
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Cheng-Gui Han | + 2063 word(s) | 2063 | 2021-09-18 08:22:33 | | | |
| 2 | Beatrix Zheng | + 88 word(s) | 2151 | 2021-10-03 09:48:26 | | |
Video Upload Options
Brassica yellows virus (BrYV) is a tentative species of the genus Polerovirus, which occurs widely, and mostly damages Brassicaceae plants in East Asia. Because BrYV cannot be transmitted mechanically, an insect-based transmission method is required for further virus research. Here, a reliable and unrestricted method is described, in which non-viruliferous aphids (Myzus persicae) acquired BrYV from transgenic Arabidopsis thaliana, harboring the full-length viral genome germinated from seeds and its frozen leaves. The aphids then transmitted the virus to healthy plants. There was no significant difference in acquisition rates between fresh and frozen infected leaves, although the transmission rate from frozen infected leaves was lower compared to fresh infected leaves. This simple novel method may be used to preserve viral inocula, evaluate host varietal resistance to BrYV, and investigate interactions among BrYV, aphids, and hosts.
1. Introduction
2. Analysis on Results
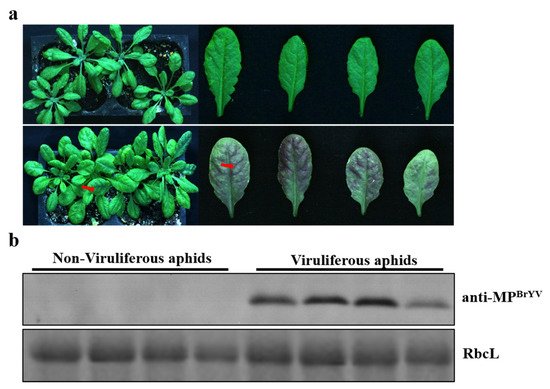
2.1. Viral Inocula Were Ready for Transmission
| Position | Efficiency (%) |
|---|---|
| Inoculated leaves (n = 58 plant) | 86.34 |
| Systemic leaves (n = 58 plant) | 46.75 |
2.2. The Aphid Species Was Confirmed and a Non-Viruliferous Aphid Clonal Population Was Obtained
2.3. Acquisition and Transmission of BrYV by the Aphids Were Available
| Treatment | Total No. of Aphids | No. of Surviving Aphids p < 0.001 |
Proportion of Viruliferous Aphids (n = 48) (%) p = 0.0183 |
Proportion of Infected Plants (n = 48) (%) p = 0.0942 |
|---|---|---|---|---|
| Frozen for 180 d | 180 (60 each) | 106 | 87.5 | 16.6 |
| Frozen for 270 d | 180 (60 each) | 91 | 77.08 | 10.4 |
| Fresh infected leaves | 180 (60 each) | 166 | 93.75 | 33.33 |
| Fresh healthy leaves | 180 (60 each) | 154 | - | - |
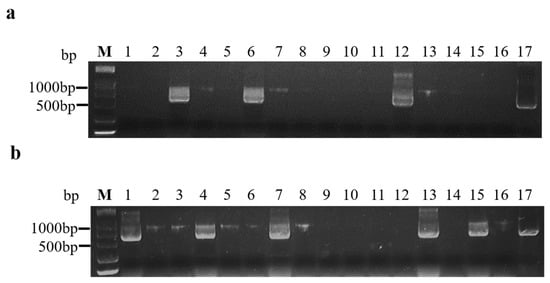
2.4. The Greatest Transmission Efficiency of BrYV Was Determined by Assessing Minimal Aphid Numbers and Inoculation Times
| Total Number of Insects | No. of Infected Plants (n = 30) |
Proportion of Infected Plants (%) |
|---|---|---|
| 1 | 9 | 30 |
| 2 | 24 | 80 |
| 4 | 27 | 90 |
| 6 | 30 | 100 |
| 10 | 30 | 100 |
| Inoculation Time (h) | Efficiency (%) (n = 16) |
|---|---|
| 6 | 12.5 |
| 12 | 50 |
| 24 | 62.5 |
| 48 | 100 |
| Host Plants | Total Number of Insects | Proportion of Infected Plants (%) n = 36 |
|---|---|---|
| Mustard | 6 | 94.4 |
| Chinese cabbage | 6 | 100 |
3. Current Insights
References
- Xiang, H.Y.; Dong, S.W.; Shang, Q.X.; Zhou, C.J.; Li, D.W.; Yu, J.L.; Han, C.G. Molecular characterization of two genotypes of a new polerovirus infecting brassicas in China. Arch. Virol. 2011, 156, 2251–2255.
- Wang, Q.; Mao, J.; Xiang, H.; Dong, L.H.; Sun, Y.H.; Liu, G.S.; Liu, H.B. First report of Brassica yellows virus on tobacco in China. Plant Dis. 2015, 99, 1192.
- Yoshida, N.; Tamada, T. Host range and molecular analysis of Beet leaf yellowing virus, Beet western yellows virus-JP and Brassica yellows virus in Japan. Plant Pathol. 2019, 68, 1045–1058.
