
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | MEHRDAD NIAKOUSARI | + 3443 word(s) | 3443 | 2021-06-24 10:54:25 |
Video Upload Options
Proteins represent one of the major food components that contribute to a wide range of biophysical functions and dictate the nutritional, sensorial, and shelf-life of food products. Different non-thermal processing technologies (e.g., irradiation, ultrasound, cold plasma, pulsed electric field, and high-pressure treatments) can affect the structure of proteins, and thus their solubility as well as their functional properties.
1. Introduction
2. Proteins and Amino Acids
3. Food Processing
3.1. Conventional Thermal Processing
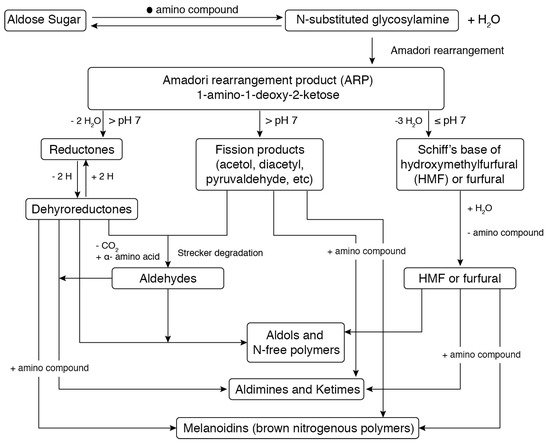
3.2. Emerging Non-Thermal Processing
4. Impact of Non-Thermal Processing on Proteins
4.1. Surface Hydrophobicity
|
Treatment |
Substrate |
Condition |
Results |
Reference |
|---|---|---|---|---|
|
Ultrasound |
Corn gluten meal |
40 kHz, pulsed on- 10 s and off 3 s, 40 min and 20 °C. |
|
[27] |
|
Soy protein |
20 kHz, power 65 W, 0.5, 1, 5 & 15 min |
Protein extraction yield enhanced due to increasing in the solubility |
[28] |
|
|
Beef proteins |
2.39, 6.23, 11.32 and 20.96 Wcm−2, 30, 60, 90 and 120 min |
|
[26] |
|
|
Myofibrillar proteins |
200, 400, 600, 800 and 1000 W, 88, 117, 150, 173 and 193 Wcm−2 |
Increase in S0, decrease in particle size |
[29] |
|
|
Squid (Dosidicus gigas) mantle proteins |
20 kHz, 0, 20, and 40%), 0, 30, 60, and 90 s |
|
[30] |
|
|
Chicken myofibrillar protein |
240 w, 0, 3, 6, 9, 12 and 15 min) |
|
[31] |
|
|
Duck liver protein isolate |
24 kHz, 266 W by a pulsed on-time of 2 s and off-time of 3 s for 42 min |
|
[32] |
|
|
Β-Lg In Cow Milk |
9.5 W, 135 W/cm2 |
No significant alteration in allergenicity |
[33] |
|
|
Tropomyosin from shrimp |
30 Hz, 800 W for 30–180 min |
Allergenicity was reduced |
[34] |
|
|
High pressure processing |
Hongqu Rice wines |
200 and 550 MPa, 25 °C, 30 min |
Free amino acids content was decreased after 6 months storage |
[35] |
|
Brown rice |
0.1–500 MPa,10 min |
Free amino acids especially essential ones were increased |
[36] |
|
|
Tropomyosin from shrimp |
200, 400 and 600 MPa at 20 °C for 20 min |
|
[37] |
|
|
Soy allergen (Glycinin) |
100, 200 and 300 MPa for 15 min |
|
[38] |
|
|
Brussels sprouts |
200 and 800 MPa for 3 min, 5 °C |
|
[39] |
|
|
Cold plasma |
Whey protein isolate |
70 kV, 1, 5, 10, 15, 30, and 60 min |
|
[40] |
|
Grain rice flour |
- |
|
[41] |
|
|
Pulsed ultraviolet light |
Soy protein isolate (SPI) |
1, 2, 4 and 6 min Three pulses per second with a width of 300 μs |
Vanishing Gly m5 & Gly m6 bands after few minutes and decrease in allergenicity |
[42] |
|
Cold atmospheric pressure plasma |
1, 2.5, 5, 7.5 and 10 min without stirring |
Reduction in immunoreactivity of SPI |
||
|
Gamma-irradiation |
Target doses were 3, 5, 10, 25, 50, and 100 kGy |
Decrease in SPI allergenicity (Gly m5 & Gly m6) was dependent on the irradiation dose |
||
|
Pulsed electric field |
Grape juice |
4 µs width and with a field strength of 35 kV/cm, 1000 Hz and the total time 1 ms |
|
[43] |
|
Radiation |
Β-Lg in cow milk |
3, 5, and 10 kGy |
Protein aggregation and alteration of IgE binding epitopes |
[44] |
Gly: Glycinin, SPI: Soy protein isolate, kGy: Kilo gray
4.2. Structural Changes and Aggregation
4.3. Particle Size/Molecular Weight Distribution and Zeta Potential
4.4. Solubility and Gel-Forming/Stability
4.5. Emulsifying Properties
4.6. Reduction in Allergens
References
- Meade, S.J.; Reid, E.A.; Gerrard, J.A. The impact of processing on the nutritional quality of food proteins. J. AOAC Int. 2005, 88, 904–922.
