
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | David Levin | + 2649 word(s) | 2649 | 2021-12-02 03:27:39 |
Video Upload Options
Polyethylene (PE) is the most abundant synthetic, petroleum-based plastic materials produced globally, and one of the most resistant to biodegradation, resulting in massive accumulation in the environment. Although the microbial degradation of polyethylene has been reported, complete biodegradation of polyethylene has not been achieved, and rapid degradation of polyethylene under ambient conditions in the environment is still not feasible.
1. Introduction
|
Authors |
Year of Publication |
Topic |
References |
|---|---|---|---|
|
Shimao |
2001 |
Biodegradation of plastics |
[9] |
|
Koutny et al. |
2006 |
Biodegradation of polyethylene films with prooxidant additives |
[10] |
|
Arutchelvi et al. |
2008 |
Biodegradation of polyethylene and polypropylene |
[11] |
|
Shah et al. |
2008 |
Biological degradation of plastics |
[12] |
|
Lucas et al. |
2008 |
Polymer biodegradation: Mechanisms and estimation techniques |
[13] |
|
Tokiwa et al. |
2009 |
Biodegradability of Plastics |
[14] |
|
Sivan |
2011 |
New perspectives in plastic biodegradation |
[15] |
|
Ammala et al. |
2011 |
An overview of degradable and biodegradable polyolefin |
[16] |
|
Restrepo-Flórez et al. |
2014 |
Microbial degradation and deterioration of polyethylene |
[17] |
|
Sen and Raut |
2015 |
Microbial degradation of low density polyethylene |
[18] |
|
Raziyafathima et al. |
2016 |
Microbial Degradation of Plastic Waste: A Review |
[19] |
|
Emadian et al. |
2017 |
Biodegradation of bioplastics in natural environments |
