
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Lorenza Putignani | + 10621 word(s) | 10621 | 2021-07-23 05:49:56 | | | |
| 2 | Peter Tang | -6057 word(s) | 4564 | 2021-11-02 05:08:58 | | | | |
| 3 | Lindsay Dong | Meta information modification | 4564 | 2021-11-12 02:31:52 | | |
Video Upload Options
Autism spectrum disorder (ASD) is a complex behavioral syndrome that is characterized by speech and language disorders, intellectual impairment, learning and motor dysfunctions. Several genetic and environmental factors are suspected to affect the ASD phenotype including air pollution, exposure to pesticides, maternal infections, inflammatory conditions, dietary factors or consumption of antibiotics during pregnancy. Many children with ASD shows abnormalities in gastrointestinal (GI) physiology, including increased intestinal permeability, overall microbiota alterations, and gut infection. Moreover, they are “picky eaters” and the existence of specific sensory patterns in ASD patients could represent one of the main aspects in hampering feeding. GI disorders are associated with an altered composition of the gut microbiota. Gut microbiome is able to communicate with brain activities through microbiota-derived signaling molecules, immune mediators, gut hormones as well as vagal and spinal afferent neurons. Since the diet induces changes in the intestinal microbiota and in the production of molecules, such as the SCFA, we wanted to investigate the role that nutritional intervention can have on GI microbiota composition and thus on its influence on behavior, GI symptoms and microbiota composition and report which are the beneficial effect on ASD condition.
