Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Materials Science, Biomaterials
The combination of nanomaterials with cell-based drug delivery systems has shown tremendous advantages in cancer treatment.
- cell therapy
- biomimetic strategy
- nanomaterial
- cancer treatment
1. Introduction
Recent decades have witnessed the tremendous progress of nanobiotechnology in cancer treatment [1,2]. Nano-based drug delivery systems (DDS) are one of the most widely investigated strategies to improve the targetability of therapeutic molecules, increase circulation time, and enhance the total bioavailability [3]. As important constituents of nano-based DDS, various nanomaterials like organic nanomaterials, inorganic nanomaterials, and hybrid nanomaterials have been intensively explored in anticancer drug delivery, owing to their unique properties [4,5,6]. The delivery of therapeutic agents using nanomaterials holds numerous advantages over their free drug counterparts, which can not only protect the encapsulated drugs from degradation or inactivation before reaching sites of action, but also enable the controlled drug release in specific environments. In addition, both passive and active targeting can be achieved via the enhanced permeability and retention (EPR) phenomenon, or by means of extra modification [7,8]. Moreover, owing to the EPR effect, nanosystems possess the ability to improve the accumulation of chemotherapeutics both as single agent and in combination, which largely elevates the amounts of drugs in target tissue [9,10,11,12]. When it comes to active targeting, two main approaches are currently adopted to improve tumor accumulation of nanoparticles. One is to apply targeting molecules to endow the nanosystems with targetability [13,14]. The other is to modulate the protein corona of nanocarriers to provide a “natural targeting” towards TME [15,16,17]. In theory, nano-based DDS can be employed as an ideal vehicle in cancer treatment. However, many obstacles still impede the wide application of nanomedicine. For instance, although the EPR effect and active targeting approaches can modulate the biodistribution of nanomedicine to a certain extent, only a part of nanomedicine can reach the tumor sites while the majority of them are cleared by the reticuloendothelial system (RES) [18]. Moreover, EPR effect tends to be more efficient in some angiogenic tumor models with leaky blood, there are still some cases that are not suitable for EPR effect [19]. Besides, modification using targeting ligands could potentially compromise the stealth ability of the nano-based DDS [20]. Therefore, other novel strategies in combination with nanotechnology is of great necessity to achieve improved therapeutic performance.
Hopefully, cell-mediated drug delivery has become a promising approach in addressing the aforementioned limitations. This innovative strategy takes advantage of the natural properties of various cells such as prolonged circulation time in blood stream, specific targetability to tumor cells, the ability to cross challenging biological barriers, abundant surface ligands, flexible morphology, and cellular signaling or metabolism [21]. Very recently, d’Avanzo et al. reported a kind of peptide-functionalized-liposomes that were able to selectively bind breast cancer cells, which in vitro demonstrated the ability of the resultant liposomes to target M2-macrophages to exploit a potential hitchhiking effect in vivo, bridging the gap between conventional nanosystems and cell-derived ones [22]. Therefore, cooperating advanced nanomaterials with cell-based therapies can largely strengthen the total therapeutic efficacy through maximizing the advantages of both, while minimizing their inherent shortcomings. In this review, a number of novel DDSs, based on various cell types including leucocytes, erythrocytes, platelets, stem cells, and adipocytes will be highlighted (Figure 1). Different cell types possess distinctive properties, which enables their multifunctional application in personalized cancer treatment. It is discussed how nanomaterials empower the field of cell-based treatment and how cellular characteristics improve the performance of nanomedicine. Diverse delivery strategies that utilize living cell internalization or cell membrane-cloaking in cooperation with multiple treatment modalities including chemotherapy, phototherapy, gene therapy and immunotherapy will be introduced in detail. Table 1 is a summary of nanomaterials in cell-based drug delivery for cancer treatment. In addition, compared to the existing review articles about cell-based therapies, the novelty of this review is that we comprehensively summarize the most widely used cell types in cyto-pharmaceuticals, which highlights the combinational strategy of innovative nanomaterials and cell-derived vectors with very latest research examples [21,23]. Moreover, the current limitations and future orientations for cell-based therapies are also discussed in this paper to provide more detailed instruction for the development of cell therapies.