[20] |
|
Harrison et al. |
2018 |
Biodegradability standards for carrier bags and plastic films in aquatic environments: A critical review |
[21] |
|
Genus (and Species) |
Source |
Experiment Duration |
Experiment Condition |
Biodegradation Result |
Reference |
|---|---|---|---|---|---|
|
Acinetobacter bumannii |
Municipal landfill |
30 days |
37 °C Non-pretreated PE |
Biomass production |
[42] |
|
Arthobacter defluvii |
Dumped soil area |
1 month |
PE bags |
20%–30% W.L. * |
[48] |
|
Bacillus amyloliquefaciens Bacillussubtilis |
|||||
|
Bacillus pumilus Bacillus subtillis |
Pelagic waters |
30 days |
PE bags |
1.5%–1.75% W.L. |
[2] |
|
Bacillus ssp. |
Waste coal, a forest and an extinct volcano crater |
225 days |
Modified PE |
Reduction of mechanical properties by 98% No W.L. detected |
[29] |
|
Bacillus sphericus |
Shallow waters of ocean |
1 year |
HDPE and LDPE; Untreated and Heat treated |
3.5% and 10% 9% and 19% |
[43] |
|
Bacillus megaterium Bacillus subtilis Bacillus cereus (MIX together) |
Soil |
90 days |
45 °C photo-degraded oxobiodegradable PE |
7%–10% mineralization |
[31] |
|
Bacillus amyloliquefaciens |
Solid waste dumped |
60 days |
LDPE |
11%–16% |
[49] |
|
Bacillus subtilis |
MCC No. 2183 |
30 days |
Adding Biosurfactant Unpretreated 18 μm thickness PE |
9.26% W.L. |
[50] |
|
Bacillus pumilus M27 Bacillus subtilis H1584 |
Pelagic waters |
30 days |
PE bags |
1.5–1.75 W.L. % |
[2] |
|
Brevibacillus borstelensis |
DSMZ |
90 days |
50 °C Irradiated LDPE |
17% W.L. |
[51] |
|
Brevibacillus |
Waste disposal site |
3 weeks |
Pretreated PE |
37.5% W.L. |
[41] |
|