1. Introduction
2. The role of Nutrition and Interventions in ASD
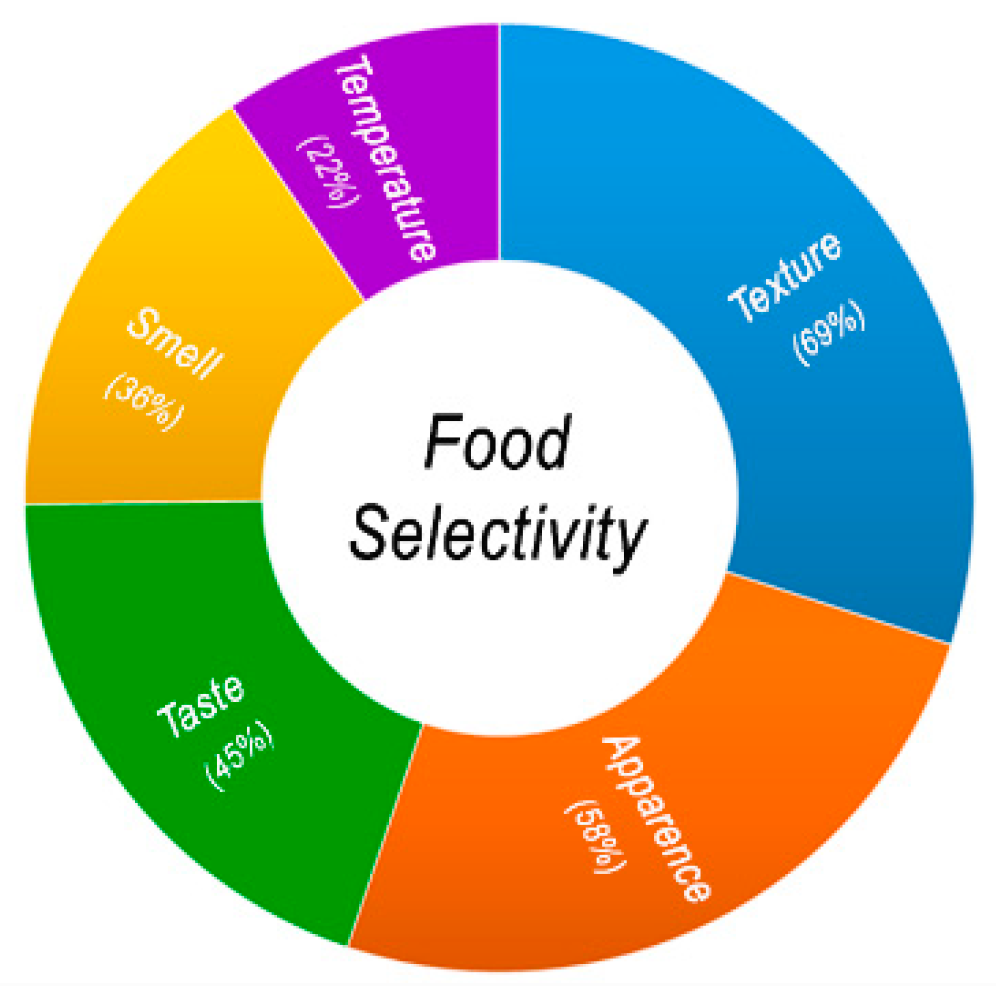
2.1. Food Selectivity and ASD
2.2. Nutrient Intake and ASDs.
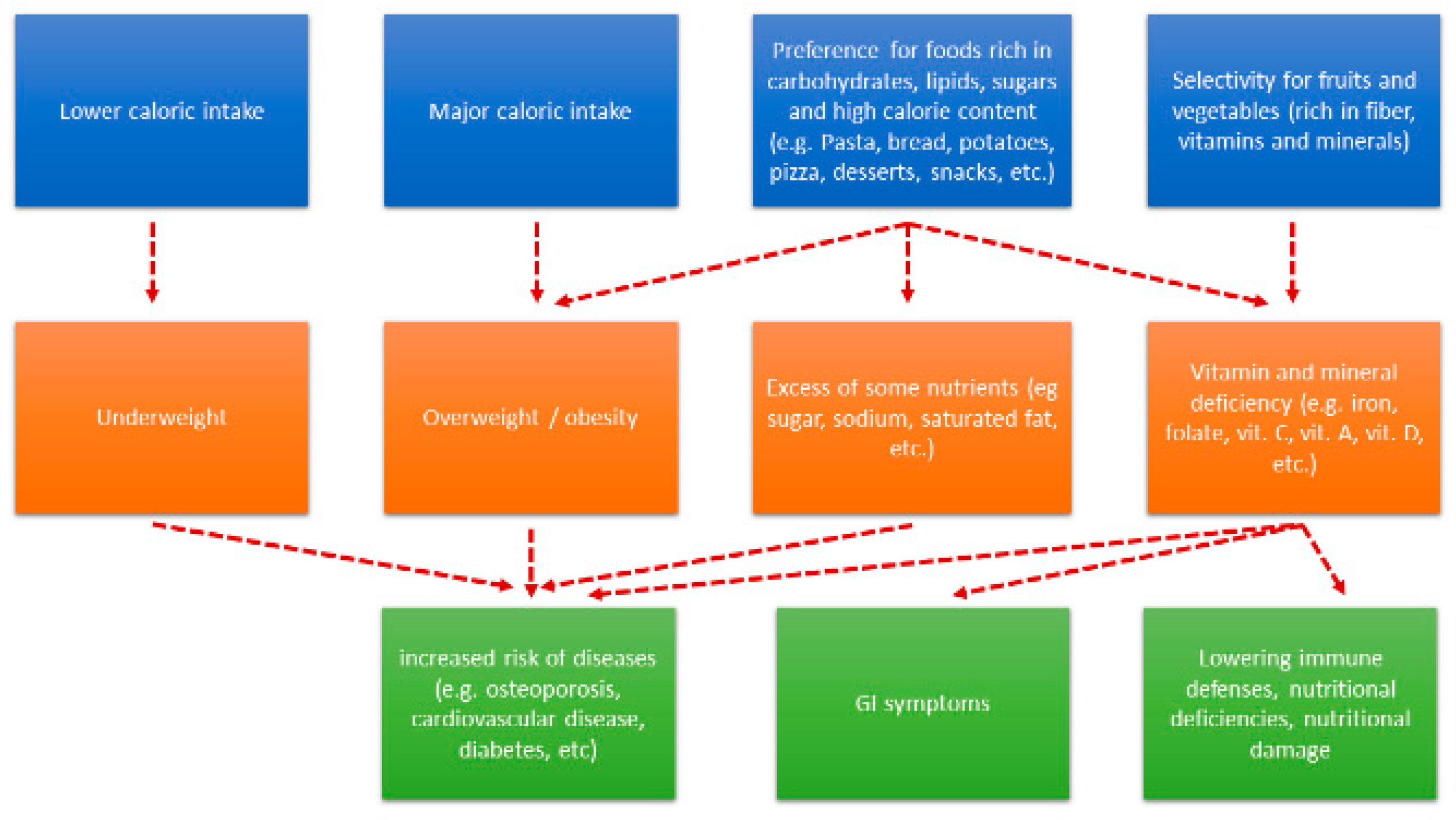
2.3. Effects of Dietary Interventions in ASD
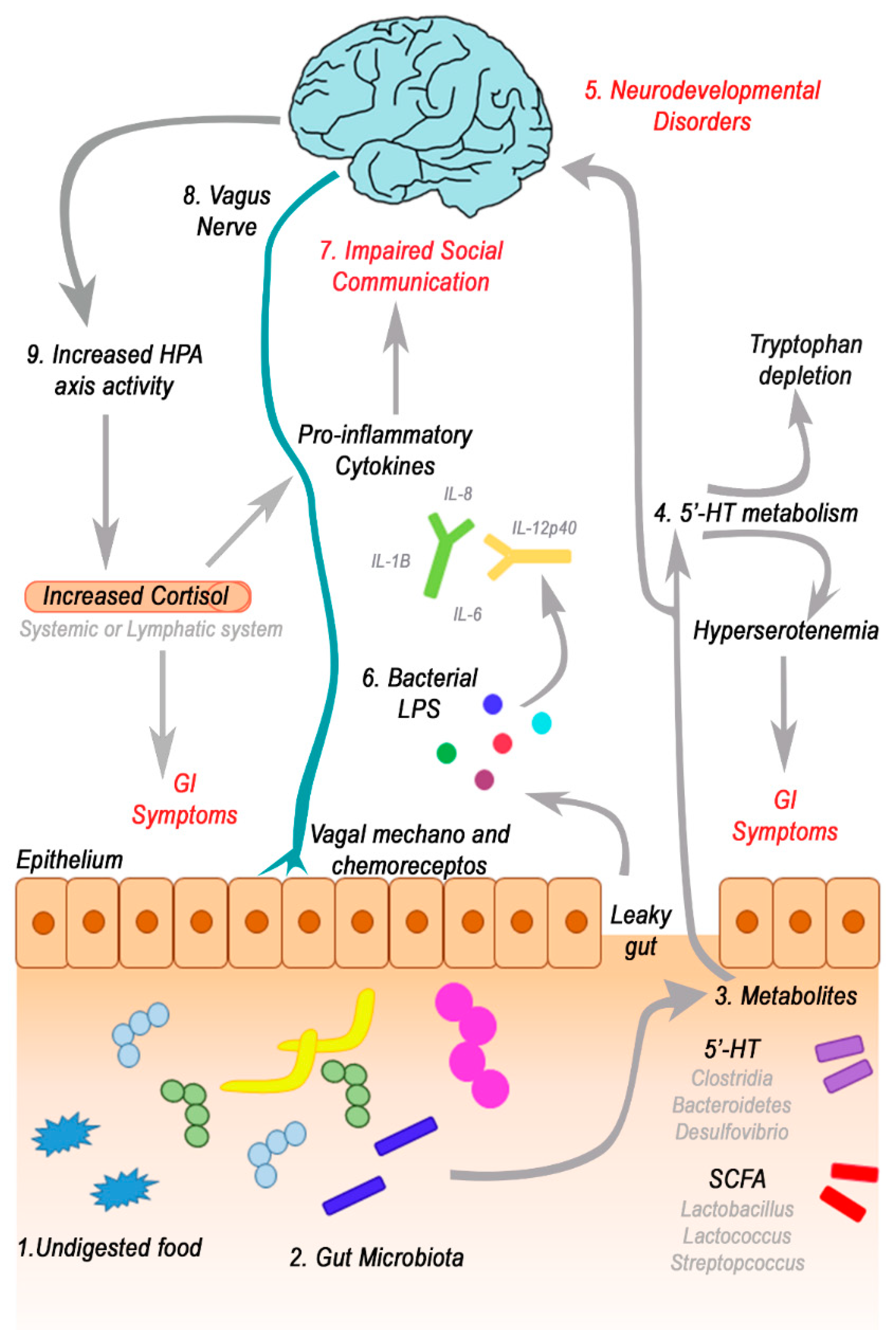
3. The role of GI Symptoms, Gut Microbiota and Gut-Brain Axis in ASD
3.1. GI symptoms in Children with Autism
3.2. GI Disorders, Microbiota and Microbiota-Gut-Brain Axis Alteration in ASD
3.3. Focus on Bacterial Metabolites and Gut-Brain Axis
3.4. Short-Chain Fatty Acids (SCFAs) and Gut-Microbial Metabolites
3.5. Neurotransmitters
4. Conclusions
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013; ISBN 978-0-89042-555-8.
- Munson, J.; Dawson, G.; Sterling, L.; Beauchaine, T.; Zhou, A.; Elizabeth, K.; Lord, C.; Rogers, S.; Sigman, M.; Estes, A.; et al. Evidence for latent classes of IQ in young children with autism spectrum disorder. Am. J. Ment. Retard. 2008, 113, 439–452.
- Baio, J.; Wiggins, L.; Christensen, D.L.; Maenner, M.J.; Daniels, J.; Warren, Z.; Kurzius-Spencer, M.; Zahorodny, W.; Robinson Rosenberg, C.; White, T.; et al. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. MMWR Surveill. Summ. 2018, 67, 1–23.