- Korhonen, H.; Pihlanto-Leppäla, A.; Rantamäki, P.; Tupasela, T. Impact of processing on bioactive proteins and peptides. Trends Food Sci. Technol. 1998, 9, 307–319.
- Halász, A.; Baráth, Á.; Simon-Sarkadi, L.; Holzapfel, W. Biogenic amines and their production by microorganisms in food. Trends Food Sci. Technol. 1994, 5, 42–49.
- Bermúdez-Aguirre, D.; Barbosa-Cánovas, G.V. An update on high hydrostatic pressure, from the laboratory to industrial applications. Food Eng. Rev. 2011, 3, 44–61.
- Barba, F.J.; Parniakov, O.; Pereira, S.A.; Wiktor, A.; Grimi, N.; Boussetta, N.; Saraiva, J.A.; Raso, J.; Martin-Belloso, O.; Witrowa-Rajchert, D.; et al. Current applications and new opportunities for the use of pulsed electric fields in food science and industry. Food Res. Int. 2015, 77(Pt. 4), 773–798.
- Barba, F.J.; SantAna, A.S.; Orlien, V.; Koubaa, M. Innovative Technologies for Food Preservation: Inactivation of Spoilage and Pathogenic Microorganisms; Academic Press: Cambridge, MA, USA, 2018; pp. 1–315.
- Young, V.R.; Pellett, P.L. Plant proteins in relation to human protein and amino acid nutrition. Am. J. Clin. Nutr. 1994, 59, 1203S–1212S.
- Kent, S.B.H. Total chemical synthesis of proteins. Chem. Soc. Rev. 2009, 38, 338–351.
- Zhang, Y.; Baranov, P.V.; Atkins, J.F.; Gladyshev, V.N. Pyrrolysine and selenocysteine use dissimilar decoding strategies. J. Biol. Chem. 2005, 280, 20740–20751.
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17.
- Müntz, K. Deposition of storage proteins. In Protein Trafficking in Plant Cells; Springer: Rotterdam, The Netherlands, 1998; pp. 77–99.
- Floros, J.D.; Newsome, R.; Fisher, W.; Barbosa-Cánovas, G.V.; Chen, H.; Dunne, C.P.; German, J.B.; Hall, R.L.; Heldman, D.R.; Karwe, M.V.; et al. Feeding the World today and tomorrow: The Importance of food science and technology. Compr. Rev. Food Sci. Food Saf. 2010, 9, 572–599.
- What is a Processed Food? You Might be Surprised! Understanding Our Food Communications Tool Kit; International Food Information Council Foundation: Washington, DC, USA, 2010.
- Van Boekel, M.; Fogliano, V.; Pellegrini, N.; Stanton, C.; Scholz, G.; Lalljie, S.; Somoza, V.; Knorr, D.; Jasti, P.R.; Eisenbrand, G. A review on the beneficial aspects of food processing. Mol. Nutr. Food Res. 2010, 54, 1215–1247.
- Weaver, C.M.; Dwyer, J.; Fulgoni, V.L.; King, J.C.; Leveille, G.A.; MacDonald, R.S.; Ordovas, J.; Schnakenberg, D. Processed foods: Contributions to nutrition. Am. J. Clin. Nutr. 2014, 99, 1525–1542.
- Rahaman, T.; Vasiljevic, T.; Ramchandran, L. Effect of processing on conformational changes of food proteins related to allergenicity. Trends Food Sci. Technol. 2016, 49, 24–34.
- Mauron, J. Influence of processing on protein quality. J. Nutr. Sci. Vitaminol. 1990, 36 (Suppl. 1), S57–S69.
- Renzone, G.; Arena, S.; Scaloni, A. Proteomic characterization of intermediate and advanced glycation end-products in commercial milk samples. J. Proteom. 2015, 117, 12–23.
