
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Dmitry Evtuguin | + 3471 word(s) | 3471 | 2021-11-18 03:19:14 | | | |
| 2 | Peter Tang | + 17 word(s) | 3488 | 2021-11-26 03:54:16 | | |
Video Upload Options
Ellagitannins (ETs), characterized by their diversity and chemical complexity, belong to the class of hydrolysable tannins that, via hydrolysis under acidic or alkaline conditions, can yield ellagic acid (EA). They are mostly found as a part of extractives in angiosperms. As known antioxidants and chelators, EA and EA derivatives are drawing an increasing interest towards extensive technical and biomedical applications.
1. Introduction
2. The Chemistry of Ellagic Acid and Ellagitannins
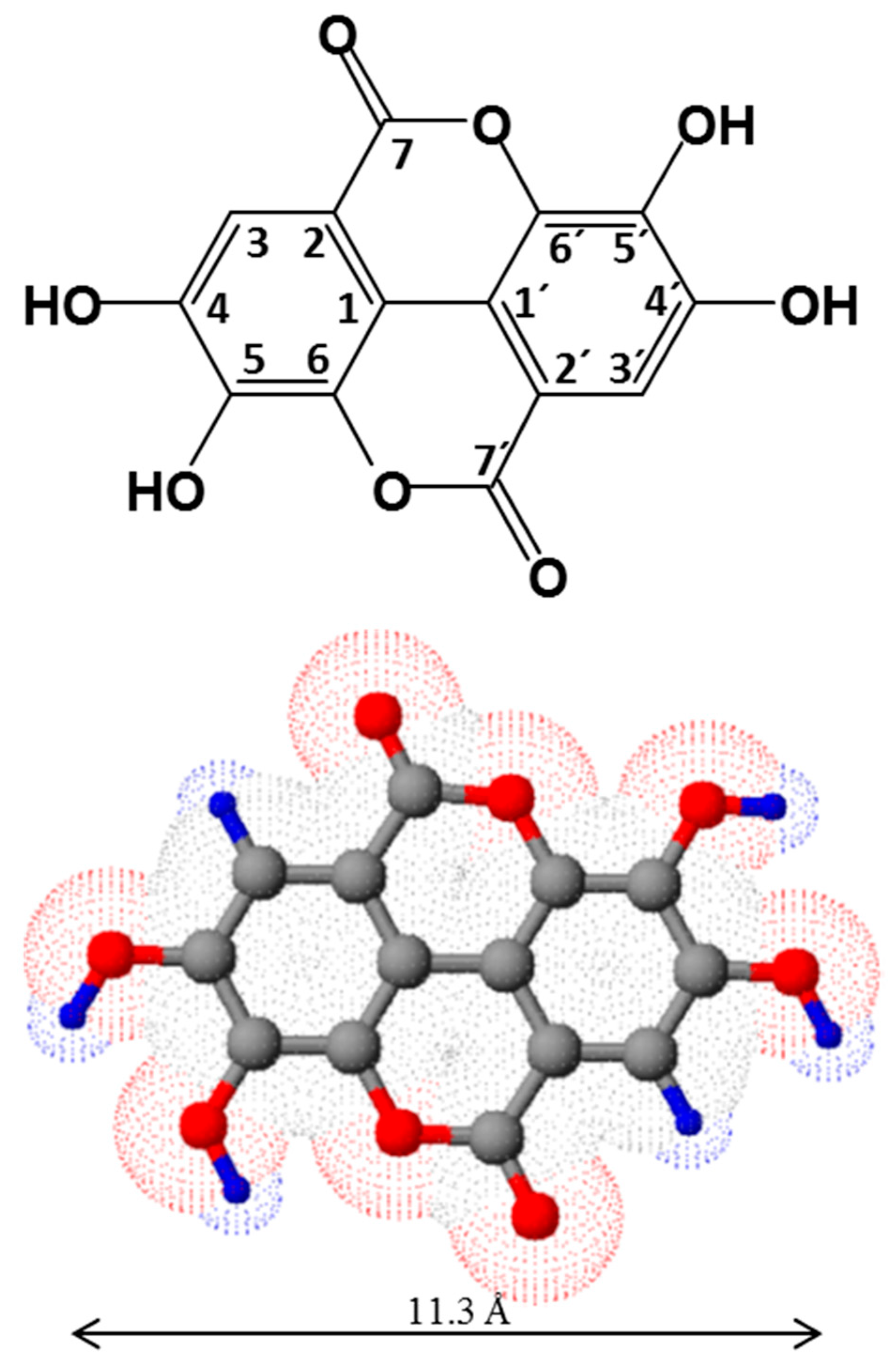
2.1. Structure and Physico-Chemical Properties of Ellagic Acid
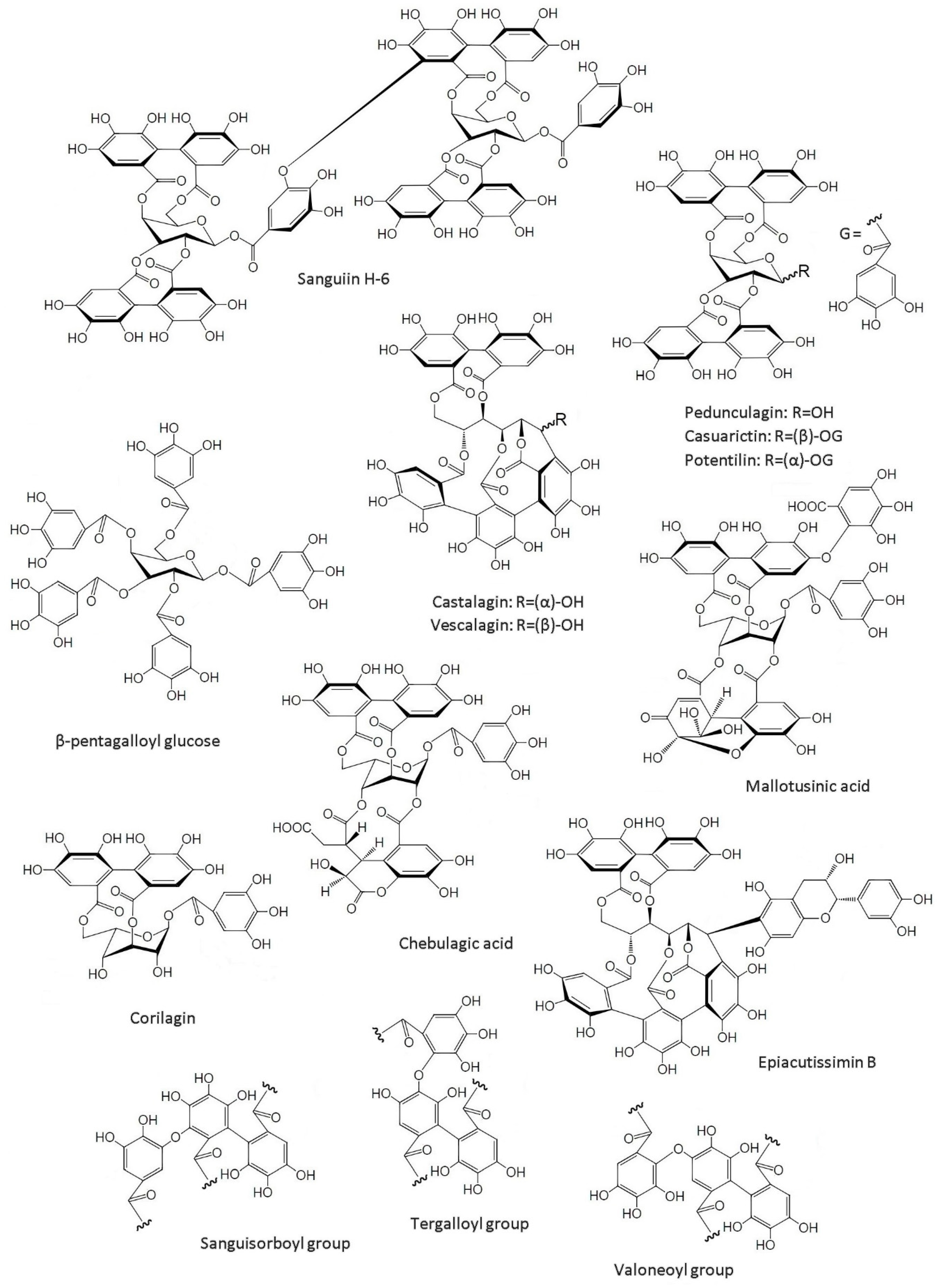
2.2. Structure of Ellagitannins
3. Sources of Ellagic Acid and Ellagitannins
|
Source |
Latin Name |
Total ET/EA # |
Free EA |
Ref. |
|---|---|---|---|---|
|
Fruits |
||||
|
Arctic bramble |
Rubus arcticus |
3900 (fw) |
- |
[29] |
|
Blackberry |
Rubus ursinus |
1500 ± 140 (dw) |
- |
[47] |
|
Camu-camu fruit: |
Myrciaria dubia |
[34] |
||
|
Pulp powder |
258.5 ± 4.3 (dw) * |
56.0 ± 1.1 (dw) |
||
|
Flour |
5656.6 ± 11.3 (dw) * |
764.9 ± 4.9 (dw) |
||
|
Peel |
71.4 (fw) * |
Nd |
||
|
Pulp |
67.3 (fw) * |
Nd |
||
|
Seeds |
2819.8 (fw) * |
50.4 (fw) |
||
|
Cloudberry |
Rubus chamaemorus |
