
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Tsutomu Fukuwatari | + 812 word(s) | 812 | 2020-05-29 09:51:11 | | | |
| 2 | Nicole Yin | -1 word(s) | 811 | 2020-11-06 03:39:00 | | |
Video Upload Options
Kynurenic acid, a metabolite of the kynurenine pathway of tryptophan catabolism, acts as antagonist for both the α7 nicotinic acetylcholine receptor and glycine co-agonist sites of the N-methyl-D-aspartic acid receptor at endogenous brain concentrations. Elevation of brain kynurenic acid levels reduces the release of neurotransmitters such as dopamine and glutamate, and kynurenic acid is considered to be involved in psychiatric disorders such as schizophrenia and depression.
1. Introduction
In 1989, Kessler et al. found that KYNA competitively inhibited glycine coagonist site of the NMDA receptor at low concentration with an IC50 of 8 μmol/L[1]. A decade later, Hilmas et al. found that KYNA noncompetitively inhibited α7nAchRs with an IC50 of 7 μmol/L using the patch-clamp technique with cultured hippocampal neurons[2]. Furthermore, Wang et al. found that KYNA is ligand for GPR35, whose EC50s are 10.7, 7.4, and 39.2 μmol/L in mouse, rat, and human, respectively[3]. Since physiological concentrations of brain KYNA are 5 pmol/g wet wt, 15 pmol/g wet wt, and 150 pmol/g wet wt in mouse, rat and human, respectively[4] [8], elevation of brain KYNA has been considered to affect these receptors. Effects of KYNA increase on the neurotransmitter release were investigated using microdialysis technique, and KYNA concentration-dependently and reversibly reduced extracellular glutamate, dopamine, and γ-aminobutyric acid (GABA) to less than 50% of baseline concentrations[5][6][7]. Conversely, inhibition of endogenous KYNA formation by reverse dialysis of KYNA synthesis inhibitor (S)-4-(ethylsulfonyl) benzoylalanine (S-ESBA) reversibly increases dopamine, glutamate, and GABA levels in the rodent brain[7][8][9]. Although these findings suggest that changes of brain KYNA levels affect neurotransmitter release via modulation of above receptors, understanding the mechanism of action of KYNA is difficult. There is disagreement about the interaction between KYNA and α7nAchRs, because several studies failed to reproduce evidence for action of KYNA on nicotinic receptors.[10]. Schematic representation of the interaction between KYNA and the receptors is shown in Figure 1.
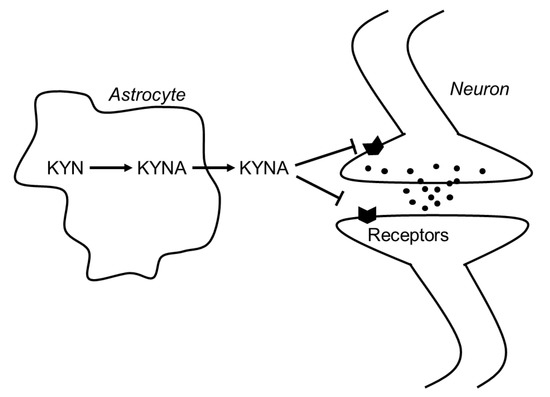
Figure 1. Schematic representation of the interaction between KYNA and neurotransmitters in the brain. Abbreviations: KYN: kynurenine; KYNA: kynurenic acid.
2. Function of Kynurenic Acid in the Brain
Behavioral studies showed that changes in brain KYNA levels affect several psychiatric functions in experimental animals. Elevation of endogenous KYNA concentration produces disruptions in prepulse inhibition[11] and habituation of auditory-evoked potentials[12], indicating that elevated KYNA levels interfere with normal reductions in processing and responding to irrelevant stimuli. Elevation of brain KYNA levels also affects cognitive function. For example, rats with elevations of endogenous KYNA exhibit spatial working memory deficits in a radial arm maze task[13]. These rats also exhibit impaired contextual fear memory consisting of two pairings of a tone and foot shock, and are slower to learn to discriminate between different contexts with or without foot shock[14]. On the other hand, reduction of endogenous KYNA levels by genetic and pharmacological manipulation improves cognitive functions. Mice with a targeted deletion of kynurenine aminotransferase II (KAT II), a major biosynthetic enzyme of brain KYNA, show reduced brain KYNA levels and significantly increased performance in three cognitive paradigms that rely in part on the integrity of hippocampal function, namely, object exploration and recognition, passive avoidance, and spatial discrimination[15]. Intracerebroventricular administration of selective KAT II inhibitor S-ESBA improves kynurenine-induced cognitive deficits on performance in the Morris water maze[16]. Systemic administration of KAT II inhibitor, PF-04859989, also dose-dependently reduces brain KYNA, prevents amphetamine- and ketamine-induced disruption of auditory gating, and improves performance in a sustained attention task[17]. It also prevents ketamine-induced disruption of performance in a working memory task and a spatial memory task in rodents and nonhuman primates, respectively. These findings support the hypotheses that endogenous KYNA impacts cognitive function and that inhibition of KAT II, and consequent lowering of endogenous brain KYNA levels, improves cognitive performance under conditions considered relevant for schizophrenia.
