1000/1000
Hot
Most Recent

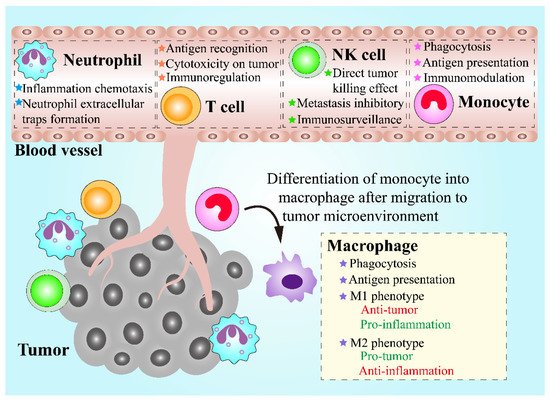
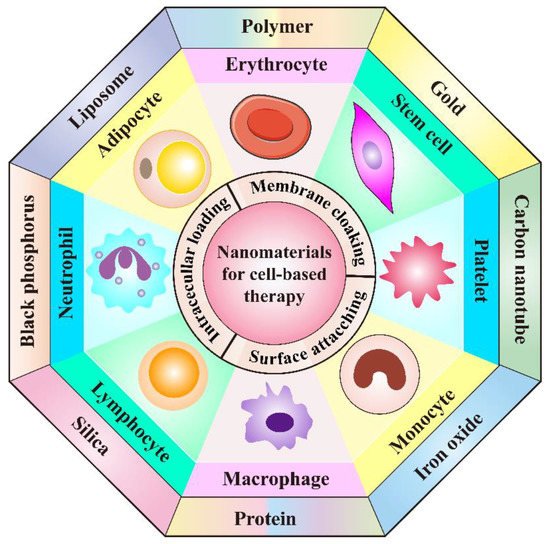
The combination of nanomaterials with cell-based drug delivery systems has shown tremendous advantages in cancer treatment.
| Type of Living Cells/Cell Membranes | Combined Nanomaterials/Applied Targeting Molecules | Advantages | Therapeutic Agents | Tumor Model | Therapeutic Performance | Refs. |
|---|---|---|---|---|---|---|
| Neutrophil membrane | PLGA | Achieve efficient tumor targeting, prolong circulation time and promote cellular internalization | Paclitaxel | Human ovarian adenocarcinoma | Inhibit tumor growth and prolong the survival rate | [24] |
| Neutrophil | PEG-b-PLGA and bacteria-secreted outer membrane vesicles | Improve tumor targeting, combine chemotherapy with PTT | Cisplatin | Murine breast cancer | Completely eradicate tumors | [25] |
| T cell | Gold nanospheres | Improve tumor targeting through the recognition of tumor-associated antigens | AuNPs | Human lymphoma | Achieve specific tumor AuNPs accumulation | [26] |
| Lipid nanocapsules | Achieve lymphoid organ-specific targeting | SN-38 | Murine lymphoma | Reduce tumor burden significantly | [27] | |
| CAR T cell membrane | Mesoporous silica | Improve tumor targeting, prolong circulation time | IR780 | Human hepatocellular carcinoma | Possess significant photothermal antitumor effect and tumor imaging | [28] |
| CAR NK cell | Cross-linked multilamellar liposomal vesicles | Improve tumor targeting | Paclitaxel | Human ovarian cancer | Inhibit tumor growth | [29] |
| NK cell membrane | Liposome | Improve tumor targeting, prolong circulation time | Doxorubicin | Human breast cancer | Inhibit tumor growth | [30] |
| Macrophage | Liposome | Improve tumor targeting, promote cellular internalization, recruited to tumor sites by CCL-2 | Resveratrol and Paclitaxel | Murine breast cancer | Inhibit tumor recurrence | [31] |
| Engineered macrophage | Lipopolysaccharide | Improve tumor targeting, induce secretion of TNF-α | Doxorubicin | Human lung cancer | Increase the inhibitory effects on tumor growth and metastasis | [32] |
| Monocyte | Polymer | Improve tumor targeting | Conjugated polymer NPs (CPNs) | Murine glioblastoma | Efficiently deliver CPNs into glioblastoma sites and improve PDT effect | [33] |
| N/A | Cross endothelial barriers and improve tumor targeting | Doxorubicin | Human glioblastoma | Induce cancer cell damage | [34] | |
| Chitosan polymeric micelles | Improve tumor targeting | N/A | Murine breast cancer | Increase NPs accumulation within tumor sites, enhance antitumor efficacy | [35] | |
| Gold-silver nanorods (AuNRs) | Improve tumor targeting, promote immunostimulation | AuNRs and CpG | Murine lymphoma | Ablate primary tumors and elicit a potent immunity to prevent tumors from metastasis and recurrence | [36] | |
| Erythrocyte | PLGA | Enable lung physiology-assisted shear-responsive targeted delivery | Doxorubicin | Murine melanoma | Inhibit tumor growth and metastasis | [37] |
| Iron oxide-based super-paramagnetic NPs | Improve tumor targeting, prolong circulation time | Monoclonal antibody mAb198.3 | Human colon-rectal cancer | Inhibit tumor growth | [38] | |
| Erythrocyte membrane | DSPE-PEG-mannose | Improve tumor targeting, possess outstanding mobility | Antigen peptides self-assembled NPs | Human breast cancer | Promote DC maturation and CTL activation, achieving broad-spectrum breast cancer inhibition | [39] |
