Brassica yellows virus (BrYV) is a tentative species of the genus Polerovirus, which occurs widely, and mostly damages Brassicaceae plants in East Asia. Because BrYV cannot be transmitted mechanically, an insect-based transmission method is required for further virus research. Here, a reliable and unrestricted method is described, in which non-viruliferous aphids (Myzus persicae) acquired BrYV from transgenic Arabidopsis thaliana, harboring the full-length viral genome germinated from seeds and its frozen leaves. The aphids then transmitted the virus to healthy plants. There was no significant difference in acquisition rates between fresh and frozen infected leaves, although the transmission rate from frozen infected leaves was lower compared to fresh infected leaves. This simple novel method may be used to preserve viral inocula, evaluate host varietal resistance to BrYV, and investigate interactions among BrYV, aphids, and hosts.
- Brassica yellows virus
- Myzus persicae
- transgenic plants
- frozen BrYV-infected plants
- acquisition and transmission
1. Introduction
2. Analysis on Results
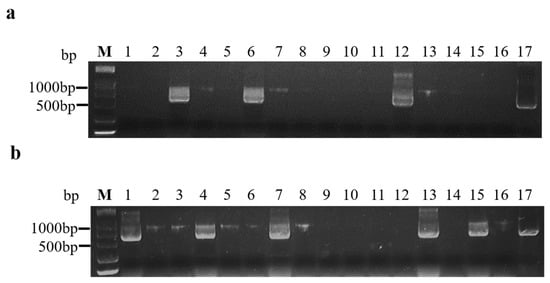
2.1. Viral Inocula Were Ready for Transmission
| Position | Efficiency (%) |
|---|---|
| Inoculated leaves (n = 58 plant) | 86.34 |
| Systemic leaves (n = 58 plant) | 46.75 |
2.2. The Aphid Species Was Confirmed and a Non-Viruliferous Aphid Clonal Population Was Obtained
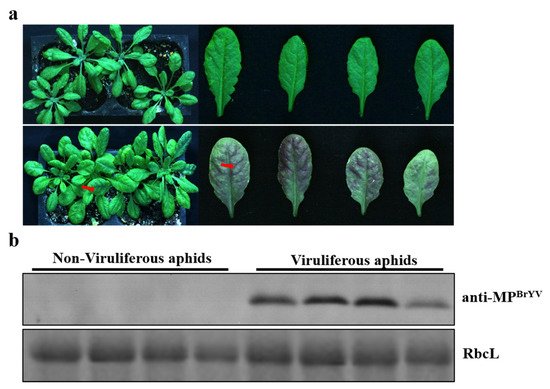
2.3. Acquisition and Transmission of BrYV by the Aphids Were Available
| Treatment | Total No. of Aphids | No. of Surviving Aphids p < 0.001 |
Proportion of Viruliferous Aphids (n = 48) (%) p = 0.0183 |
Proportion of Infected Plants (n = 48) (%) p = 0.0942 |
|---|---|---|---|---|
| Frozen for 180 d | 180 (60 each) | 106 | 87.5 | 16.6 |
| Frozen for 270 d | 180 (60 each) | 91 | 77.08 | 10.4 |
| Fresh infected leaves | 180 (60 each) | 166 | 93.75 | 33.33 |
| Fresh healthy leaves | 180 (60 each) | 154 | - | - |
2.4. The Greatest Transmission Efficiency of BrYV Was Determined by Assessing Minimal Aphid Numbers and Inoculation Times
| Total Number of Insects | No. of Infected Plants (n = 30) |
Proportion of Infected Plants (%) |
|---|---|---|
| 1 | 9 | 30 |
| 2 | 24 | 80 |
| 4 | 27 | 90 |
| 6 | 30 | 100 |
| 10 | 30 | 100 |
| Inoculation Time (h) | Efficiency (%) (n = 16) |
|---|---|
| 6 |
| Host Plants | Total Number of Insects | Proportion of Infected Plants (%) n = 36 |
|---|---|---|
| Mustard | 6 | 94.4 |
| 12.5 | ||
| 12 | 50 | |
| 24 | 62.5 | |
| 48 | 100 |
| Chinese cabbage | |
| 6 | 100 |