Chryseobacterium gleum |
Waste water activated sludge soil |
1 month |
UV-radiated LLDPE |
- |
[44] |
|
Comamonas sp. |
Plastic debris in soil |
90 days |
Non-treated LDPE |
Changing in chemical properties |
[8] |
|
Delftia sp. |
Plastic debris in soil |
90 days |
Non-treated LDPE |
Changing in chemical properties |
[8] |
|
Kocuria palustris M16, |
Pelagic waters |
30 days |
PE bags |
1% |
[2] |
|
Microbacterium paraoxydans |
Having Gene bank ID |
2 months |
Pretreated LDPE |
61% W.L. |
[52] |
|
Pseudomonas sp. |
Mangrove soil |
1 month |
PE |
20.54% W.L. |
[30] |
|
Pseudomonas aeroginosa |
Petroleum contaminated beach soil |
80 days |
LMWPE |
40.8% W.L. |
[45] |
|
Pseudomonas sp. |
Beach soil contaminated with crude oil |
80 days |
37 °C LMWPE |
4.9%–28.6% CO2 production |
[46] |
|
Pseudomonas sp. |
Garbage soil |
6 months |
PE bags |
37.09% W.L. |
[34] |
|
Pseudomonas citronellolis |
Municipal Landfill |
4 days |
LDPE |
17.8% W.L. |
[38] |
|
Pseudomonas sp. |
Having Gene bank ID |
2 months |
Pretreated LDPE |
50.5% W.L. |
[52] |
|
Pseudomonas aeroginosa Pseudomonas putida Pseudomonas siringae |
ATCC |
120 days |
Untreated PE |
9%–20% |
[53] |
|
Pseudomonas sp. |
Waste disposal site |
3 weeks |
Pretreated PE |
40.5% W.L. |
[41] |
|
Rhodococcus ruber |
PE agricultural waste in soil |
4 weeks |
Treated LDPE |
Up to 8% W.L. |
[36] |
|
Rhodococcus ruber |
PE agricultural waste in soil |
60 days |
LDPE |
0.86% W.L./week |
[54] |
|
Rhodococcus ruber |
PE agricultural waste in soil |
30 days |
LDPE |
1.5%–2.5% W.L. Reduction of 20%.in Mw and 15%.in Mn |
[55] |
|
Rhodococcus rhorocuros |
ATCC |
6 months |
