
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mario Bauer | + 2412 word(s) | 2412 | 2021-10-15 09:52:38 | | | |
| 2 | Conner Chen | Meta information modification | 2412 | 2021-10-21 05:37:44 | | |
Video Upload Options
GPR15 as a member of the Class A (rhodopsin) orphan G protein-coupled receptor (GPCR) family has been recently deorphanized by identification of two endogenous receptor-activating ligands in human. Interestingly, in vascular tissue they interact apparently with different cell types. While one ligand triggers a cytoprotective effect on endothelial cells from the luminal site of vessels, the other ligand is rather responsible for the homing of GPR15-expressing lymphocytes into the colon. Thus, in addition to the role of GPR15 as a co-receptor for the human immunodeficiency virus (HIV) or to the expansion of GPR15-expressing lymphocytes in blood by chronic smoking this review will summarize findings to the role of GPR15 for vascular tissue based on new described receptor-ligand interactions.
1. Endogenous GPR15 Ligands and Binding Protein
The first activating binding partner for GPR15 was found to be exogenous particles in the form of viruses (Figure 1 and Figure 2). In particular, many simian immunodeficiency virus (SIV) and HIV-2 strains and HIV-1 strains to a lesser extent are able to use GPR15 as an alternative co-receptor to enter cells [1]. The interaction between GPR15 and virus is performed by the envelope protein gp120, particularly with its variable loop 3 (V3) region [2]. Several single amino acid substitutions in V3 completely abrogated the infectivity of mutant virus in GPR15+ cells, indicating the specificity of V3 for GPR15 binding.
With respect to endogenous binding partners, to date, there have been reports describing two different GPR15-activating ligands: one ligand of physiological origin, named C10orf99, most highly expressed in the colon [3], and the other ligand of synthesized origin (recombinant human soluble thrombomodulin, ART-123) applied as an anticoagulant [4] (Figure 1 and Figure 2). In addition to these ligands, a cystatin C fragment in blood was found to inhibit the entry of GPR15-tropic viruses by binding but not activating GPR15 [5] (Figure 1).
Surprisingly, there are no homologies in protein sequences between the four reported GPR15 binding proteins (Table 1).
Table 1. Sequences of GPR15-interacting proteins/peptides. Color code for amino acid residues: green, hydrophobic uncharged; red, acidic; blue, basic; black, other; underline, signal peptide; black boxes, residues essential for binding to GPR15; bluish colored background, cysteine residue.
| Amino Acid Residues | Reference | |
|---|---|---|
| GPR15 activating protein (ligand) | ||
| C10orf99 (GPR15L) | MRLLVLSSLLCILLLCFSIFSTEGKRRPAKAWSGRRTRLCCHRVPSPNSTNLKGHHVRLCKPCKLEPEPRLWVVPGALPQV | [6] |
| TME5 | QMFCNQTACPADCDPNTQASCECPEGYILDDGFIC | [7] |
| TME5C | ECPEGYILDDGFICTDIDE | [7] |
| gp120 V3(HIV) | CTRPNNNTRKGVHIGPEKVYF TTSIIGDIRQAHC | [2] |
| GPR15 binding protein | ||
| CysC95-146 | GRTTCTKTQPNLDNCPFHDQPHLKRKAFCSFQIYAVPWQGTMTLSKSTCQDA | [5] |
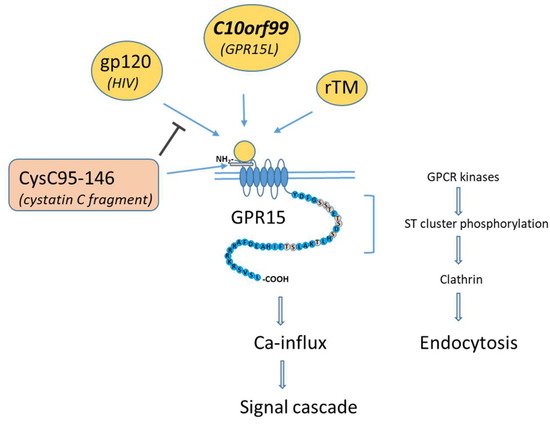
Figure 1. Protein interaction with GPR15, adapted to Okamoto et al., 2017 [8]. GPR15-activating ligands (yellow circles) bind mainly at the extracellular N terminus and to the first extracellular loop (ECL1) of GPR15. The binding protein CysC95-146 is unable to activate GPR15 but can inhibit binding to protein (gp120) of the GPR15-tropic human immunodeficiency virus (HIV). Conformational changes of ligand-bound GPR15 leads to Ca-influx and ligand-dependent endocytosis. Endocytosis is evoked by clathrin-activating phosphorylation of the ST cluster of the C-terminus of GPR15 by GPCR kinases.
