Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Chad VanSant-Webb | + 2050 word(s) | 2050 | 2021-11-08 08:03:20 | | | |
| 2 | Jessie Wu | + 13 word(s) | 2063 | 2021-11-22 03:15:48 | | | | |
| 3 | Jessie Wu | Meta information modification | 2063 | 2021-11-25 03:36:37 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Vansant-Webb, C. Intratumor Heterogeneity in Hepatocellular Carcinoma: Challenges and Opportunities. Encyclopedia. Available online: https://encyclopedia.pub/entry/16213 (accessed on 07 February 2026).
Vansant-Webb C. Intratumor Heterogeneity in Hepatocellular Carcinoma: Challenges and Opportunities. Encyclopedia. Available at: https://encyclopedia.pub/entry/16213. Accessed February 07, 2026.
Vansant-Webb, Chad. "Intratumor Heterogeneity in Hepatocellular Carcinoma: Challenges and Opportunities" Encyclopedia, https://encyclopedia.pub/entry/16213 (accessed February 07, 2026).
Vansant-Webb, C. (2021, November 19). Intratumor Heterogeneity in Hepatocellular Carcinoma: Challenges and Opportunities. In Encyclopedia. https://encyclopedia.pub/entry/16213
Vansant-Webb, Chad. "Intratumor Heterogeneity in Hepatocellular Carcinoma: Challenges and Opportunities." Encyclopedia. Web. 19 November, 2021.
Copy Citation
Hepatocellular carcinoma (HCC) represents a leading cause of cancer-related death, but it remains difficult to treat. Intratumor genetic and phenotypic heterogeneity are inherent properties of breast, skin, lung, prostate, and brain tumors, and intratumor heterogeneity (ITH) helps define prognosis and therapeutic response in these cancers. Several recent studies estimate that ITH is inherent to HCC and attribute the clinical intractability of HCC to this heterogeneity.
intratumor heterogeneity
Hepatocellular carcinoma
beta catenin
TERT
CTNNB1
1. Introduction
Hepatocellular carcinoma (HCC) is a leading cause of cancer-related mortality [1]. Regardless of etiology, HCC has few druggable targets, which makes treatment of advanced HCC challenging. Patients with early-stage HCC benefit from surgical interventions which can be curative, whereas late-stage patients are treated with locoregional treatments like chemoembolization and/or systemic chemotherapeutics. The multikinase inhibitor sorafenib extends survival by 4.3 months (95% CI, 4.0 to 5.6), and the combination of the vascular endothelial growth factor (VEGF) inhibitor bevacizumab and the immune checkpoint inhibitor atezolizumab extends overall survival by 6.8 months (95% CI, 5.7 to 8.3) [2]. Not only do these first-line chemotherapies only modestly improve survival, but molecular testing for guiding HCC treatment is used little, if at all [3], underscoring the need for new drug development strategies in HCC.
Intratumor heterogeneity (ITH) influences tumor progression, aggression, therapeutic resistance, and disease relapse across cancer types [4][5]. Although cancer was considered a clonal disease for several decades, ideas of genomic diversity within single tumors emerged in the 1950s [4]. Subsequently, mouse mammary tumor studies in the 1970s and 1980s documented phenotypic heterogeneity, with cells from different sections of the same tumor varying in growth rate, immunogenicity, ability to metastasize, and response to drugs [6]. The first studies demonstrating clonal interaction and cooperation emerged in the 1980s and 1990s [6]. The widespread use of next-generation sequencing (NGS) and other computational tools on multiregional and liquid biopsies have propelled in-depth investigation into the tumor type-specific and context-driven characteristics of ITH.
2. Tumor Microenvironment ITH
Variations in stromal cells represent another important type of ITH. HCC occurs and progresses in the context of a dynamically evolving tumor microenvironment that includes tumor-resident stem/progenitor cells, activated hepatic stellate cells, carcinoma-associated fibroblasts, myofibroblasts, endothelial cells, pericytes, dendritic cells, and tumor-infiltrating immune cells [7]. The development of innovative, more effective therapies for HCC requires an improved understanding of the entire hepatic milieu, which forms the objective of some recent investigations.