In humans, elevated KYNA levels are observed in the cerebrospinal fluid and cortex of patients with schizophrenia and bipolar disorder[18][19][20][21][22][23]. In the brain, kynurenine and KYNA levels in schizophrenic cases are 1.5 times higher than matched control subjects[20]. Similar observations reported that kynurenine and KYNA concentrations in the cerebrospinal fluid (CSF) were 2 and 1.5 times higher in patients with schizophrenia, respectively, than with healthy volunteers, whereas tryptophan concentrations did not differ between the groups[22]. Patients with bipolar disorder have 1.5 times increased levels of KYNA in their CSF compared with healthy volunteers, and the levels of KYNA are positively correlated with age among bipolar patients but not in healthy volunteers[24]. Haplotype analysis shows an association between kynurenine 3-monoxygenase (KMO) gene polymorphisms and CSF concentrations of KYNA in patients with schizophrenia[25]. In the bipolar disorder and schizophrenia patients, KMO mRNA levels are reduced in the brain compared with nonpsychotic patients and controls, and the KMO Arg452 allele is associated with increased levels of CSF KYNA and reduced brain KMO expression[26]. KMO is the primary enzyme responsible for kynurenine degradation. These results support the hypothesis that KYNA is involved in the pathophysiology of psychiatric diseases such as schizophrenia and bipolar disorder.
References
- M. Kessler; T. Terramani; G. Lynch; M. Baudry; A Glycine Site Associated with N-Methyl-d-Aspartic Acid Receptors: Characterization and Identification of a New Class of Antagonists. Journal of Neurochemistry 1989, 52, 1319-1328, 10.1111/j.1471-4159.1989.tb01881.x.
- Corey Hilmas; Edna F. R. Pereira; Manickavasagom Alkondon; Arash Rassoulpour; Robert Schwarcz; Edson X. Albuquerque; The Brain Metabolite Kynurenic Acid Inhibits α7 Nicotinic Receptor Activity and Increases Non-α7 Nicotinic Receptor Expression: Physiopathological Implications. The Journal of Neuroscience 2001, 21, 7463-7473, 10.1523/jneurosci.21-19-07463.2001.
- Jinghong Wang; Nicole Simonavicius; Xiaosu Wu; Gayathri Swaminath; Jeff Reagan; Hui Tian; Lei Ling; Kynurenic Acid as a Ligand for Orphan G Protein-coupled Receptor GPR35. Journal of Biological Chemistry 2006, 281, 22021-22028, 10.1074/jbc.m603503200.
- F. Moroni; P. Russi; G. Lombardi; M. Beni; V. Carlà; Presence of Kynurenic Acid in the Mammalian Brain. Journal of Neurochemistry 1988, 51, 177-180, 10.1111/j.1471-4159.1988.tb04852.x.
- Carpenedo, R.; Pittaluga, A.; Cozzi, A.; Attucci, S.; Galli, A.; Raiteri, M.; Moroni, F. Presynaptic kynurenate-sensitive receptors inhibit glutamate release. Eur. J. Neurosci. 2001, 13, 2141–2147.
- Rassoulpour, A.; Wu, H.Q.; Ferré, S.; Schwarcz, R. Nanomolar concentrations of kynurenic acid reduce extracellular dopamine levels in the striatum. J. Neurochem. 2005, 93, 762–765.
- Beggiato, S.; Tanganelli, S.; Fuxe, K.; Antonelli, T.; Schwarcz, R.; Ferraro, L. Endogenous kynurenic acid regulates extracellular GABA levels in the rat prefrontal cortex. Neuropharmacology 2014, 82, 11–18.
- Amori, L.; Wu, H.Q.; Marinozzi, M.; Pellicciari, R.; Guidetti, P.; Schwarcz, R. Specific inhibition of kynurenate synthesis enhances extracellular dopamine levels in the rodent striatum. Neuroscience 2009, 159, 196–203.
- Wu, H.Q.; Pereira, E.F.; Bruno, J.P.; Pellicciari, R.; Albuquerque, E.X.; Schwarcz, R. The astrocyte-derived α7 nicotinic receptor antagonist kynurenic acid controls extracellular glutamate levels in the prefrontal cortex. J. Mol. Neurosci. 2010, 40, 204–210.
- Trevor W Stone; Does kynurenic acid act on nicotinic receptors? An assessment of the evidence. Journal of Neurochemistry 2019, 152, 627-649, 10.1111/jnc.14907.
- Sophie Erhardt; Lilly Schwieler; Carolina Emanuelsson; Mark Geyer; Endogenous kynurenic acid disrupts prepulse inhibition. Biological Psychiatry 2004, 56, 255-260, 10.1016/j.biopsych.2004.06.006.
- Paul D Shepard; Brian Joy; Lucy Clerkin; Robert Schwarcz; Micromolar Brain Levels of Kynurenic Acid are Associated with a Disruption of Auditory Sensory Gating in the Rat. Neuropsychopharmacology 2003, 28, 1454-1462, 10.1038/sj.npp.1300188.