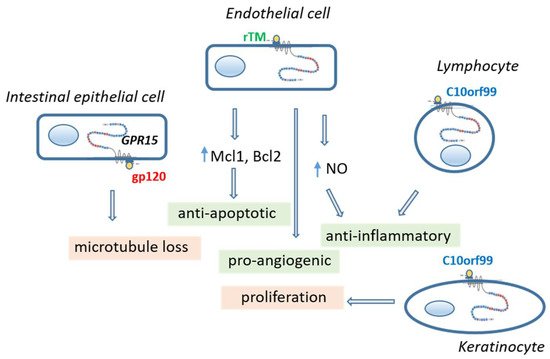
Figure 2. Consequences of GPR15 activation in different cell types by different agonists. Activation of GPR15 in vascular endothelial cell and lymphocytes leads to a greater cell-protecting effect. In contrast, activation of the intestinal epithelial cells by gp120 of HIV causes leakage in the epithelial layer and activation of keratinocytes by C10orf99 causes adverse proliferation. C10orf99 chromosome 10 open reading frame 99, gp120 glycoprotein 120 of human immunodeficiency virus, GPR15 orphan G protein-coupled receptor 15, Mcl1/Bcl2 anti-apoptotic proteins, NO nitric oxide, rTM recombinant thrombomodulin.
1.1. Physiological Ligand Found in Colon—C10orf99 (GPR15L)
On the basis on the specific homing of GPR15+ lymphocytes into the colon, it was proposed that ligands could be found in this specific tissue. One natural ligand was identified in 2017 from porcine colon extracts in an in vitro model using human GPR15-expressing Chinese hamster ovary (CHO) cells engineered to express the promiscuous G protein Gα16 to follow ligand binding by means of calcium release [9]. The human transcript orthologue of this identified pig protein was annotated to the chromosome 10 open reading frame 99 gene, C10orf99.
C10orf99 encodes for a short basic amphiphilic secreted peptide (GPR15L) of 81 amino acid residues initially called “9 kilodalton CC-motif containing cationic polypeptide AP57/colon-derived sushi containing domain-2 binding factor (CSBF)”. Because of its protein characteristics and features, it is proposed that this is a new type of multifunctional antimicrobial peptide. It is found mainly on mucosal and skin epithelium as well as in some tumor and/or their adjacent tissues, such as esophageal cancer, hepatocellular carcinoma, squamous cell carcinoma, and invasive ductal carcinoma [6]. Additionally, it is also present in low abundance in blood or cerebrospinal fluid [10]. The general physiological role of C10orf99 is not well understood but seems to be tissue dependent. In mucosal epithelium, C10orf99 is more constitutively expressed and less affected by inflammation or the presence of microbiota [11], although there is a strong specific homing of GPR15-expressing T cells mainly into the colon.
In contrast, C10orf99 (GPR15L) was upregulated in skin in all examined inflammatory diseases, including psoriasis, atopic dermatitis, contact eczema, and lichen planus [12][13]. It must be checked on a case-by-case basis whether C10orf99 primarily acts via GPR15. Despite the induction of C10orf99 in psoriasiform dermatitis in mice, GPR15+ cells did not accumulate in the skin, which led to the proposal of a more GPR15-independent pathway by the author [14]. In contrast, C10orf99 was considered to act in a GPR15-dependent pathway on keratinocytes in psoriasis [15].