3600 (fw) |
- |
[29] |
|
3151 (fw) |
- |
[28] |
||
|
Cranberries |
Vaccinium |
120 ± 4 (dw) |
- |
[47] |
|
Guava |
Psidium guajava L. |
57.2–306 (dw) |
- |
[48] |
|
Kakadu plum |
Terminalia ferdinandiana |
30,510–140,250 (dw) |
- |
[30] |
|
8796.0 ± 156.0 (dw) |
6206.0 ± 22.0 (dw) |
[31] |
||
|
Muscadine grapes |
Vitis rotundifolia |
360–912 (fw) |
- |
[36] |
|
Pomegranate: |
Punica granatum |
[35] |
||
|
Mesocarp |
40,595.4 ± 4434.2 (dw) |
234.2 ± 13.0 (dw) |
||
|
Peel |
43,979.0 ± 394.8 (dw) |
637.7 ± 32.8 (dw) |
||
|
Red raspberry |
Rubus idaeus |
1500 ± 100 (dw) |
- |
[47] |
|
1900–2700 (fw) |
- |
[29] |
||
|
2637–3309 (fw) |
- |
[28] |
||
|
Rose hip |
Rosa rugosa |
1096 (fw) |
- |
[28] |
|
Strawberry |
Fragaria ananassa |
630 ± 90 (dw) |
- |
[47] |
|
650–850 (fw) |
- |
[29] |
||
|
683–853 (fw) |
- |
[28] |
||
|
Processed Fruits |
||||
|
Pomegranate juice |
- |
87–2118.3 (mg·L−1) |
2.1–7.7 (mg·L−1) |
[35] |
|
Raspberry jam |
- |
764 (fw) |
- |
[28] |
|
Strawberry jam |
- |
245 (fw) |
- |
[28] |
|
Seeds and Nuts |
||||
|
Pecans |
Carya illinoensis |
330 ± 0.3 (dw) |
- |
[47] |
|
Walnuts |
Juglans nigra |
590 ± 0.3 (dw) |
- |
[47] |
|
Wood |
||||
|
Blue gum |
Eucalyptus globulus |
- |
500–1700 (dw) |
[41] |
|
Common Oak |
Quercus robur |
- |
81–228 (dw) |
[49] |
|
Pyrenean oak |
Quercus pyrenaica |
- |
66–219 (dw) |
[49] |
|
Rose gum |
Eucalyptus grandis |
- |
280–512 (dw) |
[40] |
|
Sessile oak |