- Amy C. Chess; Michael K. Simoni; Torey E. Alling; David J. Bucci; Elevations of Endogenous Kynurenic Acid Produce Spatial Working Memory Deficits. Schizophrenia Bulletin 2007, 33, 797-804, 10.1093/schbul/sbl033.
- Amy C. Chess; Allison M. Landers; David J. Bucci; L-kynurenine treatment alters contextual fear conditioning and context discrimination but not cue-specific fear conditioning. Behavioural Brain Research 2009, 201, 325-331, 10.1016/j.bbr.2009.03.013.
- Michelle C Potter; Greg I Elmer; Richard Bergeron; Edson X Albuquerque; Paolo Guidetti; Hui-Qiu Wu; Robert Schwarcz; Reduction of Endogenous Kynurenic Acid Formation Enhances Extracellular Glutamate, Hippocampal Plasticity, and Cognitive Behavior. Neuropsychopharmacology 2010, 35, 1734-1742, 10.1038/npp.2010.39.
- Ana Pocivavsek; Hui-Qiu Wu; Michelle C Potter; Greg I Elmer; Roberto Pellicciari; Robert Schwarcz; Fluctuations in Endogenous Kynurenic Acid Control Hippocampal Glutamate and Memory. Neuropsychopharmacology 2011, 36, 2357-2367, 10.1038/npp.2011.127.
- Rouba Kozak; Brian M. Campbell; Christine A. Strick; Weldon Horner; William E. Hoffmann; Tamas Kiss; Douglas S. Chapin; Dina McGinnis; Amanda L. Abbott; Brooke M. Roberts; et al.Kari FonsecaVictor GuanowskyDamon A. YoungPatricia A. SeymourAmy DounayM HajósGraham V. WilliamsStacy A. Castner Reduction of Brain Kynurenic Acid Improves Cognitive Function. Journal of Neuroscience 2014, 34, 10592-10602, 10.1523/jneurosci.1107-14.2014.
- Heinrichs, R.W.; Zakzanis, K.K. Neurocognitive deficit in schizophrenia: A quantitative review of the evidence. Neuropsychology 1998, 12, 426–445.
- Erhardt, S.; Blennow, K.; Nordin, C.; Skogh, E.; Lindstrom, L.H.; Engberg, G. Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci. Lett. 2001, 313, 96–98.
- Schwarcz, R.; Rassoulpour, A.; Wu, H.Q.; Medoff, D.; Tamminga, C.A.; Roberts, R.C. Increased cortical kynurenate content in schizophrenia. Biol. Psychiatry 2001, 50, 521–530.
- Sathyasaikumar, K.V.; Stachowski, E.K.; Wonodi, I.; Roberts, R.C.; Rassoulpour, A.; McMahon, R.P.; Schwarcz, R. Impaired kynurenine pathway metabolism in the prefrontal cortex of individuals with schizophrenia. Schizophr. Bull. 2011, 37, 1147–1156.
- Linderholm, K.R.; Skogh, E.; Olsson, S.K.; Dahl, M.L.; Holtze, M.; Engberg, G.; Samuelsson, M.; Erhardt, S. Increased levels of kynurenine and kynurenic acid in the CSF of patients with schizophrenia. Schizophr. Bull. 2012, 38, 426–432.
- Kindler, J.; Lim, C.K.; Weickert, C.S.; Boerrigter, D.; Galletly, C.; Liu, D.; Jacobs, K.R.; Balzan, R.; Bruggemann, J.; O’Donnell, M.; et al. Dysregulation of kynurenine metabolism is related to proinflammatory cytokines, attention, and prefrontal cortex volume in schizophrenia. Mol. Psychiatry 2019.
- Olsson, S.K.; Samuelsson, M.; Saetre, P.; Lindström, L.; Jönsson, E.G.; Nordin, C.; Engberg, G.; Erhardt, S.; Landén, M. Elevated levels of kynurenic acid in the cerebrospinal fluid of patients with bipolar disorder. J. Psychiatry Neurosci. 2010, 35, 195–199.
- Holtze, M.; Saetre, P.; Engberg, G.; Schwieler, L.; Werge, T.; Andreassen, O.A.; Hall, H.; Terenius, L.; Agartz, I.; Jönsson, E.G.; et al. Kynurenine 3-monooxygenase polymorphisms: Relevance for kynurenic acid synthesis in patients with schizophrenia and healthy controls. J. Psychiatry Neurosci. 2012, 37, 53–57.
- Catharina Lavebratt; S Olsson; Lena Backlund; L Frisén; Cm Sellgren; L Priebe; P Nikamo; L Träskman-Bendz; Sven Cichon; M P Vawter; et al.U OsbyG EngbergM. LandénSophie ErhardtM SchallingMe KegelSe BergenCj EkmanM LarssonPf SullivanP SklarJw SmollerPke MagnussonCm HultmanL Walther-JallowCi SvenssonP Lichtenstein The KMO allele encoding Arg452 is associated with psychotic features in bipolar disorder type 1, and with increased CSF KYNA level and reduced KMO expression. Molecular Psychiatry 2013, 19, 334-341, 10.1038/mp.2013.11.