Recently, it has been shown that post-translational modifications (PTMs) of the GPR15 receptor, such as sulfated N-terminal tyrosine residue(s) or the disruption of O-glycosylation on the N-terminal threonine or serine residues, or the removal of α2,3-linked sialic acids from O-glycans, enhances binding to C10orf99 [16]. For C10orf99 activity, its extreme C-terminal residue and its hydrophobicity were considered to be necessary for optimal receptor–ligand interaction.
1.2. The Synthesized Ligand Applied as Anticoagulant—Thrombomodulin
Analyzing the cause of the cytoprotective effect of the recombinant human soluble thrombomodulin (rTM, ART-123) on endothelial cells, in the same year, in 2017, an additional ligand for GPR15 was found [12]. The rTM has been used as an anticoagulant to treat patients undergoing disseminated intravascular coagulation (DIC) [17]. Specifically, immunoprecipitation of membrane proteins of human umbilical vein endothelial cells (HUVECs) with the fifth epidermal growth factor-like region (TME5) of TM revealed GPR15 as a binding partner. Similar binding effects of this short TME5 (35 amino acids residues) have also been found for the greater rTM, suggesting E5 of TM as the main binding region for GPR15. More specifically, the third loop of TME5, called TME5C, composed of 19 amino acids activates GPR15 signaling [7]. Comparing the protein sequence of the 19 amino acids of TME5C or 35 amino acids of TME5 with that of the 81 amino acids of C10orf99, homologies between both ligands are surprisingly absent (Table 1). Homologies with the most preserved amino acids of C10orf99 from different species have also not been found for the TME5C sequence.
Apart from the rTM, heterogeneous soluble TM fragments from vascular endothelial cells circulate in the plasma and are found at increased levels in various diseases, such as cardiovascular disease and diabetes, and in ischemic and/or inflammatory endothelial injuries [18]. It has been shown that these soluble TM fragments retain significant anticoagulant activities. Since the fourth and fifth regions of an EGF-like domain (TME45) act as an anticoagulant by binding thrombin [19], it can be assumed that these TM fragments may have effects on endothelial cells via the GPR15 receptor.
1.3. The GPR15 Binding Protein—Cystatin C Fragment
In 2021, a C-terminal fragment of cystatin C (named CysC95-146) but not the full-length cystatin C was identified to bind to GPR15 [5] by screening a hemofiltrate (HF)-derived peptide library containing peptides and small proteins circulating in human blood in their final processed and physiologically relevant forms. It has been shown that this fragment inhibits the entry of GPR15-tropic derivatives of HIV and SIV in human osteosarcoma (GHOST) cells that stably express CD4 and GPR15. Virus entry is indicated in ghost cells by a green fluorescent protein (GFP) gene that is under the control of the virus. Similarly, in peripheral blood mononuclear cells (PBMCs) stimulated with PHA and IL-2 to enhance the frequency of GPR15+ cells, this inhibition of virus entry was replicated but is more pronounced for SIV compared to GPR15-tropic derivatives of HIV-1 or HIV-2. Interestingly, the GPR15 ligand C10orf99 did not affect the inhibitory effect of CysC95-146 in PBMCs, which indicates different binding sites. Competing experiments with anti-GPR15 antibodies revealed that CysC95-146 binds to the extracellular N terminus and to the first extracellular loop (ECL1) of GPR15. Binding to these GPR15 regions prevents viral entry but does not activate the GPR15 receptor and does not inhibit binding of C10orf99. Essential binding sites were found by amino acid exchanges at positions G69A, K94A, and Q100A, which completely abrogated inhibition of viral entry by CysC95-146.