Quercus petraea |
- |
109–198 (dw) |
[49] |
|
Sweet chestnut |
Castanea sativa |
- |
74–140 (dw) |
[49] |
|
White oak |
Quercus alba |
- |
132–277 (dw) |
[49] |
|
Wood bark |
||||
|
Blue gum |
Eucalyptus globulus |
- |
471 (dw) |
[50] |
|
(Hybrid) eucalypt |
Eucalyptus urograndis |
- |
2243–2307 (dw) |
[51] |
|
Maidens Gum |
Eucalyptus maidenii |
- |
1130–1178 (dw) |
[51] |
|
Oak |
Quercus robur + Quercus petraea |
- |
2200–3700 (dw) |
[52] |
|
Sweet chestnut |
Castanea sativa |
- |
4300–9300 (dw) |
[53] |
|
Rose Gum |
Eucalyptus grandis |
- |
2639–2721 (dw) |
[51] |
|
Other sources |
||||
|
Eucalypt leaves |
Eucalyptus globulus |
3320.0 ± 80.0 (dw) |
- |
[54] |
|
Filtrates from unbleached kraft wood |
Eucalyptus globulus |
- |
98 ± 0.7 (mg/L) |
[41] |
|
Sulphite spent liquor |
Eucalyptus globulus |
- |
1165.5 (mg/L) |
[55] |
4. Technical Applications of Ellagic Acid
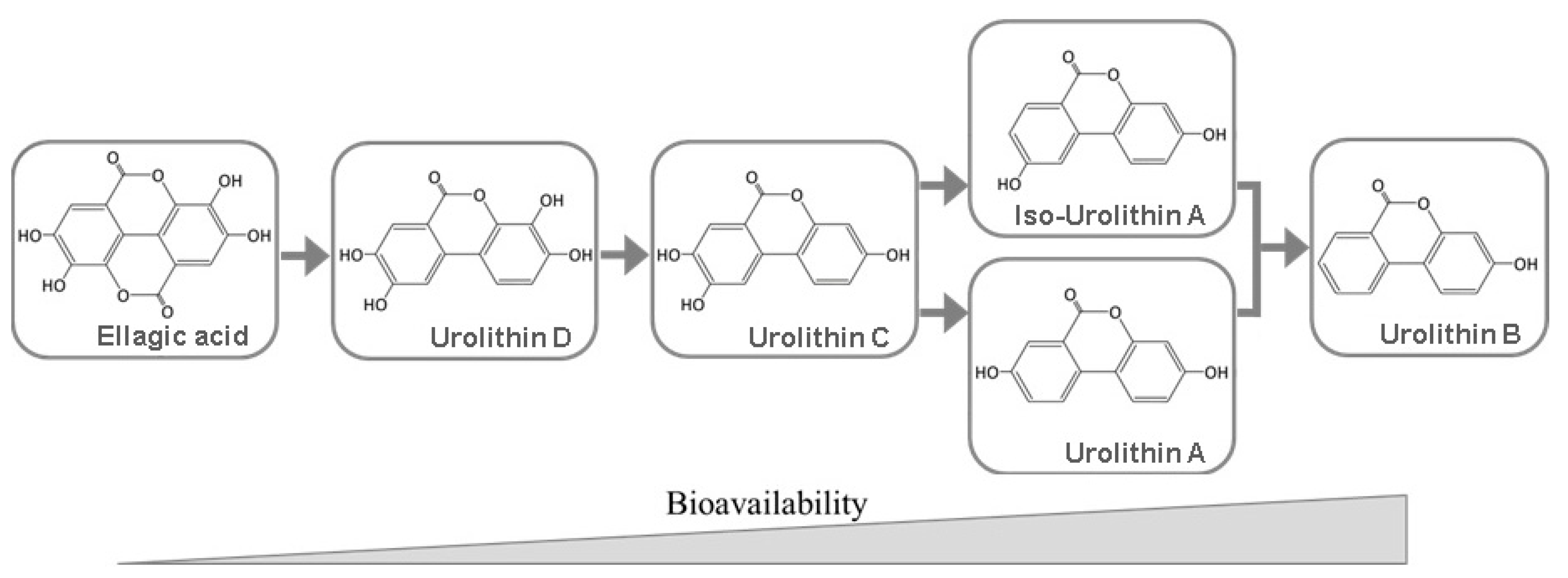
5. Bioavailability of Ellagitannins and Ellagic Acid
6. Biomedical Applications
The above-mentioned structural features of EA, ETs and derivatives have a vital role in maintaining cellular homeostasis and bestowing these compounds with preventive and protective properties in many biological systems and cell types. It has been reported a wide range of possible biomedical/pharmaceutical applications, which are briefly summarized in Table 2.
Table 2. Possible biological effects of EA and its derivatives.