Most studies examining tumor microenvironment ITH have focused on immune cells. Through single-cell transcriptomic profiling of 5063 T-cells from peripheral blood, tumor, and adjacent normal tissues of six patients with HCC, Zheng et al. identified 11 distinct subtypes of tumor-infiltrating lymphocytes (TILs) [8]. Tregs and exhausted CD8+ T-cells accumulated within HCC tumors; 82% of the Treg cells were unique and did not share T-cell receptors (TCRs) with tumor CD4+ cells or adjacent normal tissue Tregs, thus demonstrating T-cell ITH [8]. Kurebayashi et al. characterized the tumor immune microenvironment of 919 regions of 158 HCCs and found that the tumor microenvironment could be classified based on B- and T-cell infiltration levels into three immune subtypes—high, medium, and low. Although all patients could be categorized into one of the three immune subtypes based on the predominant pattern, over 50% of patients exhibited ITH with respect to these subtypes [9].
Losic et al. leveraged a combination of RNA sequencing, DNA sequencing, T-cell receptor sequencing, and single-nucleotide polymorphism array data to characterize cancer-cell-immune cell interactions across multiple regions of HCC specimens from 14 patients [10]. In three patient tumors, the authors observed significant ITH in tumor infiltrating lymphocyte (TIL) burden in different regions of the tumor, as measured by the number of RNA-seq reads that mapped to the VDJ loci. Tumor regions from two patients also showed ITH with respect to TIL architecture and the presence of tertiary lymphoid structures. These structures are correlated with a lower risk of HCC recurrence [11], thereby implicating a prognostic value for immune cell ITH. The authors next investigated the relationship between tumor cell mutational status and immunogenicity by using in silico predicted neoepitope–HLA allele binding affinities to quantify the likelihood of neoepitope presentation and recognition by a T-cell. In five out of 11 patients, these binding affinities varied significantly between regions from the same tumor, indicating that tumor cell mutational heterogeneity likely underlies heterogeneity in T-cell response. In one patient, branch mutations were associated with significantly higher immunogenicity scores compared to trunk mutations, in line with the notion that early driver mutations are immune-evasive.
Tumor microenvironment ITH can potentially influence treatment eligibility in cancer patients. In a recent study, Shen et al. used subdivided single-sample biopsies to analyze ITH within the immune tumor microenvironment [12]. They performed IHC and RNAseq on 77 regional samples from 13 individual HCC biopsies, 12 of which were of viral etiology. One of the eight IHC markers used was programmed death ligand 1 (PD-L1), a marker expressed by cancer cells to inhibit the immune system. Their study identified eight cases (62%) which had no PD-L1 expression in any tumor sample, one case (7%) which had homogenous PD-L1 expression in all regional samples, and four cases (30%) which had heterogeneous PD-L1 staining between tumor regions. Furthermore, they noted regional differences in tertiary lymphoid structures in 57% of cases [12]. Overall, the study found a single biopsy could accurately survey the tumor immune microenvironment in 60% to 70% of cases of HCC. Biopsy of cases with heterogeneous PD-L1 staining could potentially miss PD-L1 expression, affecting treatment decisions. While PD-L1 expression is not yet used in HCC to determine eligibility for immunotherapies, Food and Drug Administration (FDA) approvals for immune checkpoint inhibitor therapy are linked to specific levels of PD-L1 expression for several other cancers including non-small-cell lung cancer and bladder cancer [13].
HCC is a highly vascularized malignancy [14], but there are conflicting data with regard to microvessel density (MVD) and microvascular invasion (MVI) as predictive indicators for patient prognosis [14][15]. One possible explanation for these discrepant results is that the amount of vascular ITH may influence tumor behavior independent from the degree of MVD or MVI. Two recent imaging studies have addressed this possibility, using coded harmonic angio ultrasound (CHA) [16] and fractal density analysis of contrast enhanced-CT (CE-CT) [17] to analyze the inter- and intratumoral vasculature. Utilizing CHA to compare liver nodules of HCCs, hepatic metastases, and benign lesions, Jang et al. identified that the majority of HCCs (69%) had a heterogeneous, “irregular branching” pattern, and 27% showed “random stippling” [16]. The “irregular branching” pattern was seen exclusively in HCCs, and CHA could clearly distinguish HCCs from other hepatic nodules. In a phase II clinical trial, Hayano et al. used CE-CT with fractal density analysis to identify ITH and to determine the effects of sunitinib on intratumoral vasculature [17]. They found that patients with favorable progression-free survival had significantly lower fractal density (less ITH and less abnormal vasculature) at baseline. Furthermore, Hayano et al. determined that greater reduction in fractal density post-treatment with sunitinib significantly correlated with better progression-free survival and overall survival. Together, these studies support the hypothesis that vascular ITH is present in HCC and influences tumor behavior.