Cystatin C is an inhibitor of cysteine proteinases consisting of 146 amino acids. It is found in nearly all cells with a nucleus [20] and appears to be one of the most important extracellular inhibitors to prevent the breakdown of proteins [21]. In blood, it serves as a marker of glomerular filtration. The reference interval for plasma ranges from about 0.58–1.00 mg/L in women and 0.62–1.04 mg/L in men [22]. Reference values for the cystatin C fragments do not exist, and as a result, their physiological role has not yet been determined. Compared to blood, CysC95-146 was found at a much lower concentration of about 0.01 mg/L in the diluted hemofiltrate. An extrapolation to the plasma level cannot be carried out due to severe differences between blood and hemofiltrate. However, a half-maximal inhibitory concentration (IC50) for viral entry of about 25 mg/L (0.5 µM) has been described for CysC95-146. By greatly exceeding the normal range of the parental cystatin C in blood, GPR15-binding activities for cystatin C fragments in blood appear to be less likely. As a consequence, evidence for the active role in binding GPR15 of these fragments still needs to be provided.
2. Physiological Role of GPR15 Ligands in Vascular Tissue
The different physiological role of the two GPR15 ligands, C10orf99 and thrombomodulin species, for lymphocytes and endothelial cells is illustrated and described in more detail in Figure 3.
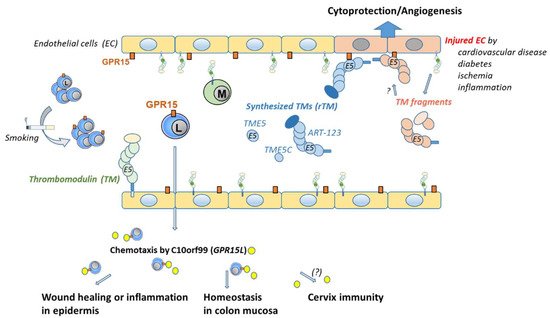
Figure 3. Distribution and binding of GPR15 by ligands in a blood vessel. GPR15 is expressed on endothelial cells (ECs) and on subtypes of lymphocytes. The frequency of GPR15+ lymphocytes can be increased by chronic cigarette smoking. If and how the GPR15 level on ECs can be influenced remains elusive. There are two different ligands for GPR15. One ligand, named C10orf99, is linked to binding on lymphocytes. It is found outside the vessel mainly in the skin, colon, and cervix. C10orf99 is responsible for chemotaxis of GPR15+ lymphocytes that are involved in wound healing or inflammation in the epidermis or homeostasis in the colon mucosa. Its role in cervix mucosa remains elusive. The second ligand is linked to thrombomodulin (TM) and ECs. TM is ubiquitously expressed on the luminal surface of ECs. Synthesized recombinant soluble fragments of TM (rTM), such as ART-123, TME5, and TME5C, were found to bind GPR15. rTM binds to GPR15 via its C-loop of the fifth epidermal growth factor-like region. TM expression decreases upon EC injury [23] and fragments are found in plasma. Binding of these natural fragments to GPR15 could be assumed but has not been confirmed. Binding of TM to GPR15 on ECs is cytoprotective and proangiogenic. ART-123 recombinant human soluble thrombomodulin, C10orf99 chromosome 10 open reading frame 99, EC endothelial cells, GPR15 orphan G protein-coupled receptor 15, L lymphocyte, M monocyte, TM thrombomodulin, TME5 fifth epidermal growth factor-like region of TM, TME5C C-loop of TME5.
2.1. C10orf99
Although C10orf99 can normally be found in plasma in low concentrations (1-6 ng/mL, manufacture notes), its major role appears to be to attract GPR15-bearing blood cells from the outside of vessels, such as the colon and skin, as mentioned above. Apart from GPR15, however, C10orf99 may act as a tumor suppressor by suppressing proliferation of several tumor cell lines via G1 arrest by interacting with another binding receptor, called sushi domain containing 2 (SUSD2) [3]. Nevertheless, its role for GPR15 signaling on vascular ECs or on attracted lymphocytes has not yet been described in detail.