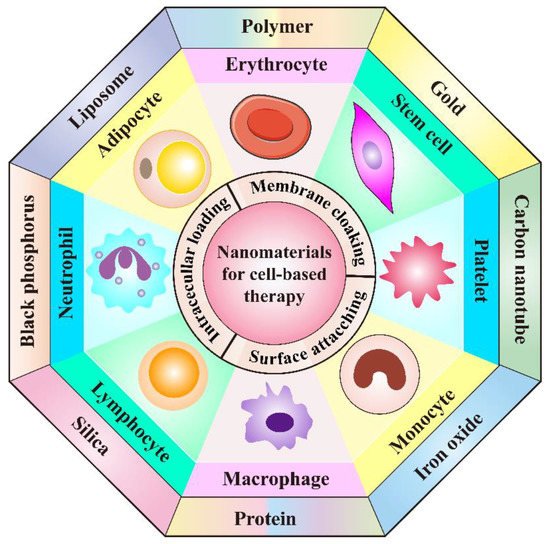
Figure 1. Schematic illustration of the cooperation of cell-based therapies with novel nanomaterials in cancer treatment.
Table 1. Overview of the combination of nanomaterials in cell-based drug delivery systems for cancer treatment.
| Type of Living Cells/Cell Membranes | Combined Nanomaterials/Applied Targeting Molecules | Advantages | Therapeutic Agents | Tumor Model | Therapeutic Performance | Refs. |
|---|---|---|---|---|---|---|
| Neutrophil membrane | PLGA | Achieve efficient tumor targeting, prolong circulation time and promote cellular internalization | Paclitaxel | Human ovarian adenocarcinoma | Inhibit tumor growth and prolong the survival rate | [24] |
| Neutrophil | PEG-b-PLGA and bacteria-secreted outer membrane vesicles | Improve tumor targeting, combine chemotherapy with PTT | Cisplatin | Murine breast cancer | Completely eradicate tumors | [25] |
| T cell | Gold nanospheres | Improve tumor targeting through the recognition of tumor-associated antigens | AuNPs | Human lymphoma | Achieve specific tumor AuNPs accumulation | [26] |
| Lipid nanocapsules | Achieve lymphoid organ-specific targeting | SN-38 | Murine lymphoma | Reduce tumor burden significantly | [27] | |
| CAR T cell membrane | Mesoporous silica | Improve tumor targeting, prolong circulation time | IR780 | Human hepatocellular carcinoma | Possess significant photothermal antitumor effect and tumor imaging | [28] |
| CAR NK cell | Cross-linked multilamellar liposomal vesicles | Improve tumor targeting | Paclitaxel | Human ovarian cancer | Inhibit tumor growth | [29] |
| NK cell membrane | Liposome | Improve tumor targeting, prolong circulation time | Doxorubicin | Human breast cancer | Inhibit tumor growth | [30] |
| Macrophage | Liposome | Improve tumor targeting, promote cellular internalization, recruited to tumor sites by CCL-2 | Resveratrol and Paclitaxel | Murine breast cancer | Inhibit tumor recurrence | [31] |
| Engineered macrophage | Lipopolysaccharide | Improve tumor targeting, induce secretion of TNF-α | Doxorubicin | Human lung cancer | Increase the inhibitory effects on tumor growth and metastasis | [32] |
| Monocyte | Polymer | Improve tumor targeting | Conjugated polymer NPs (CPNs) | Murine glioblastoma | Efficiently deliver CPNs into glioblastoma sites and improve PDT effect | [33] |
| N/A | Cross endothelial barriers and improve tumor targeting | Doxorubicin | Human glioblastoma | Induce cancer cell damage | [34] | |
| Chitosan polymeric micelles | Improve tumor targeting | N/A | Murine breast cancer | Increase NPs accumulation within tumor sites, enhance antitumor efficacy | [35] | |
| Gold-silver nanorods (AuNRs) | Improve tumor targeting, promote immunostimulation | AuNRs and CpG | Murine lymphoma | Ablate primary tumors and elicit a potent immunity to prevent tumors from metastasis and recurrence | [36] | |
| Erythrocyte | PLGA | Enable lung physiology-assisted shear-responsive targeted delivery | Doxorubicin | Murine melanoma | Inhibit tumor growth and metastasis | [37] |
| Iron oxide-based super-paramagnetic NPs | Improve tumor targeting, prolong circulation time | Monoclonal antibody mAb198.3 | Human colon-rectal cancer | Inhibit tumor growth | [38] | |
| Erythrocyte membrane | DSPE-PEG-mannose | Improve tumor targeting, possess outstanding mobility | Antigen peptides self-assembled NPs | Human breast cancer | Promote DC maturation and CTL activation, achieving broad-spectrum breast cancer inhibition | [39] |