|
Activity |
Active Compound |
Main Features |
Ref. |
|---|---|---|---|
|
Antibacterial (Gram-Positive) |
Commercial extract of pomegranate byproduct (POMx) and punicalagin |
Inhibited the growth of pathogenic Clostridium and Staphyloccocus aureus |
[78] |
|
Antibacterial (Gram-Positive) |
Ellagic acid |
Action against Bacillus luteus and Listeria monocytogenes |
[79] |
|
Antibacterial (Gram-Negative) |
Tellimagrandin I |
Time- and dose-dependent bactericidal activity against Helicobacter pylori |
[80] |
|
Antibacterial (Gram-Negative) |
Ellagic acid |
EA—cyclodextrin complex expressed activity against Escherichia coli and Pseudomonas aeruginosa |
[79] |
|
Antimycobacterial |
Punicalagin |
Inhibited the growth of Mycobacterium tuberculosis typus humanus ATCC 27294 and patient strain of Mycobacterium tuberculosis sensitive to the standard antituberculosis drugs |
[81] |
|
Antileishmanial |
Geraniin, phyllanthusiin B and elaeocarpusin |
Exhibited effect against protozoa Leishmania donovani, comparable to that of the amphotericin B |
[82] |
|
Antimalarial |
Ellagic acid |
In vitro against all Plasmodium falciparum strains. In vivo against Plasmodium vinckei petteri; potentiates the activity of chloroquine, mefloquine, artesunate and atovaquone |
[83] |
|
Antibabesial |
Ellagic acid |
In vivo against Babesia microti; EA nanoparticles as an alternative antiparasitic agent |
[84] |
|
Antifungal |
Candelitannin (ellagitannin) isolated from E. antisyphilitica Zucc. |
Effective against Alternaria alternata, Fusarium oxyzporum, Colletotrichum gloeosporoides and Rhizoctnia solani |
[85] |
|
Antifungal |
Ellagic acid |
Action against Candida albicans |
[79] |
|
Antiviral |
Castalagin, vescalagin and grandinin. |
Action against acyclovir (ACV)—resistant strains of Herpes simplex virus HSV−1 and HSV-2; synergistic effects when used in combination with ACV |
[86] |
|
Prebiotic effect |
Commercial extract of pomegranate byproduct (POMx) and punicalagin |
Enhanced growth of Bifidobacterium breve and Bifidobacterium infantis |
[78] |
|
Anti-inflammatory |
Ellagic acid, gallic acid and punicalagin A&B |
Potential inhibition of LPS-induced NO, PGE-2 and IL-6 production |
[87] |
|
Anti-inflammatory |
Ellagic acid |
Enhancement of EA’s anti-inflammatory properties in vivo by inclusion complex of EA with hydroxypropyl-β-cyclodextrin |
[88] |
|
Treatment of Type 2 diabetes mellitus |
Ellagic acid and ETs from Agrimonia pilosa Ledeb. |
Inhibition of protein tyrosine phosphatases (PTP1B) |
[13] |
|
Prevention of diabetic complications |
Ellagic acid |
ALR2 (aldose reductase) inhibition and antiglycating effect of EA could possibly delay progression of cataract |
[89] |
|
Anticancerous agent |
Ellagic acid |
Inhibition of SphK1 (sphingosine kinase 1) |
[11] |
|
Antiangiogenic and antiproliferative effect |
Ellagic acid |
Reduction in metastatic potential of bladder cancer and enhancement of the efficacy of anti-VEGF-A therapies |
[7] |
|
Gastroprotective |
Ellagitannin-rich fraction obtained from E. citriodora |
Possibly due to their antioxidant, anti-inflammatory and anti-apoptotic properties. Partially mediated by attenuating induced oxidative stress and by the reduction of pro-inflammatory markers. |
[90] |
|
Hepatoprotective |
Ellagic acid |
Suppression of caspase-3, bcl-2, NF-kB and Nrf-2 |
[6] |
|
Antiarrhythmic |
Ellagic acid |
Antilipid peroxidation property and antihyperlipidemic activity through 3-hydroxy-3 methyl glutaryl CoA reductase inhibition; cardioprotective effect |
[91] |
|
Antiasthmatic |
L. pacari extract and ellagic acid |
Effective eosinophilic inflammation suppressors |
[92] |
|
Antihyperlipidemic |
Ellagic acid |
EA-CoQ10 nanoparticles effectively attenuated induced hyperlipidemia in rats |
[93] |
|
Antiepileptic |
Ellagic acid |
Possibly achieved through increase of brain GABA levels |
[9] |
|
Antianxiety |
Ellagic acid |
Possible involvement of GABAergic system in the anxiolytic action |
[10] |
|
Antidepressant |
Ellagic acid |
Possible interaction through adrenergic and serotonergic systems or through inhibition of inducible NOS |
[8] |
|
Neuroprotective in SAD |
Ellagic acid |
Diminished oxidative stress profile, pro-inflammatory markers, acetylcholinesterase activity, and amyloid-β plaque level in induced SAD (Sporadic Alzheimer’s Disease) rats |
[12] |
|
Skin-whitening agent |
Ellagic acid |
EA acts as an alternative substrate of tyrosinase, inhibiting the melanogenesis process |
[94] |
References
- Quideau, S.; Feldman, K.S. Ellagitannin chemistry. Chem. Rev. 1996, 96, 475–504.
- Khanbabaee, K.; van Ree, T. Tannins: Classification and definition. Nat. Prod. Rep. 2001, 18, 641–649.
- Yamada, H.; Wakamori, S.; Hirokane, T.; Ikeuchi, K.; Matsumoto, S. Structural revisions in natural ellagitannins. Molecules 2018, 23, 1901.
- Covington, A.D. Modern tannins chemistry. Chem. Soc. Rev. 1997, 26, 111–126.
- Wu, X.; Gu, L.; Holden, J.; Haytowitz, D.B.; Gebhardt, S.E.; Beecher, G.; Prior, R.L. Development of a database for total antioxidant capacity in foods: A preliminary study. J. Food Compos. Anal. 2004, 17, 407–422.
- Aslan, A.; Gok, O.; Erman, O.; Kuloglu, T. Ellagic acid impedes carbontetrachloride-induced liver damage in rats through suppression of NF-kB, Bcl-2 and regulating Nrf-2 and caspase pathway. Biomed. Pharmacother. 2018, 105, 662–669.
- Ceci, C.; Tentori, L.; Atzori, M.G.; Lacal, P.M.; Bonanno, E.; Scimeca, M.; Cicconi, R.; Mattei, M.; de Martino, M.G.; Vespasiani, G.; et al. Ellagic acid inhibits bladder cancer invasiveness and in vivo tumor growth. Nutrients 2016, 8, 744.
- Dhingra, D.; Chhillar, R. Antidepressant-like activity of ellagic acid in unstressed and acute immobilization-induced stressed mice. Pharmacol. Rep. 2012, 64, 796–807.
- Dhingra, D.; Jangra, A. Antiepileptic activity of ellagic acid, a naturally occurring polyphenolic compound, in mice. J. Funct. Foods 2014, 10, 364–369.
- Girish, C.; Raj, V.; Arya, J.; Balakrishnan, S. Involvement of the GABAergic system in the anxiolytic-like effect of the flavonoid ellagic acid in mice. Eur. J. Pharmacol. 2013, 710, 49–58.