- Nomi, J.S.; Uddin, L.Q. Developmental changes in large-scale network connectivity in autism. Neuroimage Clin. 2015, 7, 732–741.
- Uddin, L.Q.; Supekar, K.; Menon, V. Reconceptualizing functional brain connectivity in autism from a developmental perspective. Front. Hum. Neurosci. 2013, 7, 458.
- Gaugler, T.; Klei, L.; Sanders, S.J.; Bodea, C.A.; Goldberg, A.P.; Lee, A.B.; Mahajan, M.; Manaa, D.; Pawitan, Y.; Reichert, J.; et al. Most genetic risk for autism resides with common variation. Nat. Genet. 2014, 46, 881–885.
- Huguet, G.; Ey, E.; Bourgeron, T. The Genetic Landscapes of Autism Spectrum Disorders. Annu. Rev. Genom. Hum. Genet. 2013, 14, 191–213.
- Sandin, S.; Lichtenstein, P.; Kuja-Halkola, R.; Hultman, C.; Larsson, H.; Reichenberg, A. The Heritability of Autism Spectrum Disorder. JAMA 2017, 318, 1182–1184.
- Chaste, P.; Leboyer, M. Autism risk factors: Genes, environment, and gene-environment interactions. Dialogues Clin. Neurosci. 2012, 14, 281–292.
- Cross-Disorder Group of the Psychiatric Genomics Consortium Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat. Genet. 2013, 45, 984–994.
- Durand, C.M.; Betancur, C.; Boeckers, T.M.; Bockmann, J.; Chaste, P.; Fauchereau, F.; Nygren, G.; Rastam, M.; Gillberg, I.C.; Anckarsäter, H.; et al. Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat. Genet. 2007, 39, 25–27.
- Griswold, A.J.; Ma, D.; Cukier, H.N.; Nations, L.D.; Schmidt, M.A.; Chung, R.H.; Jaworski, J.M.; Salyakina, D.; Konidari, I.; Whitehead, P.L.; et al. Evaluation of copy number variations reveals novel candidate genes in autism spectrum disorder-associated pathways. Hum. Mol. Genet. 2012, 21, 3513–3523.
- Guilmatre, A.; Huguet, G.; Delorme, R.; Bourgeron, T. The emerging role of SHANK genes in neuropsychiatric disorders: SHANK Genes in Neuropsychiatric Disorders. Dev. Neurobiol. 2014, 74, 113–122.
- Leblond, C.S.; Nava, C.; Polge, A.; Gauthier, J.; Huguet, G.; Lumbroso, S.; Giuliano, F.; Stordeur, C.; Depienne, C.; Mouzat, K.; et al. Meta-analysis of SHANK Mutations in Autism Spectrum Disorders: A Gradient of Severity in Cognitive Impairments. PLoS Genet. 2014, 10, e1004580.
- Peça, J.; Feliciano, C.; Ting, J.T.; Wang, W.; Wells, M.F.; Venkatraman, T.N.; Lascola, C.D.; Fu, Z.; Feng, G. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature 2011, 472, 437–442.
- Jonsson, L.; Zettergren, A.; Pettersson, E.; Hovey, D.; Anckarsäter, H.; Westberg, L.; Lichtenstein, P.; Lundström, S.; Melke, J. Association study between autistic-like traits and polymorphisms in the autism candidate regions RELN, CNTNAP2, SHANK3, and CDH9/10. Mol. Autism 2014, 5, 55.
- Poot, M.; Beyer, V.; Schwaab, I.; Damatova, N.; van’t Slot, R.; Prothero, J.; Holder, S.E.; Haaf, T. Disruption of CNTNAP2 and additional structural genome changes in a boy with speech delay and autism spectrum disorder. Neurogenetics 2010, 11, 81–89.
- Wilkinson, B.; Grepo, N.; Thompson, B.L.; Kim, J.; Wang, K.; Evgrafov, O.V.; Lu, W.; Knowles, J.A.; Campbell, D.B. The autism-associated gene chromodomain helicase DNA-binding protein 8 (CHD8) regulates noncoding RNAs and autism-related genes. Transl. Psychiatry 2015, 5, e568.
- Obrenovich, M.E.; Shamberger, R.J.; Lonsdale, D. Altered heavy metals and transketolase found in autistic spectrum disorder. Biol. Trace Elem. Res. 2011, 144, 475–486.
- Splawski, I.; Yoo, D.S.; Stotz, S.C.; Cherry, A.; Clapham, D.E.; Keating, M.T. CACNA1H mutations in autism spectrum disorders. J. Biol. Chem. 2006, 281, 22085–22091.
- Raz, R.; Roberts, A.L.; Lyall, K.; Hart, J.E.; Just, A.C.; Laden, F.; Weisskopf, M.G. Autism spectrum disorder and particulate matter air pollution before, during, and after pregnancy: A nested case-control analysis within the Nurses’ Health Study II Cohort. Environ. Health Perspect. 2015, 123, 264–270.
- Herbert, M.R. Contributions of the environment and environmentally vulnerable physiology to autism spectrum disorders. Curr. Opin. Neurol. 2010, 23, 103–110.
- Schmidt, R.J.; Lyall, K.; Hertz-Picciotto, I. Environment and Autism: Current State of the Science. Cut Edge Psychiatry Pract. 2014, 1, 21–38.
- Lyall, K.; Munger, K.L.; O’Reilly, É.J.; Santangelo, S.L.; Ascherio, A. Maternal Dietary Fat Intake in Association with Autism Spectrum Disorders. Am. J. Epidemiol. 2013, 178, 209–220.
- DeVilbiss, E.A.; Gardner, R.M.; Newschaffer, C.J.; Lee, B.K. Maternal folate status as a risk factor for autism spectrum disorders: A review of existing evidence. Br. J. Nutr. 2015, 114, 663–672.
- Horvath, K.; Perman, J.A. Autistic disorder and gastrointestinal disease. Curr. Opin. Pediatr. 2002, 14, 583–587.
- Cermak, S.A.; Curtin, C.; Bandini, L.G. Food Selectivity and Sensory Sensitivity in Children with Autism Spectrum Disorders. J. Am. Diet. Assoc. 2010, 110, 238–246.
- McElhanon, B.O.; McCracken, C.; Karpen, S.; Sharp, W.G. Gastrointestinal Symptoms in Autism Spectrum Disorder: A Meta-analysis. Pediatrics 2014, 133, 872–883.
- Adams, J.B.; Johansen, L.J.; Powell, L.D.; Quig, D.; Rubin, R.A. Gastrointestinal flora and gastrointestinal status in children with autism—Comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 2011, 11, 22.