2.2. Thrombomodulin Peptides
The cellular consequences of GPR15 activation on ECs were exclusively described for the rTM, in particular for TME5 and its C-loop TME5C, but not for the natural ligand C10orf99. By supposing soluble TM fragments as a putative natural ligand of GPR15 on EC, it has been shown that this receptor–ligand linkage mediates both cytoprotection and pro-angiogenic activity on ECs [24][7][25]. Cytoprotection was defined as the attenuation of growth inhibition and apoptosis caused by the calcineurin inhibitor FK506 or cyclosporinA (CsA). It has been shown that TME5 and TME5C induce activation of extracellular signal-regulated kinase (ERK) (p-ERK) and AKT serine/threonine kinase 1 (p-Akt) in human umbilical vein ECs (HUVECs), leading to upregulation of the anti-apoptotic myeloid cell leukemia sequence 1 (Mcl-1) protein. As a consequence, TME5 and TME5C could block calcineurin inhibitor-induced capillary leakage. The effect of TME5 or TME5C was exclusively mediated by GPR15, as cytoprotective and pro-angiogenic effects of these TM species were absent in ECs from Gpr15 knock-out mice.
The cellular consequences of GPR15 activation on circulating lymphocytes in human blood has not been studied to a sufficient extent. However, the first indications of a greater anti-inflammatory effect of TME5 have been described in mice [26][27]. It has been shown that TME5 alleviated murine graft-versus-host disease [26] or LPS-induced sepsis [27] in a GPR15-dependent manner. Anti-inflammatory signs were given by the TME5-induced increase in the number of induced regulatory T cells (iTreg) in a mixed lymphocyte reaction, by suppressed upregulation of pro-inflammatory IL-6 in association with an inhibited NF-kB pathway in activated T cells or a reduced activation of dendritic cells. Thus, in addition to the anti-inflammatory effect of thrombomodulin through the lectin-like domain [28], the C-loop of the fifth region of the EGF-like domain of TM (TME5C) preserves anti-inflammatory activity through the GPR15 receptor.
Interestingly, besides constitutive expression of TM in ECs, TM is also found at low expression levels in monocytes. Under certain pathological conditions, such as the inflammatory environment in the bone marrow of patients with low-risk myelodysplastic syndromes (MDS), TM is overexpressed, especially in classical monocytes in the bone marrow but also in peripheral blood [29]. The anti-inflammatory effect of TM was confirmed by induction of a more anti-inflammatory profile of CD4+ T cells in the presence of TM+ monocytes compared to TM- monocytes.
References
- Blaak, H.; Boers, P.H.; Gruters, R.A.; Schuitemaker, H.; van der Ende, M.E.; Osterhaus, A.D. CCR5, GPR15, and CXCR6 are major coreceptors of human immunodeficiency virus type 2 variants isolated from individuals with and without plasma viremia. J. Virol. 2005, 79, 1686–1700.
- Xiang, Y.; Liu, W.; Chen, Y.; Zhang, C.; Su, W.; Zhang, Y.; Sun, J.; Gao, F.; Jiang, C. The variable loop 3 in the envelope glycoprotein is critical for the atypical coreceptor usage of an HIV-1 strain. PLoS ONE 2014, 9, e98058.
- Pan, W.; Cheng, Y.; Zhang, H.; Liu, B.; Mo, X.; Li, T.; Li, L.; Cheng, X.; Zhang, L.; Ji, J.; et al. CSBF/C10orf99, a novel potential cytokine, inhibits colon cancer cell growth through inducing G1 arrest. Sci. Rep. 2014, 4, 6812.
- Gomi, K.; Zushi, M.; Honda, G.; Kawahara, S.; Matsuzaki, O.; Kanabayashi, T.; Yamamoto, S.; Maruyama, I.; Suzuki, K. Antithrombotic effect of recombinant human thrombomodulin on thrombin-induced thromboembolism in mice. Blood 1990, 75, 1396–1399.