| PLGA | Prolong circulation time, efficiently load and deliver oxygen to hypoxic tumor | Perfluorocarbon | Murine breast cancer | Promote cancer radiotherapy | [40] | |
| Erythrocyte-cancer cell hybrid membrane | N/A | Prolong blood circulation, improve targetability and PTT effect | Melanin nanoparticle | Human breast cancer | Inhibit tumor growth | [41] |
| Platelet | Anti-CD22 antibody | Prolong circulation time and achieve previse delivery of DOX to tumor cells | Doxorubicin | Human lymphoma | Inhibit tumor growth and attenuate cardiotoxicity of DOX | [42] |
| Anti-PD-L1 antibody | Excellent inflammatory targeting ability | N/A | Murine breast cancer | Reduce residual tumor growth and metastasis | [43] | |
| Transferrin | Effectively target melanoma | Doxorubicin | Murine melanoma | Reduce melanoma cell growth and inhibit tumor progression | [44] | |
| Platelet membrane | DSPE-PEOz liposome | Enhance tumor affinity and achieve selective drug release in acidic microenvironment | Doxorubicin | Murine colon cancer, breast cancer and pancreatic carcinoma | Inhibit tumor growth | [45] |
| PLGA | Achieve active targeting and immune evasion abilities | Doxorubicin | Murine breast cancer | Eliminate tumor completely and enhance multimodal imaging | [46] | |
| Fe3O4 NPs | Promote targetability to tumor metastasis | Sulfasalazine | Murine breast cancer | Inhibit the metastatic tumor growth | [47] | |
| BPQDs | Improve drug loading efficiency, enhance biocompatibility and targetability | Hederagenin | Human breast cancer | Inhabit tumor growth and decrease the side effects of myelosuppression | [48] | |
| Mesenchymal stem cell | Carbon nanotubes | Improve tumor homing ability | Doxorubicin | Human lung cancer | Promote lung cancer cell apoptosis and eliminate lung tumor after treatment | [49] |
| Liposome | Enhance the intercellular delivery of DOX and improve tumor targeting ability | Doxorubicin | Murine colon adenocarcinoma | Significantly inhibit tumor proliferation, Suppress primary tumor growth and lung metastasis |
[50] | |
| Genome engineered mesenchymal stem cells | PEI-coated MSNs | Increase tumor homing ability, reduce undesired side effects of anticancer treatment | A suicide fusion gene and uracil phosphoribosyl transferase | murine NMU mammary tumor | Induce NMU cancer cells death | [51] |
| Reconstituted high-density lipoprotein | Increase therapeutic efficiency and tumor targetability | pDNA encoding TRAIL | Murine melanoma | Induce cancer cell apoptosis, inhibit pulmonary metastasis tumor growth | [52] | |
| Adipocyte | N/A | Achieve local and sustained release of chemotherapeutics within the TME | Rumenic acid and doxorubicin prodrug | Murine melanoma | Promote antitumor efficacy, downregulate of PD-L1 expression | [53] |
| Adipose-derived stem cell | Superparamagnetic iron oxide NPs | Improve selective delivery | Paclitaxel | Murine brain tumor | Enhance therapeutic efficacy and prolong survival time | [54] |
| PLGA | Achieve sustained drug release and increase tumor targeting ability | Pirarubicin | Human pancreatic cancer | Inhibit tumor growth, induce the apoptosis of tumor cells, cause minimal side effects | [55] | |
| Lipid droplet | N/A | Promote anticancer therapy through metabolic intervention | Pyrolipid | Human ovarian cancer | Inhibit tumor growth | [56] |
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics13111888
This entry is offline, you can click here to edit this entry!