- Gupta, P.; Mohammad, T.; Khan, P.; Alajmi, M.F.; Hussain, A.; Rehman, M.T.; Hassan, M.I. Evaluation of ellagic acid as an inhibitor of sphingosine kinase 1: A targeted approach towards anticancer therapy. Biomed. Pharmacother. 2019, 118, 109245.
- Jha, A.B.; Panchal, S.S.; Shah, A. Ellagic acid: Insights into its neuroprotective and cognitive enhancement effects in sporadic Alzheimer’s disease. Pharmacol. Biochem. Behav. 2018, 175, 33–46.
- Nguyen, D.H.; Seo, U.M.; Zhao, B.T.; Le, D.D.; Seong, S.H.; Choi, J.S.; Min, B.S.; Woo, M.H. Ellagitannin and flavonoid constituents from Agrimonia pilosa Ledeb. with their protein tyrosine phosphatase and acetylcholinesterase inhibitory activities. Bioorg. Chem. 2017, 72, 293–300.
- Braconnot, H. Observations sur la préparation et la purification de l′acide gallique, et sur l′existence d′un acide nouveau dans la noix de galle. Ann. Chim. Phys. 1818, 9, 181–189.
- Berzelius, J.J. Acide ellagique (Acidum ellagicum) (I). In Traité de Chimie Minerale, Végétale et Animale, 2nd ed.; Esslinger, M., Hoefer, F., Eds.; Chez Firmin Didot frères: Paris, France, 1849; Volume 5, pp. 425–429.
- Mathieson, A.M.; Poppleton, B.J. The crystal structure of ellagic acid. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1968, 24, 1456–1461.
- Rossi, M.; Erlebacher, J.; Zacharias, D.E.; Carrell, H.L.; Iannucci, B. The crystal and molecular structure of ellagic acid dihydrate: A dietary anti-cancer agent. Carcinogenesis 1991, 12, 2227–2232.
- Goriparti, S.; Harish, M.N.K.; Sampath, S. Ellagic acid—a novel organic electrode material for high capacity lithium ion batteries. Chem. Commun. 2013, 49, 7234–7236.
- Li, X.C.; Elsohly, H.N.; Hufford, C.D.; Clark, A.M. NMR assignments of ellagic acid derivatives. Magn. Reson. Chem. 1999, 37, 856–859.
- Bala, I.; Bhardwaj, V.; Hariharan, S.; Kumar, M.N.V.R. Analytical methods for assay of ellagic acid and its solubility studies. J. Pharm. Biomed. Anal. 2006, 40, 206–210.
- Musialik, M.; Kuzmicz, R.; Pawcowski, T.S.; Litwinienko, G. Acidity of hydroxyl groups: An overlooked influence on antiradical properties of flavonoids. J. Org. Chem. 2009, 74, 2699–2709.
- Simić, A.Z.; Verbić, T.Ž.; Sentić, M.N.; Vojić, M.P.; Juranić, I.O.; Manojlović, D.D. Study of ellagic acid electro-oxidation mechanism. Monatsh. Chem. 2013, 144, 121–128.
- Marković, Z.; Milenković, D.; Đorović, J.; Dimitrić Marković, J.M.; Lučić, B.; Amić, D. A DFT and PM6 study of free radical scavenging activity of ellagic acid. Monatsh. Chem. Chem. Mon. 2013, 144, 803–812.
- Nenadis, N.; Tsimidou, M.Z. Contribution of DFT computed molecular descriptors in the study of radical scavenging activity trend of natural hydroxybenzaldehydes and corresponding acids. Food Res. Int. 2012, 48, 538–543.
- Okuda, T.; Yoshida, T.; Hatano, T.; Ito, H. Ellagitannins renewed the concept of tannins. In Chemistry and Biology of Ellagitannins: An Underestimated Class of Bioactive Plant Polyphenols; Quideau, S., Ed.; World Scientific Publishing: Singapore, 2009; pp. 1–54. ISBN 978-981-279-740-7.
- Quideau, S.; Jourdes, M.; Saucier, C.; Glories, Y.; Pardon, P.; Baudry, C. DNA topoisomerase inhibitor acutissimin a and other flavano-ellagitannins in red wine. Angew. Chem. Int. Ed. Engl. 2003, 42, 6012–6014.
- Niemetz, R.; Gross, G.G. Enzymology of gallotannin and ellagitannin biosynthesis. Phytochemistry 2005, 66, 2001–2011.
- Koponen, J.M.; Happonen, A.M.; Mattila, P.H.; Törrönen, A.R. Contents of anthocyanins and ellagitannins in selected foods consumed in Finland. J. Agric. Food Chem. 2007, 55, 1612–1619.
- Määttä-Riihinen, K.R.; Kamal-Eldin, A.; Törrönen, A.R. Identification and quantification of phenolic compounds in berries of Fragaria and Rubus species (family Rosaceae). J. Agric. Food Chem. 2004, 52, 6178–6187.
- Konczak, I.; Maillot, F.; Dalar, A. Phytochemical divergence in 45 accessions of Terminalia ferdinandiana (Kakadu plum). Food Chem. 2014, 151, 248–256.
- Williams, D.J.; Edwards, D.; Pun, S.; Chaliha, M.; Sultanbawa, Y. Profiling ellagic acid content: The importance of form and ascorbic acid levels. Food Res. Int. 2014, 66, 100–106.
- Fukuda, T.; Ito, H.; Yoshida, T. Antioxidative polyphenols from walnuts (Juglans regia L.). Phytochemistry 2003, 63, 795–801.
- Villarreal-Lozoya, J.E.; Lombardini, L.; Cisneros-Zevallos, L. Phytochemical constituents and antioxidant capacity of different pecan cultivars. Food Chem. 2007, 102, 1241–1249.
- Fracassetti, D.; Costa, C.; Moulay, L.; Tomás-Barberán, F.A. Ellagic acid derivatives, ellagitannins, proanthocyanidins and other phenolics, vitamin C and antioxidant capacity of two powder products from camu-camu fruit (Myrciaria dubia). Food Chem. 2013, 139, 578–588.
