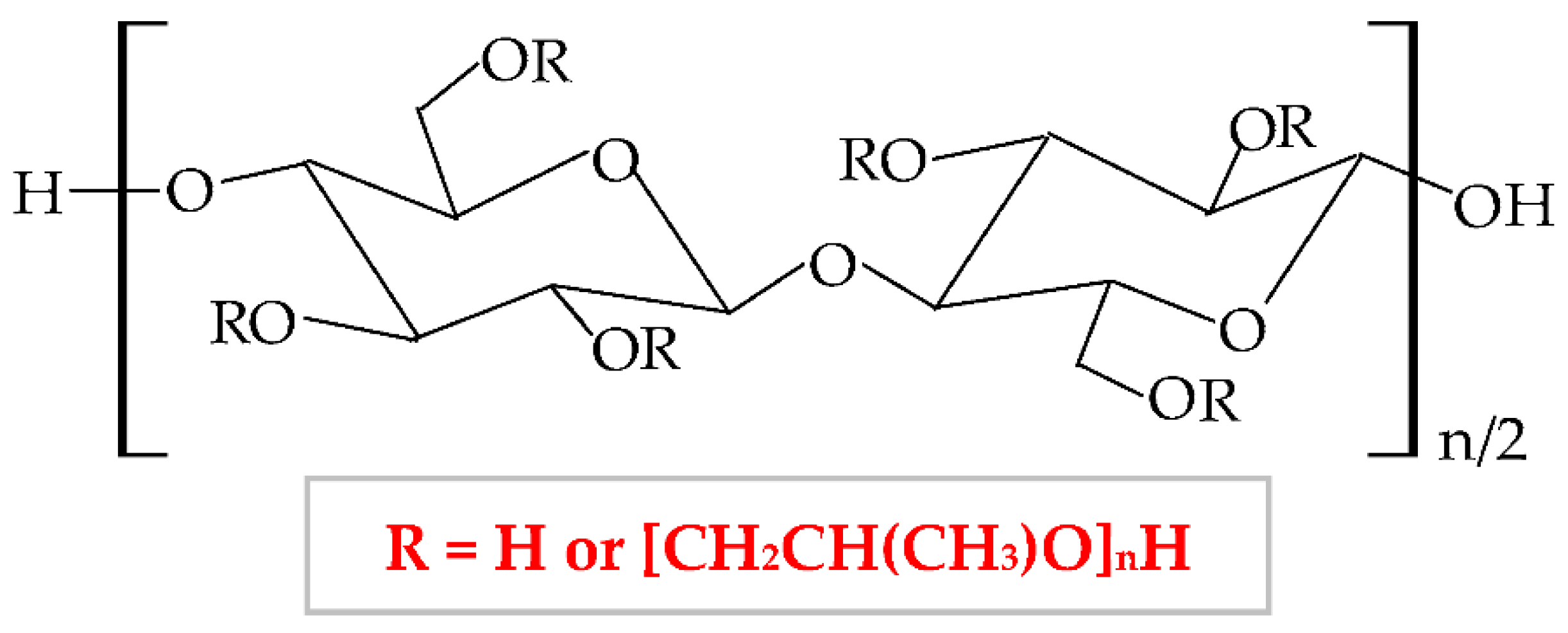
It has numerous advantageous properties, such as amphiphilicity, low price, electrical neutrality, biocompatibility, biodegradability, non-toxicity, high power of swelling the wounds exudate [173][174], adequate chemical strength, and film-forming efficiency [175]. At a high temperature and in a concentrated aqueous solution, HPC generates a cholesteric liquid crystalline network, depending on its concentration [176]. HPC exhibits a thermoplastic behavior and develops temperature-responsive hydrogels [100][177]. Regarding the HPC-based films, these are defined by high flexibility, good impermeability for oil and fat, and a low value of Tg (glass transition temperature) at excessive humidity. The LCST (lower critical solution temperature) water value is about 41°C. At a slightly higher temperature than LCST, HPC presents a phase change because the water solution of this biopolymer generates metastable nanosphere aggregates [178]. Moreover, the solubility of HPC is influenced by LCST values. At a lower temperature than LCST, HPC dissolves easily in water and at a higher temperature than LCST, HPC does not dissolve [179]. Thus, this cellulose derivative is an optimal material to be used in biomedical and pharmaceutical fields as a binding, disintegrating, emulsifying, thickening, filler, and coating agent [180][181] and in the construction domain [182]. It can also be used in the food industry because the United States Food and Drug Administration (FDA) authorized HPC as a safe food additive [183].
HPC can be blended with other polymers to improve the physicochemical and mechanical properties and thus, to extend its applicability. For instance, Veerapur et al., combined HPC and chitosan, and the new formulated composite presented higher hydrophilicity, swelling capacity, and permeation rate [184]. By mixing HPC with cellulose acetate phthalate resulted a composite with higher properties than compounds: increased pseudoplasticity and viscoelastic behavior [185]. Gan et al., prepared a high-performance hydrogel with enhanced tensile strength, toughness, biocompatibility, wear resistance, and low friction coefficient from HPC, sodium alginate, and poly(vinyl alcohol); these excellent characteristics extend the area of use to biosensors and nerve replacement [186]. Lu et al., blended HPC with poly(vinyl alcohol) to obtain a new scaffold with augmented toughness, elasticity, conductivity, and mechanical strength that is a promising material for the development of biosensors and interaction between humans and machines [187].| Biopolymer/-s | Active Pharmaceutical Ingredient (Natural or Synthetic Substances) |
Type of Wound Dressing | Main Findings | References |
|---|---|---|---|---|
| EC/HPMC | Paromomycin and Gentamicin | Film | Optimum drugs release and inhibition of Leishmania tropica growth. | [188] |
| Aloe vera | Nanofibers | Nanofibers with 10% Aloe vera showed suitable mechanical properties, biocompatibility, bioadhesion, and suitable antibacterial activity. | [189] | |
| NaCMC/HPMC | Grapefruit seed extract | Film | Suitable elongation at break, stability in water, and proper antibacterial action. | [190] |
| CuO | Film | Good biocompatibility and antibacterial effect. | [191] | |
| Tetracycline/Methylene blue | Film | Nanoporous network, increased Tg and elongation at break, sustained drug release for 72 h and high antibacterial effect. | [192] | |
| ZnO NPs | Film | Biocompatibility and optimum antibacterial action. | [193] | |
| NaCMC/HPMC/ CAB |
Resveratrol | Membrane | Excellent adhesive capacity, hydration efficiency, and higher porous structure; in vivo studies showed accelerated wound healing. | [194] |
| NaCMC/MC | Simvastatin | Membrane | In a ratio of 2:1, the membrane exhibited appropriate flexibility, viscosity, stability, and sponginess; optimal drug delivery for suppurating injuries. | [195] |