- Buie, T.; Campbell, D.B.; Fuchs, G.J.; Furuta, G.T.; Levy, J.; Vandewater, J.; Whitaker, A.H.; Atkins, D.; Bauman, M.L.; Beaudet, A.L.; et al. Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASDs: A consensus report. Pediatrics 2010, 125, S1–S18.
- Nikolov, R.N.; Bearss, K.E.; Lettinga, J.; Erickson, C.; Rodowski, M.; Aman, M.G.; McCracken, J.T.; McDougle, C.J.; Tierney, E.; Vitiello, B.; et al. Gastrointestinal Symptoms in a Sample of Children with Pervasive Developmental Disorders. J. Autism Dev. Disord. 2009, 39, 405–413.
- Onore, C.; Careaga, M.; Ashwood, P. The role of immune dysfunction in the pathophysiology of autism. BrainBehav. Immun. 2012, 26, 383–392.
- Mead, J.; Ashwood, P. Evidence supporting an altered immune response in ASD. Immunol. Lett. 2015, 163, 49–55.
- D’Eufemia, P.; Celli, M.; Finocchiaro, R.; Pacifico, L.; Viozzi, L.; Zaccagnini, M.; Cardi, E.; Giardini, O. Abnormal intestinal permeability in children with autism. Acta Paediatr. 1996, 85, 1076–1079.
- De Magistris, L.; Familiari, V.; Pascotto, A.; Sapone, A.; Frolli, A.; Iardino, P.; Carteni, M.; De Rosa, M.; Francavilla, R.; Riegler, G.; et al. Alterations of the intestinal barrier in patients with autism spectrum disorders and in their first-degree relatives. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 418–424.
- De Angelis, M.; Piccolo, M.; Vannini, L.; Siragusa, S.; De Giacomo, A.; Serrazzanetti, D.I.; Cristofori, F.; Guerzoni, M.E.; Gobbetti, M.; Francavilla, R. Fecal Microbiota and Metabolome of Children with Autism and Pervasive Developmental Disorder Not Otherwise Specified. PLoS ONE 2013, 8, e76993.
- Finegold, S.M.; Dowd, S.E.; Gontcharova, V.; Liu, C.; Henley, K.E.; Wolcott, R.D.; Youn, E.; Summanen, P.H.; Granpeesheh, D.; Dixon, D.; et al. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe 2010, 16, 444–453.
- Mulle, J.G.; Sharp, W.G.; Cubells, J.F. The Gut Microbiome: A New Frontier in Autism Research. Curr. Psychiatry Rep. 2013, 15, 337.
- Parracho, H.M.R.T.; Bingham, M.O.; Gibson, G.R.; McCartney, A.L. Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J. Med. Microbiol. 2005, 54, 987–991.
- Diaz Heijtz, R.; Wang, S.; Anuar, F.; Qian, Y.; Björkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052.
- Elsden, S.R.; Hilton, M.G.; Waller, J.M. The end products of the metabolism of aromatic amino acids by Clostridia. Arch. Microbiol. 1976, 107, 283–288.
- Finegold, S.M.; Molitoris, D.; Song, Y.; Liu, C.; Vaisanen, M.L.; Bolte, E.; McTeague, M.; Sandler, R.; Wexler, H.; Marlowe, E.M.; et al. Gastrointestinal microflora studies in late-onset autism. Clin. Infect. Dis. 2002, 35, S6–S16.
- Selmer, T.; Andrei, P.I. p-Hydroxyphenylacetate decarboxylase from Clostridium difficile. A novel glycyl radical enzyme catalysing the formation of p-cresol. Eur. J. Biochem. 2001, 268, 1363–1372.
- Song, Y.L.; Liu, C.X.; McTeague, M.; Summanen, P.; Finegold, S.M. Clostridium bartlettii sp. nov., isolated from human faeces. Anaerobe 2004, 10, 179–184.
- Berding, K.; Donovan, S.M. Microbiome and nutrition in autism spectrum disorder: Current knowledge and research needs. Nutr. Rev. 2016, 74, 723–736.
- De Theije, C.G.M.; Wu, J.; da Silva, S.L.; Kamphuis, P.J.; Garssen, J.; Korte, S.M.; Kraneveld, A.D. Pathways underlying the gut-to-brain connection in autism spectrum disorders as future targets for disease management. Eur. J. Pharmacol. 2011, 668, S70–S80.
- Carruth, B.R.; Ziegler, P.J.; Gordon, A.; Barr, S.I. Prevalence of picky eaters among infants and toddlers and their caregivers’ decisions about offering a new food. J. Am. Diet. Assoc. 2004, 104, s57–s64.
- Carruth, B.R.; Skinner, J.D. Revisiting the picky eater phenomenon: Neophobic behaviors of young children. J. Am. Coll. Nutr. 2000, 19, 771–780.
- Bryant-Waugh, R.; Markham, L.; Kreipe, R.E.; Walsh, B.T. Feeding and eating disorders in childhood. Int. J. Eat. Disord. 2010, 43, 98–111.
- Kreipe, R.E.; Palomaki, A. Beyond picky eating: Avoidant/restrictive food intake disorder. Curr. Psychiatry Rep. 2012, 14, 421–431.
- Twachtman-Reilly, J.; Amaral, S.C.; Zebrowski, P.P. Addressing feeding disorders in children on the autism spectrum in school-based settings: Physiological and behavioral issues. Lang. Speech Hear. Serv. Sch. 2008, 39, 261–272.
- Williams, P.G.; Dalrymple, N.; Neal, J. Eating habits of children with autism. Pediatr. Nurs. 2000, 26, 259–264.
- Schreck, K.A.; Williams, K. Food preferences and factors influencing food selectivity for children with autism spectrum disorders. Res. Dev. Disabil. 2006, 27, 353–363.
- Klein, U.; Nowak, A.J. Characteristics of patients with autistic disorder (AD) presenting for dental treatment: A survey and chart review. Spec. Care Dentist. 1999, 19, 200–207.
- Marí-Bauset, S.; Zazpe, I.; Mari-Sanchis, A.; Llopis-González, A.; Morales-Suárez-Varela, M. Food selectivity in autism spectrum disorders: A systematic review. J. Child Neurol. 2014, 29, 1554–1561.
- Sharp, W.G.; Berry, R.C.; McCracken, C.; Nuhu, N.N.; Marvel, E.; Saulnier, C.A.; Klin, A.; Jones, W.; Jaquess, D.L. Feeding problems and nutrient intake in children with autism spectrum disorders: A meta-analysis and comprehensive review of the literature. J. Autism. Dev. Disord. 2013, 43, 2159–2173.
- Curtin, C.; Anderson, S.E.; Must, A.; Bandini, L. The prevalence of obesity in children with autism: A secondary data analysis using nationally representative data from the National Survey of Children’s Health. BMC Pediatr. 2010, 10, 11.