Cancer-associated fibroblasts (CAFs) shape the extracellular matrix and are key contributors to ITH. CAFs display an array of different phenotypes, cell surface markers, and functions reflecting diverse potential origins including activated hepatic stellate cells [18], mesenchymal stem cells [19], and endothelial cells [20]. CAFs are usually considered tumor-promoting, but they can be tumor-suppressive as well [21], indicating marked heterogeneity in function.
The effect of CAFs on tumor behavior may be dependent on which cell surface marker(s) they express. While no cell surface marker is specific to CAFs, they typically display some combination of α-smooth muscle actin (α-SMA), fibroblast activating protein (FAP), and/or vimentin [22]. When staining CAFs for α-SMA, Takamura et al. found an inverse correlation between α-SMA staining and both disease-free survival and overall survival in HCC patients [23]. A similar study with a larger cohort also found an inverse correlation between α-SMA staining and disease-free survival [24]. However, Kim et al. did not find an association between FAP-positive CAFs and HCC prognosis [25]. It is possible that different patient characteristics or methodologies could account for the contradictory results among these three studies. However, these reports also raise the intriguing possibility that α-SMA-expressing CAFs could have stronger tumor-promoting effects than FAP-expressing CAFs, supporting heterogeneity in CAF function. This hypothesis could be tested by examining the correlation between prognosis and different CAF markers including α-SMA and FAP in the same set of HCC patients.
3. Clinical Consequences of ITH for HCC
ITH presents several challenges for HCC diagnosis, prognosis, and therapy. As demonstrated by Xue et al. [26], distinct lesions from a single HCC patient can possess different genetic landscapes, implying that molecular analyses of cells derived from single-region biopsies can misrepresent tumor properties, incorrectly biasing clinical decisions. Even in the case of solitary tumor nodules, single-region biopsies may not capture subclonal mutations that might serve as druggable targets or sources of potential therapeutic resistance. ITH is even more important to investigate in the setting of multifocal HCC as each focus can be composed of subclones, harboring potentially clinically relevant aberrations, as highlighted by Xu et al. [27]. Multiregional biopsies can be technically challenging to perform in solid tumors, and there is a relatively high risk of bleeding in HCC patients who usually have chronic liver disease.
Liquid biopsies are recent additions to efforts to measure ITH, leveraging the availability of circulating tumor cells, DNA, and/or T-cell antigen receptors. Since the release of these molecules into the bloodstream by tumors is not fully characterized or uniform, findings from these measurements may need to be interpreted with caution. However, Huang et al. found that more than 80% of mutations in TP53, TERT, and CTNNB1 in circulating tumor DNA were concordant with their status in paired HCC tumor tissue [28]. Future studies will be required to determine if more heterogeneous mutations such as those in PIK3CA are well-represented by liquid biopsy.
Following assessment of ITH, the prediction of how the identified ITH will impact clinical outcomes is not straightforward. Measurement of ITH results in a steep increase in the number of variables that need to be considered when building mathematical models to forecast clinical outcome. Recent efforts begin to address this computational challenge. The DARWIN clinical trial aims to evaluate whether targeting a clonal versus subclonal mutation with the anti-PD-L1 drug atezolizumab alters progression-free survival in patients with non-small-cell lung cancer (NCT02183883). Lin et al. [29] used the MATH algorithm to demonstrate that higher methylation ITH scores may indicate worse survival outcomes for HCC patients. Further studies directly investigating the relationship between ITH and patient outcome in HCC are critical to determine how widely the MATH algorithm can be applied and to developing improved models for predicting clinical outcomes based in ITH.