- Hayn, M.; Blötz, A.; Rodríguez, A.; Vidal, S.; Preising, N.; Ständker, L.; Wiese, S.; Stürzel, C.M.; Harms, M.; Gross, R.; et al. Natural cystatin C fragments inhibit GPR15-mediated HIV and SIV infection without interfering with GPR15L signaling. Proc. Natl. Acad. Sci. USA 2021, 118, e2023776118.
- Yang, M.; Tang, M.; Ma, X.; Yang, L.; He, J.; Peng, X.; Guo, G.; Zhou, L.; Luo, N.; Yuan, Z.; et al. AP-57/C10orf99 is a new type of multifunctional antimicrobial peptide. Biochem. Biophys. Res. Commun. 2015, 457, 347–352.
- Wang, X.; Pan, B.; Honda, G.; Wang, X.; Hashimoto, Y.; Ohkawara, H.; Xu, K.; Zeng, L.; Ikezoe, T. Cytoprotective and pro-angiogenic functions of thrombomodulin are preserved in the C loop of the fifth epidermal growth factor-like domain. Haematologica 2018, 103, 1730–1740.
- Okamoto, Y.; Shikano, S. Differential phosphorylation signals control endocytosis of GPR15. Mol. Biol. Cell 2017, 28, 2267–2281.
- Suply, T.; Hannedouche, S.; Carte, N.; Li, J.; Grosshans, B.; Schaefer, M.; Raad, L.; Beck, V.; Vidal, S.; Hiou-Feige, A.; et al. A natural ligand for the orphan receptor GPR15 modulates lymphocyte recruitment to epithelia. Sci. Signal 2017, 10, 496.
- Ammitzbøll, C.; von Essen, M.R.; Börnsen, L.; Petersen, E.R.; McWilliam, O.; Ratzer, R.; Romme Christensen, J.; Oturai, A.B.; Søndergaard, H.B.; Sellebjerg, F. GPR15(+) T cells are Th17 like, increased in smokers and associated with multiple sclerosis. J Autoimmun. 2019, 97, 114–121.
- Ocon, B.; Pan, J.; Dinh, T.T.; Chen, W.; Ballet, R.; Bscheider, M.; Habtezion, A.; Tu, H.; Zabel, B.A.; Butcher, E.C. A Mucosal and Cutaneous Chemokine Ligand for the Lymphocyte Chemoattractant Receptor GPR15. Front Immunol. 2017, 8, 1111.
- Pan, B.; Wang, X.; Nishioka, C.; Honda, G.; Yokoyama, A.; Zeng, L.; Xu, K.; Ikezoe, T. G-protein coupled receptor 15 mediates angiogenesis and cytoprotective function of thrombomodulin. Sci. Rep. 2017, 7, 692.
- Reimann, E.; Lättekivi, F.; Keermann, M.; Abram, K.; Kõks, S.; Kingo, K.; Fazeli, A. Multicomponent Biomarker Approach Improves the Accuracy of Diagnostic Biomarkers for Psoriasis Vulgaris. Acta Derm. Venereol. 2019, 99, 1258–1265.
- Sezin, T.; Kempen, L.; Meyne, L.M.; Mousavi, S.; Zillikens, D.; Sadik, C.D. GPR15 is not critically involved in the regulation of murine psoriasiform dermatitis. J. Dermatol. Sci. 2019, 94, 196–204.
- Chen, C.; Wu, N.; Duan, Q.; Yang, H.; Wang, X.; Yang, P.; Zhang, M.; Liu, J.; Liu, Z.; Shao, Y.; et al. C10orf99 contributes to the development of psoriasis by promoting the proliferation of keratinocytes. Sci. Rep. 2018, 8, 8590.
- Okamoto, Y.; Shikano, S. Tyrosine sulfation and O-glycosylation of chemoattractant receptor GPR15 differentially regulate interaction with GPR15L. J. Cell. Sci. 2021, 134.