27 °C Degradable PE |
60% mineralization |
[56] |
|
Rhodococcus rhorocuros |
ATCC 29672 |
6 month |
PE containing prooxidant additives |
Different amount of mineralization |
[57] |
|
Rhodococcus sp. |
Waste disposal site |
3 weeks |
Pretreated PE |
33% W.L. |
[41] |
|
Rhodococcus sp. |
Three forest soil |
30 days |
LDPE containing prooxidant additives |
Confirmation of Adhering |
[35] |
|
Staphylococcus arlettae |
Various soil environments |
30 days |
PE |
13.6% W.L. |
[32] |
|
Stentrophomonas sp. |
Plastic debris in soil |
90 days |
Non-treated LDPE |
Changing in chemical properties |
[8] |
|
Stentrophomonas pavanii |
Solid waste dump site |
56 days |
Modified LDPE |
Confirmed by FTIR |
[40] |
|
Streptomyces spp. |
Nile River Delta |
1 month |
30 °C Heat treated degradable PE bags |
3 species showed slight W.L. |
[58] |
2. Abiotic Deterioration of PE
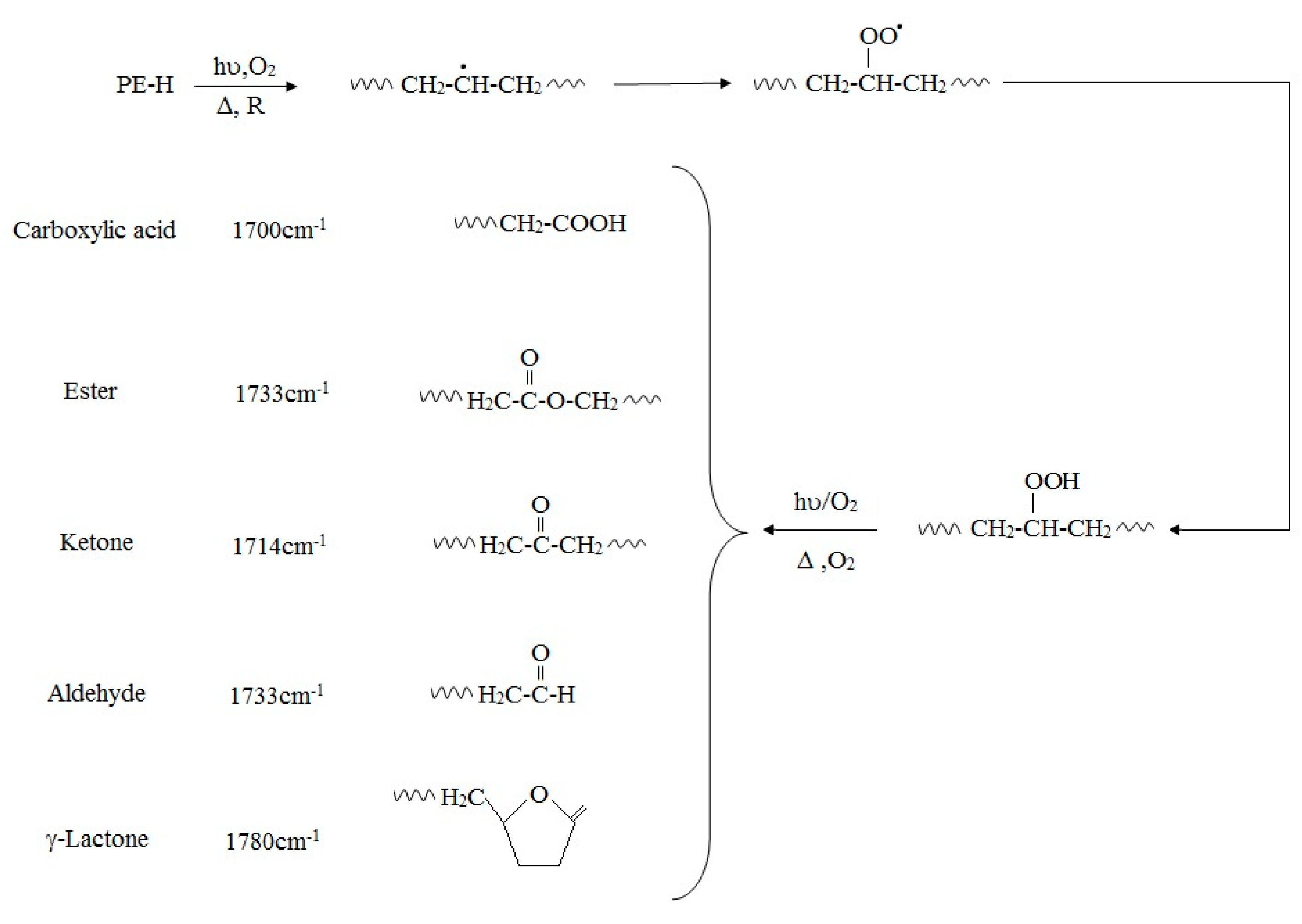
|
SI No. |
Wave Number (cm−1) |
Bond |
Functional Group |
|---|---|---|---|
|
1 |
3000–2850 |
–C–H stretch |
Alkanes |
|
2 |
2830–2695 |
H–C = O: C–H stretch |
Aldehyde |
|
3 |
1710–1665 |
–C = O stretch |
Ketones, Aldehyde |
|
4 |
1470–1450 |
–C–H Bend |
Alkanes |
|
5 |
1320–1000 |
–C–O stretch |
Alcohol, Carboxylic acid, esters, ethers |
|
6 |
1000–650 |
=C–H Bond |
Alkenes |
3. Biodeterioration of PE
4. General Overview of Biodegradation Processes
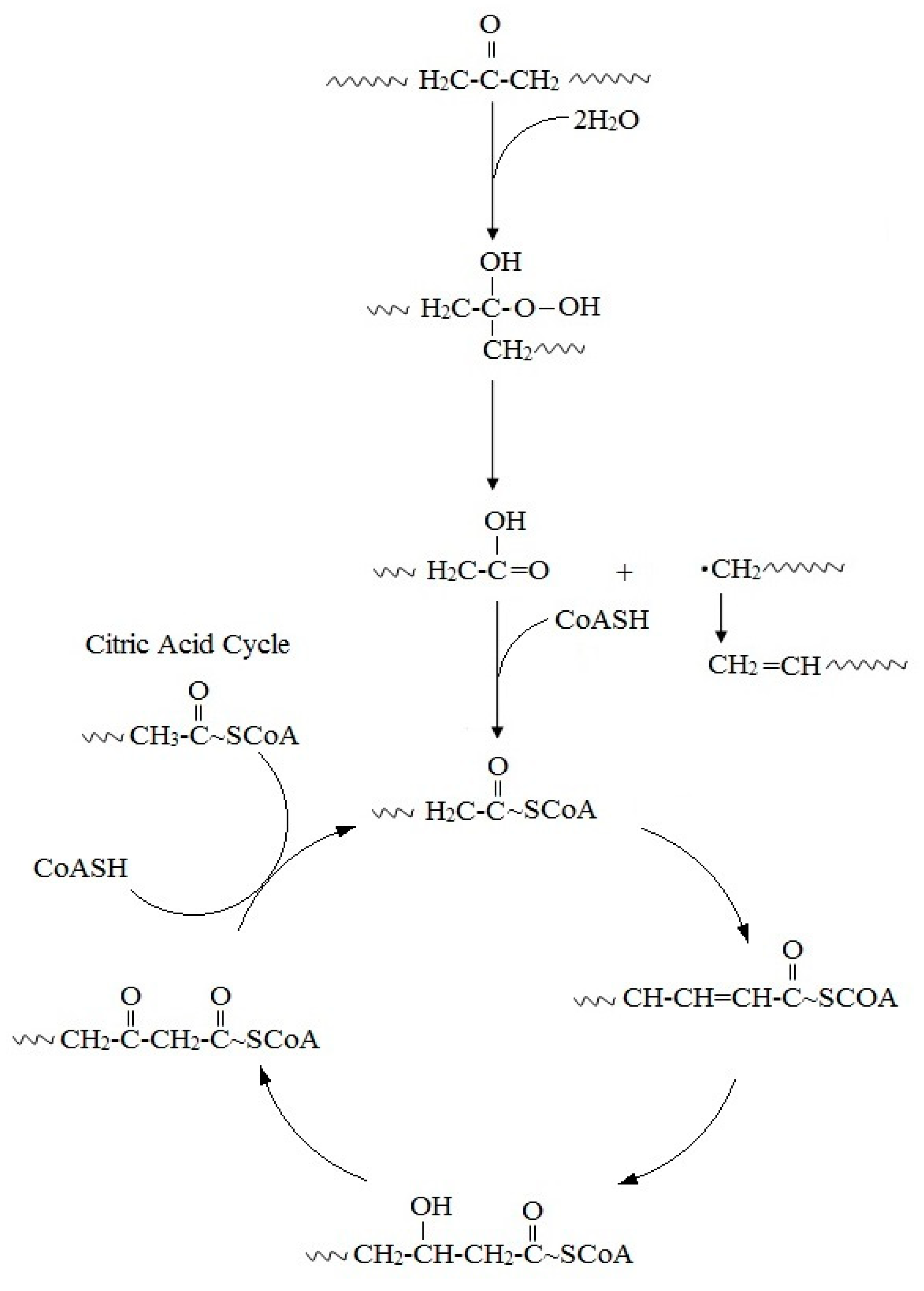
References
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58.
- Harshvardhan, K.; Jha, B. Biodegradation of low-density polyethylene by marine bacteria from pelagic waters Arabian Sea, India. Mar. Pollut. Bull. 2013, 77, 100–106.