- Fischer, U.A.; Carle, R.; Kammerer, D.R. Identification and quantification of phenolic compounds from pomegranate (Punica granatum L.) peel, mesocarp, aril and differently produced juices by HPLC-DAD-ESI/MSn. Food Chem. 2011, 127, 807–821.
- Lee, J.-H.; Johnson, J.V.; Talcott, S.T. Identification of ellagic acid conjugates and other polyphenolics in muscadine grapes by HPLC-ESI-MS. J. Agric. Food Chem. 2005, 53, 6003–6010.
- Lu, J.; Yuan, Q. A new method for ellagic acid production from pomegranate husk. J. Food Process. Eng. 2008, 31, 443–454.
- Hillis, W.E. The distribution and formation of polyphenols within the tree. In Wood Extractives and Their Significance to the Pulp and Paper Industries; Hillis, W.E., Ed.; ACADEMIC PRESS INC.: New York, NY, USA, 1962; pp. 59–131. ISBN 978-1-4832-3321-5.
- Santos, S.A.O.; Villaverde, J.J.; Sousa, A.F.; Coelho, J.F.J.; Neto, C.P.; Silvestre, A.J.D. Phenolic composition and antioxidant activity of industrial cork by-products. Ind. Crops Prod. 2013, 47, 262–269.
- Santos, S.A.O.; Vilela, C.; Domingues, R.M.A.; Oliveira, C.S.D.; Villaverde, J.J.; Freire, C.S.R.; Neto, C.P.; Silvestre, A.J.D. Secondary metabolites from Eucalyptus grandis wood cultivated in Portugal, Brazil and South Africa. Ind. Crops Prod. 2017, 95, 357–364.
- Costa, E.V.; Lima, D.L.D.; Evtyugin, D.V.; Esteves, V.I. Development and application of a capillary electrophoresis method for the determination of ellagic acid in E. globulus wood and in filtrates from E. globulus kraft pulp. Wood Sci. Technol. 2014, 48, 99–108.
- Conde, E.; Cadahia, E.; Garciavallejo, M.; Tomasbarberan, F. Low molecular weight polyphenols in wood and bark of Eucalyptus globulus. Wood Fiber Sci. 1995, 27, 379–383.
- Charrier, B.; Marques, M.; Haluk, J.P. HPLC analysis of gallic and ellagic acids in european oakwood (Quercus robur L.) and eucalyptus (Eucalyptus globulus). Holzforschung 1992, 46, 87–89.
- Elgailani, I.E.H.; Ishak, C.Y. Determination of tannins of three common Acacia species of Sudan. Adv. Chem. 2014, 1–5.
- Sanz, M.; Cadahía, E.; Esteruelas, E.; Muñoz, Á.M.; Fernández De Simón, B.; Hernández, T.; Estrella, I. Phenolic compounds in chestnut (Castanea sativa Mill.) heartwood. Effect of toasting at cooperage. J. Agric. Food Chem. 2010, 58, 9631–9640.
- Fengel, D.; Wegener, G. Extractives. In Wood—Chemistry, Ultrastructure, Reactions; Walter de Gruyter: Berlin, Germany, 1989; pp. 182–226. ISBN 3-11-012059-3.
- Daniel, E.M.; Krupnick, A.S.; Heur, Y.H.; Blinzler, J.A.; Nims, R.W.; Stoner, G.D. Extraction, stability, and quantitation of ellagic acid in various fruits and nuts. J. Food Compos. Anal. 1989, 2, 338–349.
- dos Santos, W.N.L.; da Silva Sauthier, M.C.; dos Santos, A.M.P.; de Andrade Santana, D.; Azevedo, R.S.A.; da Cruz Caldas, J. Simultaneous determination of 13 phenolic bioactive compounds in guava (Psidium guajava L.) by HPLC-PAD with evaluation using PCA and Neural Network Analysis (NNA). Microchem. J. 2017, 133, 583–592.
- Alañón, M.E.; Castro-Vázquez, L.; Díaz-Maroto, M.C.; Hermosín-Gutiérrez, I.; Gordon, M.H.; Pérez-Coello, M.S. Antioxidant capacity and phenolic composition of different woods used in cooperage. Food Chem. 2011, 129, 1584–1590.
- Santos, S.A.O.; Freire, C.S.R.; Domingues, M.R.M.; Silvestre, A.J.D.; Neto, C.P. Characterization of phenolic components in polar extracts of Eucalyptus globulus Labill. bark by high-performance liquid chromatography-mass spectrometry. J. Agric. Food Chem. 2011, 59, 9386–9393.
- Santos, S.A.O.; José, J.; Freire, C.S.R.; Domingues, M.R.M.; Pascoal, C.; Silvestre, A.J.D. Phenolic composition and antioxidant activity of Eucalyptus grandis, E. urograndis (E. grandis × E. urophylla) and E. maidenii bark extracts. Ind. Crop. Prod. 2012, 39, 120–127.
- Dedrie, M.; Jacquet, N.; Bombeck, P.L.; Hébert, J.; Richel, A. Oak barks as raw materials for the extraction of polyphenols for the chemical and pharmaceutical sectors: A regional case study. Ind. Crops Prod. 2015, 70, 316–321.
- Comandini, P.; Lerma-García, M.J.; Simó-Alfonso, E.F.; Toschi, T.G. Tannin analysis of chestnut bark samples (Castanea sativa Mill.) by HPLC-DAD-MS. Food Chem. 2014, 157, 290–295.
- Liu, Z.; Chen, Z.; Han, F.; Kang, X.; Gu, H.; Yang, L. Microwave-assisted method for simultaneous hydrolysis and extraction in obtaining ellagic acid, gallic acid and essential oil from Eucalyptus globulus leaves using Brönsted acidic ionic liquid HSO4. Ind. Crops Prod. 2016, 81, 152–161.