- Saito, H.; Maruyama, I.; Shimazaki, S.; Yamamoto, Y.; Aikawa, N.; Ohno, R.; Hirayama, A.; Matsuda, T.; Asakura, H.; Nakashima, M.; et al. Efficacy and safety of recombinant human soluble thrombomodulin (ART-123) in disseminated intravascular coagulation: Results of a phase III, randomized, double-blind clinical trial. J. Thromb. Haemost. 2007, 5, 31–41.
- Ohlin, A.K.; Larsson, K.; Hansson, M. Soluble thrombomodulin activity and soluble thrombomodulin antigen in plasma. J Thromb. Haemost. 2005, 3, 976–982.
- Dittman, W.A.; Majerus, P.W. Structure and function of thrombomodulin: A natural anticoagulant. Blood. 1990, 75, 329–336.
- Onopiuk, A.; Tokarzewicz, A.; Gorodkiewicz, E. Cystatin C: A kidney function biomarker. Adv. Clin. Chem. 2015, 68, 57–69.
- Turk, V.; Stoka, V.; Vasiljeva, O.; Renko, M.; Sun, T.; Turk, B.; Turk, D. Cysteine cathepsins: From structure, function and regulation to new frontiers. Biochim. Biophys. Acta 2012, 1824, 68–88.
- Erlandsen, E.J.; Randers, E. Reference intervals for plasma cystatin C and plasma creatinine in adults using methods traceable to international calibrators and reference methods. J. Clin. Lab. Anal. 2018, 32, e22433.
- Ikezoe, T. Advances in the diagnosis and treatment of disseminated intravascular coagulation in haematological malignancies. Int. J. Hematol. 2021, 113, 34–44.
- Bauer, M.; Hackermuller, J.; Schor, J.; Schreiber, S.; Fink, B.; Pierzchalski, A.; Herberth, G. Specific induction of the unique GPR15 expression in heterogeneous blood lymphocytes by tobacco smoking. Biomarkers 2018, 24, 217–224.
- Ikezoe, T.; Yang, J.; Nishioka, C.; Pan, B.; Xu, K.; Furihata, M.; Nakamura, K.; Yurimoto, H.; Sakai, Y.; Honda, G.; et al. The fifth epidermal growth factor-like region of thrombomodulin exerts cytoprotective function and prevents SOS in a murine model. Bone Marrow Transplant. 2017, 52, 73–79.
- Pan, B.; Wang, X.; Kojima, S.; Nishioka, C.; Yokoyama, A.; Honda, G.; Xu, K.; Ikezoe, T. The Fifth Epidermal Growth Factor-like Region of Thrombomodulin Alleviates Murine Graft-versus-Host Disease in a G-Protein Coupled Receptor 15 Dependent Manner. Biol. Blood Marrow Transpl. 2017, 23, 746–756.
- Pan, B.; Wang, X.; Kojima, S.; Nishioka, C.; Yokoyama, A.; Honda, G.; Xu, K.; Ikezoe, T. The fifth epidermal growth factor like region of thrombomodulin alleviates LPS-induced sepsis through interacting with GPR15. Thromb. Haemost. 2017, 117, 570–579.
- Conway, E.M.; Van de Wouwer, M.; Pollefeyt, S.; Jurk, K.; Van Aken, H.; De Vriese, A.; Weitz, J.I.; Weiler, H.; Hellings, P.W.; Schaeffer, P.; et al. The lectin-like domain of thrombomodulin confers protection from neutrophil-mediated tissue damage by suppressing adhesion molecule expression via nuclear factor kappaB and mitogen-activated protein kinase pathways. J. Exp. Med. 2002, 196, 565–577.
- Van Leeuwen-Kerkhoff, N.; Westers, T.M.; Poddighe, P.J.; de Gruijl, T.D.; Kordasti, S.; van de Loosdrecht, A.A. Thrombomodulin-expressing monocytes are associated with low-risk features in myelodysplastic syndromes and dampen excessive immune activation. Haematologica 2020, 105, 961–971.