- Álvarez-Hernández, C.; Cairós, C.; López-Darias, J.; Mazzetti, E.; Hernández-Sánchez, C.; González-Sálamo, J.; Hernández-Borges, J. Microplastic debris in beaches of Tenerife (Canary Islands, Spain). Mar. Pollut. Bull. 2019, 146, 26–32.
- Foley, C.J.; Feiner, Z.S.; Malinich, T.D.; Hook, T.O. A meta-analysis of the effects of exposure to microplastics on fish and aquatic invertebrates. Sci. Total Environ. 2018, 631, 550–559.
- Rochman, C.M.; Browne, M.A.; Underwood, A.J.; Van Franeker, J.A.; Thompson, R.C.; Amaral-Zettler, L.A. The ecological impacts of marine debris: Unraveling the demonstrated evidence from what is perceived. Ecology 2016, 97, 302–312.
- Shen, M.; Zhang, Y.; Zhu, Y.; Song, B.; Zeng, G.; Hu, D.; Wen, X.; Ren, X. Recent advances in toxicological research of nanoplastics in the environment: A review. Environ. Pollut. 2019, 252, 511–521.
- Bergmann, M.; Mützel, S.; Primpke, S.; Tekman, M.B.; Trachsel, J.; Gerdts, G. White and wonderful? Microplastics prevail in snow from the Alps to the Arctic. Sci. Adv. 2019, 5, eaax1157.
- Peixoto, J.; Silva, P.L.; Krüger, R.H. Brazilian Cerrado soil reveals an untapped microbial potential forunpretreated polyethylene biodegradation. J. Hazard. Mater. 2017, 324, 634–644.
- Shimao, M. Biodegradation of plastics. Curr. Opin. Biotechnol. 2001, 12, 242–247.
- Koutny, M.; Lemaire, J.; Delort, A.M. Biodegradation of polyethylene films with prooxidant additives. Chemosphere 2006, 64, 1243–1252.
- Arutchelvi, J.; Sudhakar, M.; Arkatkar, A.; Doble, M.; Bhaduri, S.; Uppara, P.V. Biodegradation of polyethylene and polypropylene. Indian J. Biotechnol. 2008, 7, 9–22.
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological degradation of plastics: A comprehensive review. Biotechnol. Adv. 2008, 26, 246–265.
- Lucas, N.; Bienaime, C.; Belloy, C.; Queneudec, M.; Silvestre, F.; Nava-Saucedo, J.E. Polymer biodegradation: Mechanisms and estimation techniques. Chemosphere 2008, 73, 429–442.
- Tokiwa, Y.; Calabia, B.P.; Ugwu, C.U.; Aiba, S. Biodegradability of plastics. Int. J. Mol. Sci. 2009, 10, 3722–3742.
- Sivan, A. New perspectives in plastic biodegradation. Curr. Opin. Biotechnol. 2011, 22, 422–426.
- Ammala, A.; Bateman, S.; Deana, K.; Petinakis, E.; Sangwan, P.; Wong, S.; Yuan, Q.; Yu, L.; Patrick, C.; Leong, K.H. An overview of degradable and biodegradable polyolefins. Prog. Polym. Sci. 2011, 36, 1015–1049.
- Restrepo-Flórez, J.M.; Bassi, A.; Thompson, M.R. Microbial degradation and deterioration of polyethylene: A review. Int. Biodeterior. Biodegrad. 2014, 88, 83–90.
- Sen, S.; Raut, S. Microbial degradation of low density polyethylene (LDPE): A review. J. Environ. Chem. Eng. 2015, 3, 462–473.
- Raziyafathima, M.; Praseetha, P.K.; Rimal Isaac, R.S. Microbial degradation of plastic waste: A review. J. Pharm. Chem. Biol. Sci. 2016, 4, 231–242.
- Emadian, S.M.; Onat, T.T.; Demirel, B. Biodegradation of bioplastics in natural environments. Waste Manag. 2017, 59, 526–536.
- Harrison, J.P.; Boardman, C.; O’Callaghan, K.; Delort, A.M.; Song, J. Biodegradability standards for carrier bags and plastic films in aquatic environments: A critical review. R. Soc. Open Sci. 2018, 5, 171792.