- Alexandri, M.; Papapostolou, H.; Vlysidis, A.; Gardeli, C.; Komaitis, M.; Papanikolaou, S.; Koutinas, A.A. Extraction of phenolic compounds and succinic acid production from spent sulphite liquor. J. Chem. Technol. Biotechnol. 2016, 91, 2751–2760.
- Rana, V.; Joshi, G.; Singh, S.P.; Gupta, P.K. Eucalypts in pulp and paper industry. In Eucalypts in India; Bhojvaid, P.P., Kaushik, S., Singh, Y.P., Kumar, D., Thapliyal, M., Barthwal, S., Eds.; ENVIS Centre on Forestry, Forest Research Institute: Dehradun, India, 2014; pp. 470–506. ISBN 978-93-5174-121-3.
- Rodrigues, P.F.; Evtyugin, D.D.; Evtuguin, D.V.; Prates, A. Extractives profiles in the production of sulphite dissolving pulp from Eucalyptus globulus wood. J. Wood Chem. Technol. 2018, 38, 397–408.
- Gardner, J.A.F.; Hillis, W.E. The influence of extractives on the pulping of wood. In Wood Extractives and Their Significance to the Pulp and Paper Industries; Hillis, W.E., Ed.; ACADEMIC PRESS INC.: New York, NY, USA, 1962; pp. 367–403. ISBN 978-1-4832-3321-5.
- Sjöström, J.; Bädenlid, R.; Norborg, M.A. Short note: Analysis of ellagic acid in pulp mill deposits. Holzforschung 1993, 47, 446–448.
- Zhang, N.Z.; Chen, Y.Y. Synthesis of macroporous ellagitannic acid resin and its chelating properties for metal ions. J. Macromol. Sci. Part A Chem. 1988, 25, 1455–1462.
- Przewloka, S.R.; Shearer, B.J. The further chemistry of ellagic acid II. Ellagic acid and water-soluble ellagates as metal precipitants. Holzforschung 2002, 56, 13–19.
- Reitze, J.D.; Przewloka, S.R.; Shearer, B.J. The further chemistry of ellagic acid I. Synthesis of tetramethylellagic acid and associated polymer precursors. Holzforschung 2001, 55, 171–175.
- Wang, H.; Xu, X.; Lee, C.; Johnson, C.; Sohlberg, K.; Ji, H.F. Highly selective sensing of nitroaromatics using nanomaterials of ellagic acid. J. Phys. Chem. C 2012, 116, 4442–4448.
- Gonçalves, S.S.L.; Rudnitskaya, A.; Sales, A.J.M.; Costa, L.M.C.; Evtuguin, D.V. Nanocomposite Polymeric Materials Based on Eucalyptus Lignoboost® Kraft Lignin for Liquid Sensing Applications. Materials 2020, 13, 1637.
- Barnaby, S.N.; Yu, S.M.; Fath, K.R.; Tsiola, A.; Khalpari, O.; Banerjee, I.A. Ellagic acid promoted biomimetic synthesis of shape-controlled silver nanochains. Nanotechnology 2011, 22, 225605.
- Frayne, S.H.; Barnaby, S.N.; Nakatsuka, N.; Banerjee, I.A. Growth and properties of CdSe nanoparticles on ellagic acid biotemplates for photodegradation applications. Mater. Express 2012, 2, 335–343.
- Kim, S.; Liu, Y.; Gaber, M.W.; Bumgardner, J.D.; Haggard, W.O.; Yang, Y. Development of chitosan-ellagic acid films as a local drug delivery system to induce apoptotic death of human melanoma cells. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 90, 145–155.
- Shaik, M.M.; Kowshik, M. Ellagic acid containing collagen-chitosan scaffolds as potential antioxidative bio-materials for tissue engineering applications. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 208–215.
- Arulmozhi, V.; Pandian, K.; Mirunalini, S. Ellagic acid encapsulated chitosan nanoparticles for drug delivery system in human oral cancer cell line (KB). Colloids Surf. B Biointerfaces 2013, 110, 313–320.
- Mady, F.M.; Shaker, M.A. Enhanced anticancer activity and oral bioavailability of ellagic acid through encapsulation in biodegradable polymeric nanoparticles. Int. J. Nanomed. 2017, 12, 7405–7417.
- Vilela, C.; Pinto, R.J.B.; Coelho, J.; Domingues, M.R.M.; Daina, S.; Sadocco, P.; Santos, S.A.O.; Freire, C.S.R. Bioactive chitosan/ellagic acid films with UV-light protection for active food packaging. Food Hydrocoll. 2017, 73, 120–128.
- Zhang, X.-K.; He, F.; Zhang, B.; Reeves, M.J.; Liu, Y.; Zhao, X.; Duan, C.-Q. The effect of prefermentative addition of gallic acid and ellagic acid on the red wine color, copigmentation and phenolic profiles during wine aging. Food Res. Int. 2018, 106, 568–579.
- González-Sarrías, A.; García-Villalba, R.; Núñez-Sánchez, M.Á.; Tomé-Carneiro, J.; Zafrilla, P.; Mulero, J.; Tomás-Barberán, F.A.; Espín, J.C. Identifying the limits for ellagic acid bioavailability: A crossover pharmacokinetic study in healthy volunteers after consumption of pomegranate extracts. J. Funct. Foods 2015, 19, 225–235.
- Cerdá, B.; Tomás-Barberán, F.A.; Espín, J.C. Metabolism of antioxidant and chemopreventive ellagitannins from strawberries, raspberries, walnuts, and oak-aged wine in humans: Identification of biomarkers and individual variability. J. Agric. Food Chem. 2005, 53, 227–235.
- Gonzalez-Sarrias, A.; Gimenez-Bastida, J.A.; Garcia-Conesa, M.T.; Gomez-Sanchez, M.B.; Garcia-Talavera, N.V.; Gil-Izquierdo, A.; Sanchez-Alvarez, C.; Fontana-Compiano, L.O.; Morga-Egea, J.P.; Pastor-Quirante, F.A.; et al. Occurrence of urolithins, gut microbiota ellagic acid metabolites and proliferation markers expression response in the human prostate gland upon consumption of walnuts and pomegranate juice. Mol. Nutr. Food Res. 2010, 54, 311–322.