- Berry, R.C.; Novak, P.; Withrow, N.; Schmidt, B.; Rarback, S.; Feucht, S.; Criado, K.K.; Sharp, W.G. Nutrition Management of Gastrointestinal Symptoms in Children with Autism Spectrum Disorder: Guideline from an Expert Panel. J. Acad. Nutr. Diet. 2015, 115, 1919–1927.
- Cornish, E. A balanced approach towards healthy eating in autism. J. Hum. Nutr. Diet. 1998, 11, 501–509.
- Nadon, G.; Feldman, D.E.; Dunn, W.; Gisel, E. Mealtime problems in children with autism spectrum disorder and their typically developing siblings: A comparison study. Autism 2011, 15, 98–113.
- Kerwin, M.E.; Eicher, P.S.; Gelsinger, J. Parental Report of Eating Problems and Gastrointestinal Symptoms in Children with Pervasive Developmental Disorders. Child. Health Care 2005, 34, 217–234.
- Schmitt, L.; Heiss, C.; Campbell, E. A Comparison of Nutrient Intake and Eating Behaviors of Boys with and Without Autism. Top. Clin. Nutr. 2008, 23, 23–31.
- Spek, A.A.; van Rijnsoever, W.; van Laarhoven, L.; Kiep, M. Eating Problems in Men and Women with an Autism Spectrum Disorder. J. Autism Dev. Disord. 2019, 24, 1–8.
- Karlsson, L.; Råstam, M.; Wentz, E. The SWedish Eating Assessment for Autism spectrum disorders (SWEAA)-Validation of a self-report questionnaire targeting eating disturbances within the autism spectrum. Res. Dev. Disabil. 2013, 34, 2224–2233.
- Lane, A.E.; Molloy, C.A.; Bishop, S.L. Classification of children with autism spectrum disorder by sensory subtype: A case for sensory-based phenotypes. Autism Res. 2014, 7, 322–333.
- Miller, L.J.; Anzalone, M.E.; Lane, S.J.; Cermak, S.A.; Osten, E.T. Concept evolution in sensory integration: A proposed nosology for diagnosis. Am. J. Occup. Ther. 2007, 61, 135–140.
- Bandini, L.G.; Anderson, S.E.; Curtin, C.; Cermak, S.; Evans, E.W.; Scampini, R.; Maslin, M.; Must, A. Food selectivity in children with autism spectrum disorders and typically developing children. J. Pediatr. 2010, 157, 259–264.
- Jen, M.; Yan, A.C. Syndromes associated with nutritional deficiency and excess. Clin. Dermatol. 2010, 28, 669–685.
- Zimmer, M.H.; Hart, L.C.; Manning-Courtney, P.; Murray, D.S.; Bing, N.M.; Summer, S. Food variety as a predictor of nutritional status among children with autism. J. Autism Dev. Disord. 2012, 42, 549–556.
- Marí-Bauset, S.; Llopis-González, A.; Zazpe-García, I.; Marí-Sanchis, A.; Morales-Suárez-Varela, M. Nutritional status of children with autism spectrum disorders (ASDs): A case-control study. J. Autism Dev. Disord. 2015, 45, 203–212.
- Lockner, D.W.; Crowe, T.K.; Skipper, B.J. Dietary intake and parents’ perception of mealtime behaviors in preschool-age children with autism spectrum disorder and in typically developing children. J. Am. Diet. Assoc. 2008, 108, 1360–1363.
- Emond, A.; Emmett, P.; Steer, C.; Golding, J. Feeding symptoms, dietary patterns, and growth in young children with autism spectrum disorders. Pediatrics 2010, 126, e337–e342.
- Herndon, A.C.; DiGuiseppi, C.; Johnson, S.L.; Leiferman, J.; Reynolds, A. Does nutritional intake differ between children with autism spectrum disorders and children with typical development? J. Autism Dev. Disord. 2009, 39, 212–222.
- Levy, S.E.; Souders, M.C.; Ittenbach, R.F.; Giarelli, E.; Mulberg, A.E.; Pinto-Martin, J.A. Relationship of dietary intake to gastrointestinal symptoms in children with autistic spectrum disorders. Biol. Psychiatry 2007, 61, 492–497.
- Xia, W.; Zhou, Y.; Sun, C.; Wang, J.; Wu, L. A preliminary study on nutritional status and intake in Chinese children with autism. Eur. J. Pediatr. 2010, 169, 1201–1206.
- Johnson, C.R.; Handen, B.L.; Mayer-Costa, M.; Sacco, K. Eating habits and dietary status in young children with autism. J. Dev. Phys. Disabil. 2008, 20, 437–448.
- Cornish, E. Gluten and casein free diets in autism: A study of the effects on food choice and nutrition. J. Hum. Nutr. Diet. 2002, 15, 261–269.
- Evans, E.W.; Must, A.; Anderson, S.E.; Curtin, C.; Scampini, R.; Maslin, M.; Bandini, L. Dietary Patterns and Body Mass Index in Children with Autism and Typically Developing Children. Res. Autism Spectr. Disord. 2012, 6, 399–405.
- Bicer, A.H.; Alsaffar, A.A. Body mass index, dietary intake and feeding problems of Turkish children with autism spectrum disorder (ASD). Res. Dev. Disabil. 2013, 34, 3978–3987.
- Hyman, S.L.; Stewart, P.A.; Schmidt, B.; Cain, U.; Lemcke, N.; Foley, J.T.; Peck, R.; Clemons, T.; Reynolds, A.; Johnson, C.; et al. Nutrient intake from food in children with autism. Pediatrics 2012, 130, S145–S153.
- Malhi, P.; Venkatesh, L.; Bharti, B.; Singhi, P. Feeding Problems and Nutrient Intake in Children with and without Autism: A Comparative Study. Indian J. Pediatr. 2017, 84, 283–288.
- Al-Farsi, Y.M.; Waly, M.I.; Deth, R.C.; Al-Sharbati, M.M.; Al-Shafaee, M.; Al-Farsi, O.; Al-Khaduri, M.M.; Gupta, I.; Ali, A.; Al-Khalili, M.; et al. Low folate and vitamin B12 nourishment is common in Omani children with newly diagnosed autism. Nutrition 2013, 29, 537–541.
- Hamlin, J.C.; Pauly, M.; Melnyk, S.; Pavliv, O.; Starrett, W.; Crook, T.A.; James, S.J. Dietary intake and plasma levels of choline and betaine in children with autism spectrum disorders. Autism Res. Treat. 2013, 2013, 578429.
- Castro, K.; Faccioli, L.S.; Baronio, D.; Gottfried, C.; Perry, I.S.; dos Santos Riesgo, R. Effect of a ketogenic diet on autism spectrum disorder: A systematic review. Res. Autism Spectr. Disord. 2015, 20, 31–38.