- Albertsson, A.C.; Karlsson, S. The Influence of biotic and abiotic environments on the degradation of polyethylene. Prog. Polym. Sci. 1990, 15, 177–192.
- Pirt, S.J. Microbial degradation of synthetic polymers. J. Chem. Technol. Biotechnol. 1980, 30, 176–179.
- Otake, Y.; Kobayashi, T.; Asabe, H.; Murakami, N. Biodegradation of low density polyethylene, polystyrene, polyvinyl chloride, and urea formaldehyde resin buried under soil for over 32 years. Appl. Polym. Sci. 1995, 56, 1789–1796.
- Chiellini, E.; Cortia, A.; Swift, G. Biodegradation of thermally-oxidized, fragmented low-density polyethylenes. Polym. Degrad. Stab. 2003, 81, 341–351.
- Yashchuk, O.; Portillo, F.S.; Hermida, E.B. Degradation of polyethylene film samples containing oxodegradable additives. Procedia Mater. Sci. 2012, 1, 439–445.
- Chiellini, E.; Corti, A.; D’Antone, S. Oxo-biodegradable full carbon backbone polymers biodegradation behaviour of thermally oxidized polyethylene in an aqueous medium. Polym. Degrad. Stab. 2007, 92, 1378–1383.
- Veethahavya, K.S.; Rajath, B.S.; Noobia, S.; Kumar, M.B. Biodegradation of low density polyethylene in aqueous media. Procedia Environ. Sci. 2016, 35, 709–713.
- Nowak, B.; Pajak, J.; Drozd-Bratkowicz, M.; Rymarz, G. Microorganisms participating in the biodegradation of modified polyethylene films in different soils under laboratory conditions. Int. Biodeterior. Biodegrad. 2011, 65, 757–767.
- Kathiresan, K. Polythene and plastics-degrading microbes from the mangrove soil. Rev. Biol. Trop. 2003, 51, 629–633.
- Abrusci, C.; Pablos, J.L.; Corrales, T.; López-Marín, J.; Marín, I.; Catalina, F. Biodegradation of photo-degraded mulching films based on polyethylenes and stearates of calcium and iron as pro-oxidant additives. Int. Biodeterior. Biodegrad. 2011, 65, 451–459.
- Divyalakshmi, S.; Subhashini, A. Screening and isolation of polyethylene degrading bacteria from various soil environments. IOSR J. Environ. Sci. Toxicol. Food Technol. 2016, 10, 1–7.
- Hassan, F.; Shah, A.A.; Hameed, A.; Ahmed, S. Synergistic effect of photo and chemical treatment on the rate of biodegradation of low density polyethylene by Fusarium sp. AF4. J. Appl. Polym. Sci. 2007, 105, 1466–1470.
- Usha, R.; Sangeetha, T.; Palaniswamy, M. Screening of polyethylene degrading microorganisms from garbage soil. Libyan Agric. Res. Cent. J. Intern. 2011, 2, 200–204.
- Koutny, M.; Amato, P.; Muchova, M.; Ruzicka, J.; Delort, A.M. Soil bacterial strains able to grow on the surface of oxidized polyethylene film containing prooxidant additives. Int. Biodeterior. Biodegrad. 2009, 63, 354–357.
- Gilan, I.; Hadar, Y.; Sivan, A. Colonization, biofilm formation and biodegradation of polyethylene by a strain of Rhodococcus ruber. Appl. Microbiol. Biotechnol. 2004, 65, 97–104.
- Montazer, Z.; Habibi-Najafi, M.B.; Mohebbi, M.; Oromiehei, A. Microbial degradation of UV-pretreated low-density polyethylene films by novel polyethylene-degrading bacteria isolated from plastic-dump soil. J. Polym. Environ. 2018, 26, 3613–3625.
- Bhatia, M.; Girdhar, A.; Tiwari, A.; Nayarisseri, A. Implications of a novel Pseudomonas species on low density polyethylene biodegradation: An in vitro to in silico approach. SpringerPlus 2014, 3, 497.