ITH plays a critical role in therapeutic resistance via various potential mechanisms, so careful consideration of ITH is helpful when formulating clinical strategies [5][30] (Figure 1). Tumor cells can develop drug resistance through genetic amplification of the therapeutic target, point mutations that affect the ability of the therapeutic to inhibit the oncogenic pathway, and/or amplification/inhibition of other genes that compensate for the drug-inhibited oncogene [31]. When devising treatment strategies for heterogeneous HCCs, it is important to consider that each cell within a single tumor could respond differentially to therapeutic stress via one or more of these resistance mechanisms. With treatment, pre-existing, undiagnosed, and untargeted ITH can result in the expansion of smaller subclonal population(s) as a result of positive selection, with the destruction of drug-responsive subclone(s) [5]. Alternatively, therapy can induce a subset of cells within the tumor to undergo resistance-conferring epigenetic changes [5]. Clonal cooperation is also thought to influence drug sensitivity [6]. In HCC, long-term treatment with sorafenib promotes epithelial-to-mesenchymal transition via the PI3 kinase/AKT-snail pathway in vitro [32], and tumors resistant to sorafenib upregulate insulin-like and fibroblast growth factor signaling pathways in vivo in animal models [33]. Further studies in animal models defining the degree of ITH in HCC before and after sorafenib administration will help elucidate which resistance mechanisms are most relevant to HCC and help determine how post-sorafenib ITH affects response to second-line therapies.
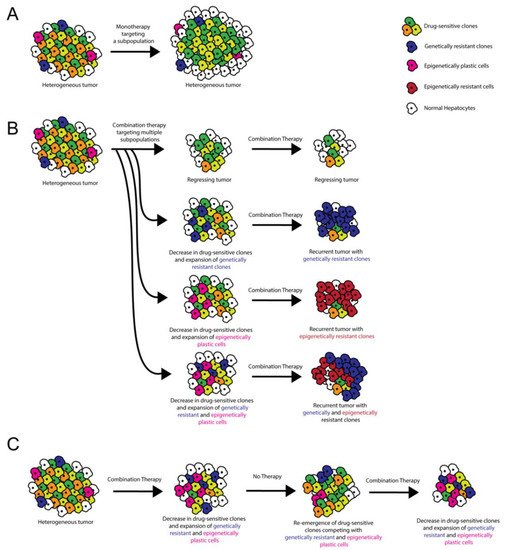
Figure 1. Role of ITH in targeted therapy resistance and treatment stratagem. (A) Monotherapies target a subset of tumor cells, resulting in incomplete tumor attrition. (B) Combination therapies target multiple oncogenic pathways, potentially resulting in tumor regression. However, recurrent tumors can occur when pre-existing ITH or ITH induced by cell–cell/cell–microenvironment interactions results in the emergence of genetically and/or epigenetically resistant clones. (C) Combination therapy administered with alternating periods without treatment allows for the disappearance and re-emergence of drug-sensitive clones. Competition between clones limits the emergence of resistant clones.
References
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular Carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6.
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905.
- Villanueva, A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462.
- McGranahan, N.; Swanton, C. Clonal Heterogeneity and Tumor Evolution: Past, Present, and the Future. Cell 2017, 168, 613–628.
- Marusyk, A.; Janiszewska, M.; Polyak, K. Intratumor Heterogeneity: The Rosetta Stone of Therapy Resistance. Cancer Cell 2020, 37, 471–484.
- Tabassum, D.P.; Polyak, K. Tumorigenesis: It Takes a Village. Nat. Rev. Cancer 2015, 15, 473–483.
- Carloni, V.; Luong, T.V.; Rombouts, K. Hepatic Stellate Cells and Extracellular Matrix in Hepatocellular Carcinoma: More Complicated than Ever. Liver Int. 2014, 34, 834–843.
- Zheng, C.; Zheng, L.; Yoo, J.-K.; Guo, H.; Zhang, Y.; Guo, X.; Kang, B.; Hu, R.; Huang, J.Y.; Zhang, Q.; et al. Landscape of Infiltrating T Cells in Liver Cancer Revealed by Single-Cell Sequencing. Cell 2017, 169, 1342–1356.e16.