- Zhang, X.; Peng, Y.; Wang, Y.; Zhang, Z.; Li, D.; Yu, J.; Han, C. Simultaneous detection and differentiation of three genotypes of Brassica yellows virus by multiplex reverse transcription-polymerase chain reaction. Virol. J. 2016, 13, 189.
- Lim, S.; Yoo, R.H.; Igori, D.; Zhao, F.; Kim, K.H.; Moon, J.S. Genome sequence of a recombinant brassica yellows virus infecting Chinese cabbage. Arch. Virol. 2015, 160, 597–600.
- Tian, C.L.; Ye, S.H.; Li, S.R.; Chen, X.; Luo, K.; Xiang, S.Y.; Wang, L.; Xie, Z.Y.; Dong, P.; Qing, L.; et al. Molecular identification of pathogens of Brassica juncea var. gemmifera and Brassica juncea var. tumida virus disease in Chongqing. Acta Hortic. Sin. 2019, 46, 738–748.
- Zhang, X.Y.; Xiang, H.Y.; Zhou, C.J.; Li, D.W.; Yu, J.L.; Han, C.G. Complete genome sequence analysis identifies a new genotype of brassica yellows virus that infects cabbage and radish in China. Arch. Virol. 2014, 159, 2177–2180.
- Zhang, X.Y.; Dong, S.W.; Xiang, H.Y.; Chen, X.R.; Li, D.W.; Yu, J.L.; Han, C.G. Development of three full-length infectious cDNA clones of distinct brassica yellows virus genotypes for agrobacterium-mediated inoculation. Virus Res. 2015, 197, 13–16.
- Chen, X.R.; Wang, Y.; Zhao, H.H.; Zhang, X.Y.; Wang, X.B.; Li, D.W.; Yu, J.L.; Han, C.G. Brassica yellows virus’ movement protein upregulates anthocyanin accumulation, leading to the development of purple leaf symptoms on Arabidopsis thaliana. Sci. Rep. 2019, 8, 16273.
- Li, Y.Y.; Sun, Q.; Zhao, T.Y.; Xiang, H.Y.; Zhang, X.Y.; Wu, Z.Y.; Zhou, C.J.; Zhang, X.; Wang, Y.; Zhang, Y.L.; et al. Interaction between Brassica yellows virus silencing suppressor P0 and plant SKP1 facilitates stability of P0 in vivo against degradation by proteasome and autophagy pathways. New Phytol. 2019, 222, 1458–1473.
- Sun, Q.; Li, Y.Y.; Wang, Y.; Zhao, H.H.; Zhao, T.Y.; Zhang, Z.Y.; Li, D.W.; Yu, J.L.; Wang, X.B.; Zhang, Y.L.; et al. Brassica yellows virus P0 protein impairs the antiviral activity of NbRAF2 in Nicotiana benthamiana. J. Exp. Bot. 2019, 69, 3127–3139.
- Hollings, M.; Stone, M. The long-term survival of some plant viruses preserved by lyophilization. Ann. Appl. Boil. 1970, 65, 411–418.
- Hollings, M.; Lelliott, R.A. Preservation of plant viruses by freeze drying. Plant Pathol. 1960, 9, 63–66.
- Heck, M.; Brault, V. Targeted disruption of aphid transmission: A vision for the management of crop diseases caused by Luteoviridae members. Curr. Opin. Virol. 2018, 33, 24–32.
- Taliansky, M.; Mayo, M.; Barker, H. Potato leafroll virus: A classic pathogen shows some new tricks. Mol. Plant Pathol. 2003, 4, 81–89.
- Mayo, M.; Ryabov, E.; Fraser, G.; Taliansky, M. Mechanical transmission of Potato leafroll virus. J. Gen. Virol. 2000, 81, 2791–2795.
- Franco-Lara, L.; McGeachy, K.D.; Commandeur, U.; Martin, R.R.; Mayo, M.A.; Barker, H. Transformation of tobacco and potato with cDNA encoding the full-length genome of Potato leafroll virus: Evidence for a novel virus distribution and host effects on virus multiplication. J. Gen. Virol. 1999, 80, 2813–2822.
- Boissinot, S.; Pichon, E.; Sorin, C.; Piccini, C.; Scheidecker, D.; Ziegler-Graff, V.; Brault, V. Systemic Propagation of a Fluorescent Infectious Clone of a Polerovirus Following Inoculation by Agrobacteria and Aphids. Viruses 2017, 9, 166.
- Zhou, T.; Wu, L.J.; Wang, Y.; Cheng, Z.B.; Ji, Y.H.; Fan, Y.J.; Zhou, Y.J. Transmission of rice black streaked dwarf virus from frozen infected leaves to healthy rice plants by small brown Planthopper (Laodelphax striatellus). Rice Sci. 2011, 18, 152–156.
- Shikata, E.; Kitagawa, Y. Rice black-streaked dwarf virus: Its properties, morphology and intracellular localization. Virology 1977, 77, 826–842.