- Hediger, M.L.; England, L.J.; Molloy, C.A.; Yu, K.F.; Manning-Courtney, P.; Mills, J.L. Reduced bone cortical thickness in boys with autism or autism spectrum disorder. J. Autism Dev. Disord. 2008, 38, 848–856.
- Neumeyer, A.M.; O’Rourke, J.A.; Massa, A.; Lee, H.; Lawson, E.A.; McDougle, C.J.; Misra, M. Brief report: Bone fractures in children and adults with autism spectrum disorders. J. Autism Dev. Disord. 2015, 45, 881–887.
- Clark, J.H.; Rhoden, D.K.; Turner, D.S. Symptomatic vitamin A and D deficiencies in an eight-year-old with autism. J. Parenter. Enter. Nutr. 1993, 17, 284–286.
- Stewart, C.; Latif, A. Symptomatic nutritional rickets in a teenager with autistic spectrum disorder. Child Care Health Dev. 2008, 34, 276–278.
- Keown, K.; Bothwell, J.; Jain, S. Nutritional implications of selective eating in a child with autism spectrum disorder. BMJ Case Rep. 2014, 2014.
- Duggan, C.P.; Westra, S.J.; Rosenberg, A.E. Case records of the Massachusetts General Hospital. Case 23-2007. A 9-year-old boy with bone pain, rash, and gingival hypertrophy. N. Engl. J. Med. 2007, 357, 392–400.
- Gongidi, P.; Johnson, C.; Dinan, D. Scurvy in an autistic child: MRI findings. Pediatr. Radiol. 2013, 43, 1396–1399.
- Duvall, M.G.; Pikman, Y.; Kantor, D.B.; Ariagno, K.; Summers, L.; Sectish, T.C.; Mullen, M.P. Pulmonary hypertension associated with scurvy and vitamin deficiencies in an autistic child. Pediatrics 2013, 132, e1699–e1703.
- Kitcharoensakkul, M.; Schulz, C.G.; Kassel, R.; Khanna, G.; Liang, S.; Ngwube, A.; Baszis, K.W.; Hunstad, D.A.; White, A.J. Scurvy revealed by difficulty walking: Three cases in young children. J. Clin. Rheumatol. 2014, 20, 224–228.
- Gulko, E.; Collins, L.K.; Murphy, R.C.; Thornhill, B.A.; Taragin, B.H. MRI findings in pediatric patients with scurvy. Skelet. Radiol. 2015, 44, 291–297.
- Ma, N.S.; Thompson, C.; Weston, S. Brief Report: Scurvy as a Manifestation of Food Selectivity in Children with Autism. J. Autism Dev. Disord. 2016, 46, 1464–1470.
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031.
- Genton, L.; Cani, P.D.; Schrenzel, J. Alterations of gut barrier and gut microbiota in food restriction, food deprivation and protein-energy wasting. Clin. Nutr. 2015, 34, 341–349.
- Tremaroli, V.; Kovatcheva-Datchary, P.; Bäckhed, F. A role for the gut microbiota in energy harvesting? Gut 2010, 59, 1589–1590.
- De Clercq, N.C.; Groen, A.K.; Romijn, J.A.; Nieuwdorp, M. Gut Microbiota in Obesity and Undernutrition. Adv. Nutr. 2016, 7, 1080–1089.
- Buie, T.; Fuchs, G.J.; Furuta, G.T.; Kooros, K.; Levy, J.; Lewis, J.D.; Wershil, B.K.; Winter, H. Recommendations for evaluation and treatment of common gastrointestinal problems in children with ASDs. Pediatrics 2010, 125, S19–S29.
- Molloy, C.A.; Manning-Courtney, P. Prevalence of chronic gastrointestinal symptoms in children with autism and autistic spectrum disorders. Autism 2003, 7, 165–171.
- Valicenti-McDermott, M.; McVicar, K.; Rapin, I.; Wershil, B.K.; Cohen, H.; Shinnar, S. Frequency of gastrointestinal symptoms in children with autistic spectrum disorders and association with family history of autoimmune disease. J. Dev. Behav. Pediatr. 2006, 27, S128–S136.
- Ibrahim, S.H.; Voigt, R.G.; Katusic, S.K.; Weaver, A.L.; Barbaresi, W.J. Incidence of gastrointestinal symptoms in children with autism: A population-based study. Pediatrics 2009, 124, 680–686.
- Wang, L.W.; Tancredi, D.J.; Thomas, D.W. The prevalence of gastrointestinal problems in children across the United States with autism spectrum disorders from families with multiple affected members. J. Dev. Behav. Pediatr. 2011, 32, 351–360.
- Gorrindo, P.; Williams, K.C.; Lee, E.B.; Walker, L.S.; McGrew, S.G.; Levitt, P. Gastrointestinal dysfunction in autism: Parental report, clinical evaluation, and associated factors. Autism Res. 2012, 5, 101–108.
- Chaidez, V.; Hansen, R.L.; Hertz-Picciotto, I. Gastrointestinal problems in children with autism, developmental delays or typical development. J. Autism Dev. Disord. 2014, 44, 1117–1127.
- Ming, X.; Brimacombe, M.; Chaaban, J.; Zimmerman-Bier, B.; Wagner, G.C. Autism spectrum disorders: Concurrent clinical disorders. J. Child Neurol. 2008, 23, 6–13.
- Adams, J.B.; Holloway, C.E.; George, F.; Quig, D. Analyses of toxic metals and essential minerals in the hair of Arizona children with autism and associated conditions, and their mothers. Biol. Trace Elem. Res. 2006, 110, 193–209.
- Horvath, K.; Papadimitriou, J.C.; Rabsztyn, A.; Drachenberg, C.; Tildon, J.T. Gastrointestinal abnormalities in children with autistic disorder. J. Pediatr. 1999, 135, 559–563.
- Field, D.; Garland, M.; Williams, K. Correlates of specific childhood feeding problems. J. Paediatr. Child Health 2003, 39, 299–304.
- Whitehouse, A.J.O.; Maybery, M.; Wray, J.A.; Hickey, M. No association between early gastrointestinal problems and autistic-like traits in the general population. Dev. Med. Child. Neurol. 2011, 53, 457–462.
- Prosperi, M.; Santocchi, E.; Balboni, G.; Narzisi, A.; Bozza, M.; Fulceri, F.; Apicella, F.; Igliozzi, R.; Cosenza, A.; Tancredi, R.; et al. Behavioral Phenotype of ASD Preschoolers with Gastrointestinal Symptoms or Food Selectivity. J. Autism Dev. Disord. 2017, 47, 3574–3588.
- Fulceri, F.; Morelli, M.; Santocchi, E.; Cena, H.; Del Bianco, T.; Narzisi, A.; Calderoni, S.; Muratori, F. Gastrointestinal symptoms and behavioral problems in preschoolers with Autism Spectrum Disorder. Dig. Liver Dis. 2016, 48, 248–254.