- Kurebayashi, Y.; Ojima, H.; Tsujikawa, H.; Kubota, N.; Maehara, J.; Abe, Y.; Kitago, M.; Shinoda, M.; Kitagawa, Y.; Sakamoto, M. Landscape of Immune Microenvironment in Hepatocellular Carcinoma and Its Additional Impact on Histological and Molecular Classification. Hepatology 2018, 68, 1025–1041.
- Losic, B.; Craig, A.J.; Villacorta-Martin, C.; Martins-Filho, S.N.; Akers, N.; Chen, X.; Ahsen, M.E.; von Felden, J.; Labgaa, I.; D’Avola, D.; et al. Intratumoral Heterogeneity and Clonal Evolution in Liver Cancer. Nat. Commun. 2020, 11, 291.
- Calderaro, J.; Petitprez, F.; Becht, E.; Laurent, A.; Hirsch, T.Z.; Rousseau, B.; Luciani, A.; Amaddeo, G.; Derman, J.; Charpy, C.; et al. Intra-Tumoral Tertiary Lymphoid Structures Are Associated with a Low Risk of Early Recurrence of Hepatocellular Carcinoma. J. Hepatol. 2019, 70, 58–65.
- Shen, Y.-C.; Hsu, C.-L.; Jeng, Y.-M.; Ho, M.-C.; Ho, C.-M.; Yeh, C.-P.; Yeh, C.-Y.; Hsu, M.-C.; Hu, R.-H.; Cheng, A.-L. Reliability of a Single-Region Sample to Evaluate Tumor Immune Microenvironment in Hepatocellular Carcinoma. J. Hepatol. 2020, 72, 489–497.
- Davis, A.A.; Patel, V.G. The Role of PD-L1 Expression as a Predictive Biomarker: An Analysis of All US Food and Drug Administration (FDA) Approvals of Immune Checkpoint Inhibitors. J. Immunother. Cancer 2019, 7, 278.
- Morse, M.A.; Sun, W.; Kim, R.; He, A.R.; Abada, P.B.; Mynderse, M.; Finn, R.S. The Role of Angiogenesis in Hepatocellular Carcinoma. Clin. Cancer Res. 2019, 25, 912–920.
- Zhang, Q.; Wu, J.; Bai, X.; Liang, T. Evaluation of Intra-Tumoral Vascularization in Hepatocellular Carcinomas. Front. Med. 2020, 7, 584250.
- Jang, H.-J.; Lim, H.K.; Lee, W.J.; Kim, S.H.; Kim, M.J.; Choi, D.; Lee, S.J.; Lim, J.H. Focal Hepatic Lesions: Evaluation with Contrast-Enhanced Gray-Scale Harmonic US. Korean J. Radiol. 2003, 4, 91.
- Hayano, K.; Yoshida, H.; Zhu, A.X.; Sahani, D.V. Fractal Analysis of Contrast-Enhanced CT Images to Predict Survival of Patients with Hepatocellular Carcinoma Treated with Sunitinib. Dig. Dis. Sci. 2014, 59, 1996–2003.
- Yin, C.; Evason, K.J.; Asahina, K.; Stainier, D.Y.R. Hepatic Stellate Cells in Liver Development, Regeneration, and Cancer. J. Clin. Invest. 2013, 123, 1902–1910.
- Sukowati, C.H.C.; Anfuso, B.; Crocé, L.S.; Tiribelli, C. The Role of Multipotent Cancer Associated Fibroblasts in Hepatocarcinogenesis. BMC Cancer 2015, 15, 188.
- Zeisberg, E.M.; Potenta, S.; Xie, L.; Zeisberg, M.; Kalluri, R. Discovery of Endothelial to Mesenchymal Transition as a Source for Carcinoma-Associated Fibroblasts. Cancer Res. 2007, 67, 10123–10128.
- Ping, Q.; Yan, R.; Cheng, X.; Wang, W.; Zhong, Y.; Hou, Z.; Shi, Y.; Wang, C.; Li, R. Cancer-Associated Fibroblasts: Overview, Progress, Challenges, and Directions. Cancer Gene Ther. 2021, 28, 984–999.