- Das, M.P.; Kumar, S. An approach to low-density polyethylene biodegradation by Bacillus amyloliquefaciens. 3 Biotech 2015, 5, 81–86.
- Mehmood, C.T.; Qazi, I.A.; Hashmi, I.; Bhargava, S.; Deepa, S. Biodegradation of low density polyethylene (LDPE) modified with dye sensitized titania and starch blend using Stenotrophomonas pavanii. Int. Biodeterior. Biodegrad. 2016, 113, 276–286.
- Nanda, S.; Sahu, S.S. Biodegradability of polyethylene by Brevibacillus, Pseudomonas, and Rhodococcus spp. N. Y. Sci. J. 2010, 3, 95–98.
- Pramila, R.; Ramesh, K.V. Potential biodegradation of low-density polyethylene (LDPE) by Acinetobacter bumannii. Afr. J. Bacteriol. Res. 2015, 7, 24–28.
- Sudhakar, M.; Doble, M.; Sriyutha Murthy, P.; Venkatesan, R. Marine microbe-mediated biodegradation of low- and high-density polyethylenes. Int. Biodeterior. Biodegrad. 2008, 61, 203–213.
- Jeon, H.J.; Kim, M.N. Degradation of linear low density polyethylene (LLDPE) exposed to UV-irradiation. Eur. Polym. J. 2014, 52, 146–153.
- Jeon, H.J.; Kim, M.N. Functional analysis of alkane hydroxylase system derived from Pseudomonas aeruginosa E7 for low molecular weight polyethylene biodegradation. Int. Biodeterior. Biodegrad. 2015, 103, 141–146.
- Yoon, M.G.; Jeon, J.H.; Kim, M.N. Biodegradation of polyethylene by a soil bacterium and AlkB cloned recombinant cell. J. Bioremed. Biodegrad. 2012, 3, 145.
- Yang, J.; Yang, Y.; Wu, W.M.; Zhao, J.; Jiang, L. Evidence of polyethylene biodegradation by bacterial strains from the guts of plastic-eating waxworms. Environ. Sci. Technol. 2014, 48, 13776–13784.
- Thakur, P. Screening of Plastic Degrading Bacteria from Dumped Soil Area. Ph.D. Thesis, National Institue of Technology of Rourkela, Odisha, India, 2012.
- Das, M.P.; Kumar, S. Influence of cell surface hydrophobicity in colonization and biofilm formation on LDPE biodegradation. Int. J. Pharm. Pharm. Sci. 2013, 4, 690–694.
- Vimala, P.P.; Mathew, L. Biodegradation of polyethylene using Bacillus subtilis. Procedia Technol. 2016, 24, 232–239.
- Hadad, D.; Geresh, S.; Sivan, A. Biodegradation of polyethylene by the thermophilic bacterium Brevibacillus borstelensis. J. Appl. Microbiol. 2005, 98, 1093–1100.
- Rajandas, H.; Parimannan, S.; Sathasivam, K.; Ravichandran, M.; Yin, L.S. A novel FTIR-ATR spectroscopy based technique for the estimation of low-density polyethylene biodegradation. Polym. Test. 2012, 3, 1094–1099.
- Kyaw, B.M.; Champakalakshmi, R.; Sakharkar, M.K.; Lim, C.S.; Sakharkar, K.R. Biodegradation of low-density polythene (LDPE) by Pseudomonas species. Indian J. Microbiol. 2012, 52, 411–419.
- Sivan, A.; Santo, M.; Pavlov, V. Biofilm development of the polyethylene-degrading bacterium Rhodococcus ruber. Appl. Microbiol. Biotechnol. 2006, 72, 346–352.
- Santo, M.; Weitsman, R.; Sivan, A. The role of the copper-binding enzyme, laccase, in the biodegradation of polyethylene by the actinomycete Rhodococcus ruber. Int. Biodeterior. Biodegrad. 2013, 84, 204–210.
- Bonhomme, S.; Cuer, A.; Delort, A.M.; Lemaire, J.; Sancelme, M.; Scott, C. Environmental biodegradation of polyethylene. Polym. Degrad. Stab. 2003, 81, 441–452.