- Kuddo, T.; Nelson, K.B. How common are gastrointestinal disorders in children with autism? Curr. Opin. Pediatr. 2003, 15, 339–343.
- Holingue, C.; Newill, C.; Lee, L.C.; Pasricha, P.J.; Daniele Fallin, M. Gastrointestinal symptoms in autism spectrum disorder: A review of the literature on ascertainment and prevalence. Autism Res. 2018, 11, 24–36.
- Adams, J.B.; Romdalvik, J.; Ramanujam, V.M.S.; Legator, M.S. Mercury, lead, and zinc in baby teeth of children with autism versus controls. J. Toxicol. Environ. Health Part A 2007, 70, 1046–1051.
- Konstantareas, M.M.; Homatidis, S. Ear infections in autistic and normal children. J. Autism Dev. Disord. 1987, 17, 585–594.
- Niehus, R.; Lord, C. Early medical history of children with autism spectrum disorders. J. Dev. Behav. Pediatr. 2006, 27, S120–S127.
- Julio-Pieper, M.; Bravo, J.A.; Aliaga, E.; Gotteland, M. Review article: Intestinal barrier dysfunction and central nervous system disorders—A controversial association. Aliment. Pharmacol. Ther. 2014, 40, 1187–1201.
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F.; et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 2013, 155, 1451–1463.
- Qin, L.; Wu, X.; Block, M.L.; Liu, Y.; Breese, G.R.; Hong, J.S.; Knapp, D.J.; Crews, F.T. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 2007, 55, 453–462.
- Ashwood, P.; Krakowiak, P.; Hertz-Picciotto, I.; Hansen, R.; Pessah, I.; Van de Water, J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav. Immun. 2011, 25, 40–45.
- Vargas, D.L.; Nascimbene, C.; Krishnan, C.; Zimmerman, A.W.; Pardo, C.A. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann. Neurol. 2005, 57, 67–81.
- Zhan, Y.; Paolicelli, R.C.; Sforazzini, F.; Weinhard, L.; Bolasco, G.; Pagani, F.; Vyssotski, A.L.; Bifone, A.; Gozzi, A.; Ragozzino, D.; et al. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat. Neurosci. 2014, 17, 400–406.
- Trevarthen, C.; Aitken, K.; Nagy, E.; Delafield-Butt, J.; Vandekerckhove, M. Collaborative Regulations of Vitality in Early Childhood: Stress in Intimate Relationships and Postnatal Psychopathology. In Developmental Psychopathology; Wiley: New York, NY, USA, 2006; Volume 2, pp. 65–126.
- Wang, Y.; Kasper, L.H. The role of microbiome in central nervous system disorders. Brain Behav. Immun. 2014, 38, 1–12.
- Keita, A.V.; Söderholm, J.D. The intestinal barrier and its regulation by neuroimmune factors. Neurogastroenterol. Motil. 2010, 22, 718–733.
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.N.; Kubo, C.; Koga, Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004, 558, 263–275.
- Neufeld, K.M.; Kang, N.; Bienenstock, J.; Foster, J.A. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol. Motil. 2011, 23, 255–264.
- Gareau, M.G.; Wine, E.; Rodrigues, D.M.; Cho, J.H.; Whary, M.T.; Philpott, D.J.; Macqueen, G.; Sherman, P.M. Bacterial infection causes stress-induced memory dysfunction in mice. Gut 2011, 60, 307–317.
- Sampson, T.R.; Mazmanian, S.K. Control of brain development, function, and behavior by the microbiome. Cell Host Microbe 2015, 17, 565–576.
- Sudo, N. Role of microbiome in regulating the HPA axis and its relevance to allergy. Chem. Immunol. Allergy 2012, 98, 163–175.
- Desbonnet, L.; Clarke, G.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. Microbiota is essential for social development in the mouse. Mol. Psychiatry 2014, 19, 146–148.
- Wong, A.C.-N.; Holmes, A.; Ponton, F.; Lihoreau, M.; Wilson, K.; Raubenheimer, D.; Simpson, S.J. Behavioral Microbiomics: A Multi-Dimensional Approach to Microbial Influence on Behavior. Front. Microbiol. 2015, 6, 1359.
- Stilling, R.M.; Ryan, F.J.; Hoban, A.E.; Shanahan, F.; Clarke, G.; Claesson, M.J.; Dinan, T.G.; Cryan, J.F. Microbes & neurodevelopment—Absence of microbiota during early life increases activity-related transcriptional pathways in the amygdala. Brain Behav. Immun. 2015, 50, 209–220.
- Desbonnet, L.; Clarke, G.; Traplin, A.; O’Sullivan, O.; Crispie, F.; Moloney, R.D.; Cotter, P.D.; Dinan, T.G.; Cryan, J.F. Gut microbiota depletion from early adolescence in mice: Implications for brain and behaviour. Brain Behav. Immun. 2015, 48, 165–173.
- Erny, D.; Hrabě de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977.
- Forsythe, P.; Bienenstock, J. Immunomodulation by commensal and probiotic bacteria. Immunol. Investig. 2010, 39, 429–448.
- Barrett, E.; Ross, R.P.; O’Toole, P.W.; Fitzgerald, G.F.; Stanton, C. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J. Appl. Microbiol. 2012, 113, 411–417.
- De Angelis, M.; Francavilla, R.; Piccolo, M.; De Giacomo, A.; Gobbetti, M. Autism spectrum disorders and intestinal microbiota. Gut Microbes 2015, 6, 207–213.
- Kang, D.-W.; Adams, J.B.; Coleman, D.M.; Pollard, E.L.; Maldonado, J.; McDonough-Means, S.; Caporaso, J.G.; Krajmalnik-Brown, R. Long-term benefit of Microbiota Transfer Therapy on autism symptoms and gut microbiota. Sci. Rep. 2019, 9, 5821.
- Dinan, T.G.; Cryan, J.F. Melancholic microbes: A link between gut microbiota and depression? Neurogastroenterol. Motil. 2013, 25, 713–719.
- Srikantha, P.; Mohajeri, M.H. The Possible Role of the Microbiota-Gut-Brain-Axis in Autism Spectrum Disorder. Int. J. Mol. Sci. 2019, 20, 2115.
- Byrne, C.S.; Chambers, E.S.; Morrison, D.J.; Frost, G. The role of short chain fatty acids in appetite regulation and energy homeostasis. Int. J. Obes. 2015, 39, 1331–1338.
- Thomas, R.H.; Meeking, M.M.; Mepham, J.R.; Tichenoff, L.; Possmayer, F.; Liu, S.; MacFabe, D.F. The enteric bacterial metabolite propionic acid alters brain and plasma phospholipid molecular species: Further development of a rodent model of autism spectrum disorders. J. Neuroinflamm. 2012, 9, 153.