- Yin, Z.; Dong, C.; Jiang, K.; Xu, Z.; Li, R.; Guo, K.; Shao, S.; Wang, L. Heterogeneity of Cancer-Associated Fibroblasts and Roles in the Progression, Prognosis, and Therapy of Hepatocellular Carcinoma. J. Hematol. Oncol. 2019, 12, 101.
- Takamura, H.; Nakanuma, S.; Hayashi, H.; Tajima, H.; Kakinoki, K.; Sakai, S.; Makino, I.; Nakagawara, H.; Miyashita, T.; Okamoto, K.; et al. Evaluation of Eligibility Criteria in Living Donor Liver Transplantation for Hepatocellular Carcinoma by α-SMA-Positive Cancer-Associated Fibroblasts. Oncol. Rep. 2013, 30, 1561–1574.
- Fang, M.; Yuan, J.; Chen, M.; Sun, Z.; Liu, L.; Cheng, G.; Ying, H.; Yang, S.; Chen, M. The Heterogenic Tumor Microenvironment of Hepatocellular Carcinoma and Prognostic Analysis Based on Tumor Neo-Vessels, Macrophages and α-SMA. Oncol. Lett. 2018, 15, 4805–4812.
- Kim, G.J.; Rhee, H.; Yoo, J.E.; Ko, J.E.; Lee, J.S.; Kim, H.; Choi, J.S.; Park, Y.N. Increased Expression of CCN2, Epithelial Membrane Antigen, and Fibroblast Activation Protein in Hepatocellular Carcinoma with Fibrous Stroma Showing Aggressive Behavior. PLoS ONE 2014, 9, e105094.
- Xue, R.; Li, R.; Guo, H.; Guo, L.; Su, Z.; Ni, X.; Qi, L.; Zhang, T.; Li, Q.; Zhang, Z.; et al. Variable Intra-Tumor Genomic Heterogeneity of Multiple Lesions in Patients with Hepatocellular Carcinoma. Gastroenterology 2016, 150, 998–1008.
- Xu, L.X.; He, M.H.; Dai, Z.H.; Yu, J.; Wang, J.G.; Li, X.C.; Jiang, B.B.; Ke, Z.F.; Su, T.H.; Peng, Z.W.; et al. Genomic and Transcriptional Heterogeneity of Multifocal Hepatocellular Carcinoma. Ann. Oncol. 2019, 30, 990–997.
- Huang, A.; Zhang, X.; Zhou, S.-L.; Cao, Y.; Huang, X.-W.; Fan, J.; Yang, X.-R.; Zhou, J. Detecting Circulating Tumor DNA in Hepatocellular Carcinoma Patients Using Droplet Digital PCR Is Feasible and Reflects Intratumoral Heterogeneity. J. Cancer 2016, 7, 1907–1914.
- Lin, D.-C.; Mayakonda, A.; Dinh, H.Q.; Huang, P.; Lin, L.; Liu, X.; Ding, L.; Wang, J.; Berman, B.P.; Song, E.-W.; et al. Genomic and Epigenomic Heterogeneity of Hepatocellular Carcinoma. Cancer Res. 2017, 77, 2255–2265.
- Craig, A.J.; von Felden, J.; Garcia-Lezana, T.; Sarcognato, S.; Villanueva, A. Tumour Evolution in Hepatocellular Carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 139–152.
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Adv. Pharm. Bull. 2017, 7, 339–348.
- Hu, G.; Zhang, Y.; Ouyang, K.; Xie, F.; Fang, H.; Yang, X.; Liu, K.; Wang, Z.; Tang, X.; Liu, J.; et al. In vivo Acquired Sorafenib-Resistant Patient-Derived Tumor Model Displays Alternative Angiogenic Pathways, Multi-Drug Resistance and Chromosome Instability. Oncol. Lett. 2018, 16, 3439–3446.
- Firtina Karagonlar, Z.; Koc, D.; Iscan, E.; Erdal, E.; Atabey, N. Elevated Hepatocyte Growth Factor Expression as an Autocrine C-Met Activation Mechanism in Acquired Resistance to Sorafenib in Hepatocellular Carcinoma Cells. Cancer Sci. 2016, 107, 407–416.
More
Information
Subjects:
Biochemistry & Molecular Biology; Pathology; Cell Biology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
772
Revisions:
3 times
(View History)
Update Date:
25 Nov 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