- Tomás-Barberán, F.A.; Espín, J.C.; García-Conesa, M.T. Ellagitannins bioavailability and metabolism of ellagic acid and ellagitannins. In Chemistry and Biology of Ellagitannins: An Underestimated Class of Bioactive Plant Polyphenols; Quideau, S., Ed.; World Scientific Publishing: Singapore, 2009; pp. 273–297. ISBN 978-981-279-740-7.
- Tomás-Barberán, F.A.; Gonzalez-Sarrias, A.; García-Villalba, R.; Núñez-Sánchez, M.Á.; Selma, M.V.; Garcia-Conesa, M.T.; Espín, J.C. Urolithins, the rescue of “old” metabolites to understand a “new” concept: Metabotypes as a nexus among phenolic metabolism, microbiota dysbiosis, and host health status. Mol. Nutr. Food Res. 2017, 61.
- Bialonska, D.; Kasimsetty, S.G.; Schrader, K.K.; Ferreira, D. The effect of pomegranate (Punica granatum L.) byproducts and ellagitannins on the growth of human gut bacteria. J. Agric. Food Chem. 2009, 57, 8344–8349.
- Savic, I.M.; Jocic, E.; Nikolic, V.D.; Popsavin, M.M.; Rakic, S.J.; Savic-Gajic, I.M. The effect of complexation with cyclodextrins on the antioxidant and antimicrobial activity of ellagic acid. Pharm. Dev. Technol. 2018, 24, 410–418.
- Funatogawa, K.; Hayashi, S.; Shimomura, H.; Yoshida, T.; Hatano, T.; Ito, H.; Hirai, Y. Antibacterial activity of hydrolyzable tannins derived from medicinal plants against Helicobacter pylori. Microbiol. Immunol. 2004, 48, 251–261.
- Asres, K.; Bucar, F.; Edelsbrunner, S.; Kartnig, T.; Höger, G.; Thiel, W. Investigations on antimycobacterial activity of some Ethiopian medicinal plants. Phyther. Res. 2001, 15, 323–326.
- Kolodziej, H.; Kayser, O.; Kiderlen, A.; Ito, H.; Hatano, T.; Yoshida, T.; Foo, L. Antileishmanial activity of hydrolyzable tannins and their modulatory effects on nitric oxide and tumour necrosis factor-alpha release in macrophages in vitro. Planta Med. 2001, 67, 825–832.
- Soh, P.N.; Witkowski, B.; Olagnier, D.; Nicolau, M.L.; Garcia-Alvarez, M.C.; Berry, A.; Benoit-Vical, F. In vitro and in vivo properties of ellagic acid in malaria treatment. Antimicrob. Agents Chemother. 2009, 53, 1100–1106.
- Beshbishy, A.M.; Batiha, G.E.; Yokoyama, N.; Igarashi, I. Ellagic acid microspheres restrict the growth of Babesia and Theileria in vitro and Babesia microti in vivo. Parasit. Vectors 2019, 12, 269.
- Ascacio-Valdés, J.; Burboa, E.; Aguilera-Carbo, A.F.; Aparicio, M.; Pérez-Schmidt, R.; Rodríguez, R.; Aguilar, C.N. Antifungal ellagitannin isolated from Euphorbia antisyphilitica Zucc. Asian Pac. J. Trop. Biomed. 2013, 3, 41–46.
- Vilhelmova-Ilieva, N.; Jacquet, R.; Quideau, S.; Galabov, A.S. Ellagitannins as synergists of ACV on the replication of ACV-resistant strains of HSV 1 and 2. Antiviral Res. 2014, 110, 104–114.
- BenSaad, L.A.; Kim, K.H.; Quah, C.C.; Kim, W.R.; Shahimi, M. Anti-inflammatory potential of ellagic acid, gallic acid and punicalagin A&B isolated from Punica granatum. BMC Complement. Altern. Med. 2017, 17, 47.
- Bulani, V.D.; Kothavade, P.S.; Nagmoti, D.M.; Kundaikar, H.S.; Degani, M.S.; Juvekar, A.R. Characterisation and anti-inflammatory evaluation of the inclusion complex of ellagic acid with hydroxypropyl-β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2015, 82, 361–372.
- Akileshwari, C.; Raghu, G.; Muthenna, P.; Mueller, N.H.; Suryanaryana, P.; Petrash, J.M.; Reddy, G.B. Bioflavonoid ellagic acid inhibits aldose reductase: Implications for prevention of diabetic complications. J. Funct. Foods 2014, 6, 374–383.
- Al-Sayed, E.; El-Naga, R.N. Protective role of ellagitannins from Eucalyptus citriodora against ethanol-induced gastric ulcer in rats: Impact on oxidative stress, inflammation and calcitonin-gene related peptide. Phytomedicine 2015, 22, 5–15.
- Kannan, M.M.; Quine, S.D. Ellagic acid inhibits cardiac arrhythmias, hypertrophy and hyperlipidaemia during myocardial infarction in rats. Metabolism 2013, 62, 52–61.
- Rogerio, A.P.; Fontanari, C.; Borducchi, É.; Keller, A.C.; Russo, M.; Soares, E.G.; Albuquerque, D.A.; Faccioli, L.H. Anti-inflammatory effects of Lafoensia pacari and ellagic acid in a murine model of asthma. Eur. J. Pharmacol. 2008, 580, 262–270.
- Ratnam, D.V.; Chandraiah, G.; Meena, A.K.; Ramarao, P.; Ravi Kumar, M.N.V. The co-encapsulated antioxidant nanoparticles of ellagic acid and coenzyme Q10 ameliorates hyperlipidemia in high fat diet fed rats. J. Nanosci. Nanotechnol. 2009, 9, 6741–6746.
- Ortiz-Ruiz, C.V.; Berna, J.; Tudela, J.; Varon, R.; Garcia-Canovas, F. Action of ellagic acid on the melanin biosynthesis pathway. J. Dermatol. Sci. 2016, 82, 115–122.