- Fontanella, S.; Bonhomme, S.; Koutny, M.; Husarova, L.; Brusso, J.M.; Courdavault, J.P.; Pitteri, S.; Pichon, S.G.; Emaire, G.J.; Delort, A.M. Comparison of the biodegradability of various polyethylene films containing pro-oxidant additives. Polym. Degrad. Stab. 2010, 95, 1011–1021.
- El-Shafei, H.; EI-Nasser, N.H.A.; Kansoh, A.L.; Ali, A.M. Biodegradation of disposable polyethylene by fungi Streptomyces species. Polym. Degrad. Stab. 1998, 62, 361–365.
- Ranjan, V.P.; Goel, S. Degradation of Low-density polyethylene film exposed to UV radiation in four environments. J. Hazard. Toxic Radioact. Waste 2019, 23, 04019015.
- Celina, M.; Linde, E.; Brunson, D.; Quintana, A.; Giron, N. Overview of accelerated aging and polymer degradation kinetics for combined radiation-thermal environments. Polym. Degrad. Stab. 2019, 166, 353–378.
- Kelkar, V.P.; Rolsky, C.B.; Pant, A.; Green, M.D.; Tongay, S.; Halden, R.U. Chemical and physical changes of microplastics during sterilization by chlorination. Water Res. 2019, 163, 114871.
- Albertsson, A.C.; Anderson, S.O.; Karlsson, S. Mechanism of biodegradation of polyethylene. Polym. Degrad. Stab. 1987, 18, 73–87.
- Abrusci, C.; Pablos, J.L.; Marín, I.; Espí, E.; Corrales, T.; Catalina, F. Comparative effect of metal stearates as pro-oxidant additives on bacterial biodegradation of thermal- and photo-degraded low density polyethylene mulching films. Int. Biodeterior. Biodegrad. 2013, 83, 25–32.
- Reddy, M.M.; Deighton, M.; Gupta, R.K.; Bhattacharya, S.N.; Parthasarathy, R. Biodegradation of montmorillonite filled oxo-biodegradable polyethylene. J. Appl. Polym. Sci. 2009, 113, 826–832.
- Yamada-Onodera, K.; Mukumoto, H.; Katsuyama, Y.; Saiganji, A.; Tani, Y. Degradation of polyethylene by a fungus, Penicillium simplicissimum YK. Polym. Degrad. Stab. 2001, 72, 323–327.
- Montazer, Z.; Habibi-Najafi, M.B.; Levin, D.B. Microbial degradation of low-density polyethylene and synthesis of polyhydroxyalkanoate polymers. Can. J. Microbiol. 2019, 65, 1–11.
- Haines, J.R. Microbial degradation of high-molecular-weight alkanes. Appl. Microbiol. 1975, 28, 1084–1085.
- Álvarez, H.M. Relationship between β-oxidation pathway and the hydrocarbon-degrading profile in actinomycetes bacteria. Int. Biodeterior. Biodegrad. 2003, 52, 35–42.
- Eubeler, J.P.; Bernhard, M.; Knepper, T.P. Environmental biodegradation of synthetic polymers: Biodegradation of different polymer groups. Trends Analyt. Chem. 2010, 29, 84–100.
- Gewert, B.; Plassmann, M.M.; MacLeod, M. Pathways for degradation of plastic polymers floating in the marine environment. Environ. Sci. Process. Impacts 2015, 17, 1513–1521.
- Jeon, H.J.; Kim, M.N. Comparison of the functional characterization between alkane monooxygenases for low-molecular-weight polyethylene biodegradation. Int. Biodeterior. Biodegrad. 2016, 114, 202–208.
- Takei, D.; Washio, K.; Morikawa, M. Identification of alkane hydroxylase genes in Rhodococcus sp. strain TMP2 that degrades a branched alkane. Biotechnol. Lett. 2008, 30, 1447–1452.
- Rojo, F. Degradation of alkanes by bacteria. Environ. Microbiol. 2009, 11, 2477–2490.