- MacFabe, D.F. Enteric short-chain fatty acids: Microbial messengers of metabolism, mitochondria, and mind: Implications in autism spectrum disorders. Microb. Ecol. Health Dis. 2015, 26, 28177.
- Al-Lahham, S.H.; Peppelenbosch, M.P.; Roelofsen, H.; Vonk, R.J.; Venema, K. Biological effects of propionic acid in humans; metabolism, potential applications and underlying mechanisms. Biochim. Biophys. Acta 2010, 1801, 1175–1183.
- Liu, F.; Li, J.; Wu, F.; Zheng, H.; Peng, Q.; Zhou, H. Altered composition and function of intestinal microbiota in autism spectrum disorders: A systematic review. Transl. Psychiatry 2019, 9, 43.
- Kratsman, N.; Getselter, D.; Elliott, E. Sodium butyrate attenuates social behavior deficits and modifies the transcription of inhibitory/excitatory genes in the frontal cortex of an autism model. Neuropharmacology 2016, 102, 136–145.
- Wang, L.; Christophersen, C.T.; Sorich, M.J.; Gerber, J.P.; Angley, M.T.; Conlon, M.A. Elevated fecal short chain fatty acid and ammonia concentrations in children with autism spectrum disorder. Dig. Dis. Sci. 2012, 57, 2096–2102.
- Conn, A.R.; Fell, D.I.; Steele, R.D. Characterization of alpha-keto acid transport across blood-brain barrier in rats. Am. J. Physiol. 1983, 245, E253–E260.
- Reigstad, C.S.; Salmonson, C.E.; Rainey, J.F.; Szurszewski, J.H.; Linden, D.R.; Sonnenburg, J.L.; Farrugia, G.; Kashyap, P.C. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015, 29, 1395–1403.
- El-Ansary, A.; Al-Ayadhi, L. Relative abundance of short chain and polyunsaturated fatty acids in propionic acid-induced autistic features in rat pups as potential markers in autism. Lipids Health Dis. 2014, 13, 140.
- Al-Ghamdi, M.; Al-Ayadhi, L.; El-Ansary, A. Selected biomarkers as predictive tools in testing efficacy of melatonin and coenzyme Q on propionic acid—Induced neurotoxicity in rodent model of autism. BMC Neurosci. 2014, 15, 34.
- Persico, A.M.; Napolioni, V. Urinary p-cresol in autism spectrum disorder. Neurotoxicol. Teratol. 2013, 36, 82–90.
- Ming, X.; Stein, T.P.; Barnes, V.; Rhodes, N.; Guo, L. Metabolic perturbance in autism spectrum disorders: A metabolomics study. J. Proteome Res. 2012, 11, 5856–5862.
- Shimmura, C.; Suda, S.; Tsuchiya, K.J.; Hashimoto, K.; Ohno, K.; Matsuzaki, H.; Iwata, K.; Matsumoto, K.; Wakuda, T.; Kameno, Y.; et al. Alteration of plasma glutamate and glutamine levels in children with high-functioning autism. PLoS ONE 2011, 6, e25340.
- Muller, C.L.; Anacker, A.M.J.; Veenstra-VanderWeele, J. The serotonin system in autism spectrum disorder: From biomarker to animal models. Neuroscience 2016, 321, 24–41.
- Gheorghe, C.E.; Martin, J.A.; Manriquez, F.V.; Dinan, T.G.; Cryan, J.F.; Clarke, G. Focus on the essentials: Tryptophan metabolism and the microbiome-gut-brain axis. Curr. Opin. Pharmacol. 2019, 48, 137–145.
- Israelyan, N.; Margolis, K.G. Serotonin as a link between the gut-brain-microbiome axis in autism spectrum disorders. Pharmacol. Res. 2018, 132, 1–6.
- Chugani, D.C.; Muzik, O.; Behen, M.; Rothermel, R.; Janisse, J.J.; Lee, J.; Chugani, H.T. Developmental changes in brain serotonin synthesis capacity in autistic and nonautistic children. Ann. Neurol. 1999, 45, 287–295.
- Melke, J.; Goubran Botros, H.; Chaste, P.; Betancur, C.; Nygren, G.; Anckarsäter, H.; Rastam, M.; Ståhlberg, O.; Gillberg, I.C.; Delorme, R.; et al. Abnormal melatonin synthesis in autism spectrum disorders. Mol. Psychiatry 2008, 13, 90–98.
- Gabriele, S.; Sacco, R.; Persico, A.M. Blood serotonin levels in autism spectrum disorder: A systematic review and meta-analysis. Eur. Neuropsychopharmacol. 2014, 24, 919–929.
- Schain, R.J.; Freedman, D.X. Studies on 5-hydroxyindole metabolism in autistic and other mentally retarded children. J. Pediatr. 1961, 58, 315–320.
- Anderson, G.M.; Freedman, D.X.; Cohen, D.J.; Volkmar, F.R.; Hoder, E.L.; McPhedran, P.; Minderaa, R.B.; Hansen, C.R.; Young, J.G. Whole blood serotonin in autistic and normal subjects. J. Child Psychol. Psychiatry 1987, 28, 885–900.
- Hanley, H.G.; Stahl, S.M.; Freedman, D.X. Hyperserotonemia and amine metabolites in autistic and retarded children. Arch. Gen. Psychiatry 1977, 34, 521–531.
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161, 264–276.
- Marler, S.; Ferguson, B.J.; Lee, E.B.; Peters, B.; Williams, K.C.; McDonnell, E.; Macklin, E.A.; Levitt, P.; Gillespie, C.H.; Anderson, G.M.; et al. Brief Report: Whole Blood Serotonin Levels and Gastrointestinal Symptoms in Autism Spectrum Disorder. J. Autism Dev. Disord. 2016, 46, 1124–1130.
- McDougle, C.J.; Naylor, S.T.; Cohen, D.J.; Aghajanian, G.K.; Heninger, G.R.; Price, L.H. Effects of tryptophan depletion in drug-free adults with autistic disorder. Arch. Gen. Psychiatry 1996, 53, 993–1000.
- Bischoff, S.C.; Mailer, R.; Pabst, O.; Weier, G.; Sedlik, W.; Li, Z.; Chen, J.J.; Murphy, D.L.; Gershon, M.D. Role of serotonin in intestinal inflammation: Knockout of serotonin reuptake transporter exacerbates 2,4,6-trinitrobenzene sulfonic acid colitis in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G685–G695.
- Kraneveld, A.D.; Szklany, K.; de Theije, C.G.M.; Garssen, J. Gut-to-Brain Axis in Autism Spectrum Disorders: Central Role for the Microbiome. Int. Rev. Neurobiol. 2016, 131, 263–287.
- Fattorusso, A.; Di Genova, L.; Dell’Isola, G.B.; Mencaroni, E.; Esposito, S. Autism Spectrum Disorders and the Gut Microbiota. Nutrients 2019, 11, 521.