| PLGA | Prolong circulation time, efficiently load and deliver oxygen to hypoxic tumor | Perfluorocarbon | Murine breast cancer | Promote cancer radiotherapy | [40] | |
| Erythrocyte-cancer cell hybrid membrane | N/A | Prolong blood circulation, improve targetability and PTT effect | Melanin nanoparticle | Human breast cancer | Inhibit tumor growth | [41] |
| Platelet | Anti-CD22 antibody | Prolong circulation time and achieve previse delivery of DOX to tumor cells | Doxorubicin | Human lymphoma | Inhibit tumor growth and attenuate cardiotoxicity of DOX | [42] |
| Anti-PD-L1 antibody | Excellent inflammatory targeting ability | N/A | Murine breast cancer | Reduce residual tumor growth and metastasis | [43] | |
| Transferrin | Effectively target melanoma | Doxorubicin | Murine melanoma | Reduce melanoma cell growth and inhibit tumor progression | [44] | |
| Platelet membrane | DSPE-PEOz liposome | Enhance tumor affinity and achieve selective drug release in acidic microenvironment | Doxorubicin | Murine colon cancer, breast cancer and pancreatic carcinoma | Inhibit tumor growth | [45] |
| PLGA | Achieve active targeting and immune evasion abilities | Doxorubicin | Murine breast cancer | Eliminate tumor completely and enhance multimodal imaging | [46] | |
| Fe3O4 NPs | Promote targetability to tumor metastasis | Sulfasalazine | Murine breast cancer | Inhibit the metastatic tumor growth | [47] | |
| BPQDs | Improve drug loading efficiency, enhance biocompatibility and targetability | Hederagenin | Human breast cancer | Inhabit tumor growth and decrease the side effects of myelosuppression | [48] | |
| Mesenchymal stem cell | Carbon nanotubes | Improve tumor homing ability | Doxorubicin | Human lung cancer | Promote lung cancer cell apoptosis and eliminate lung tumor after treatment | [49] |
| Liposome | Enhance the intercellular delivery of DOX and improve tumor targeting ability | Doxorubicin | Murine colon adenocarcinoma | Significantly inhibit tumor proliferation, Suppress primary tumor growth and lung metastasis |
[50] | |
| Genome engineered mesenchymal stem cells | PEI-coated MSNs | Increase tumor homing ability, reduce undesired side effects of anticancer treatment | A suicide fusion gene and uracil phosphoribosyl transferase | murine NMU mammary tumor | Induce NMU cancer cells death | [51] |
| Reconstituted high-density lipoprotein | Increase therapeutic efficiency and tumor targetability | pDNA encoding TRAIL | Murine melanoma | Induce cancer cell apoptosis, inhibit pulmonary metastasis tumor growth | [52] | |
| Adipocyte | N/A | Achieve local and sustained release of chemotherapeutics within the TME | Rumenic acid and doxorubicin prodrug | Murine melanoma | Promote antitumor efficacy, downregulate of PD-L1 expression | [53] |
| Adipose-derived stem cell | Superparamagnetic iron oxide NPs | Improve selective delivery | Paclitaxel | Murine brain tumor | Enhance therapeutic efficacy and prolong survival time | [54] |
| PLGA | Achieve sustained drug release and increase tumor targeting ability | Pirarubicin | Human pancreatic cancer | Inhibit tumor growth, induce the apoptosis of tumor cells, cause minimal side effects | [55] | |
| Lipid droplet | N/A | Promote anticancer therapy through metabolic intervention | Pyrolipid | Human ovarian cancer | Inhibit tumor growth | [56] |