- Martins, S.I.F.; Jongen, W.M.; van Boekel, M.A.J. A review of Maillard reaction in food and implications to kinetic modelling. Trends Food Sci. Technol. 2000, 11, 364–373.
- Dagostin, J.L.A. Blanching as an acrylamide mitigation technique. In New Perspectives on Food Blanching; Springer: Cham, Switzerland, 2017; pp. 95–122.
- Hodge, J.E. Dehydrated foods, chemistry of browning reactions in model systems. J. Agric. Food Chem. 1953, 1, 928–943.
- Barba, F.J.; Koubaa, M.; do Prado-Silva, L.; Orlien, V.; Sant’Ana, A.d.S. Mild processing applied to the inactivation of the main foodborne bacterial pathogens: A review. Trends Food Sci. Technol. 2017, 66, 20–35.
- Knorr, D.; Froehling, A.; Jaeger, H.; Reineke, K.; Schlueter, O.; Schoessler, K. Emerging technologies in food processing. Annu. Rev. Food Sci. Technol. 2011, 2, 203–235.
- Tao, Y.; Sun, D.-W. Enhancement of food processes by ultrasound: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 570–594.
- Toepfl, S.; Mathys, A.; Heinz, V.; Knorr, D. Review: Potential of high hydrostatic pressure and pulsed electric fields for energy efficient and environmentally friendly food processing. Food Rev. Int. 2006, 22, 405–423.
- Kang, D.; Zou, Y.; Cheng, Y.; Xing, L.; Zhou, G.; Zhang, W. Effects of power ultrasound on oxidation and structure of beef proteins during curing processing. Ultrason. Sonochem. 2016, 33, 47–53.
- Zhou, C.; Hu, J.; Yu, X.; Yagoub, A.E.A.; Zhang, Y.; Ma, H.; Gao, X.; Otu, P.N.Y. Heat and/or ultrasound pretreatments motivated enzymolysis of corn gluten meal: Hydrolysis kinetics and protein structure. LWT 2017, 77, 488–496.
- Preece, K.E.; Hooshyar, N.; Krijgsman, A.; Fryer, P.J.; Zuidam, N.J. Intensified soy protein extraction by ultrasound. Chem. Eng. Process. Process Intensif. 2017, 113, 94–101.
- Zhang, Z.; Regenstein, J.M.; Zhou, P.; Yang, Y. Effects of high intensity ultrasound modification on physicochemical property and water in myofibrillar protein gel. Ultrason. Sonochem. 2017, 34, 960–967.
- Higuera-Barraza, O.A.; Torres-Arreola, W.; Ezquerra-Brauer, J.M.; Cinco-Moroyoqui, F.J.; Rodríguez Figueroa, J.C.; Marquez-Ríos, E. Effect of pulsed ultrasound on the physicochemical characteristics and emulsifying properties of squid (Dosidicus gigas) mantle proteins. Ultrason. Sonochem. 2017, 38, 829–834.
- Wang, J.; Yang, Y.; Tang, X.; Ni, W.; Zhou, L. Effects of pulsed ultrasound on rheological and structural properties of chicken myofibrillar protein. Ultrason. Sonochem. 2017, 38, 225–233.
- Zou, Y.; Wang, L.; Li, P.; Cai, P.; Zhang, M.; Sun, Z.; Sun, C.; Geng, Z.; Xu, W.; Xu, X.; et al. Effects of ultrasound assisted extraction on the physiochemical, structural and functional characteristics of duck liver protein isolate. Process Biochem. 2017, 52, 174–182.
- Stanic-Vucinic, D.; Stojadinovic, M.; Atanaskovic-Markovic, M.; Ognjenovic, J.; Grönlund, H.; van Hage, M.; Lantto, R.; Sancho, A.I.; Velickovic, T.C. Structural changes and allergenic properties of β-lactoglobulin upon exposure to high-intensity ultrasound. Mol. Nutr. Food Res. 2012, 56, 1894–1905.
- Li, Z.; Lin, H.; Cao, L.; Jameel, K. Effect of high intensity ultrasound on the allergenicity of shrimp. J. Zhejiang Univ. Sci. B 2006, 7, 251–256.
- Tian, Y.; Huang, J.; Xie, T.; Huang, L.; Zhuang, W.; Zheng, Y.; Zheng, B. Oenological characteristics, amino acids and volatile profiles of Hongqu rice wines during pottery storage: Effects of high hydrostatic pressure processing. Food Chem. 2016, 203, 456–464.
- Xia, Q.; Green, B.D.; Zhu, Z.; Li, Y.; Gharibzahedi, S.M.T.; Roohinejad, S.; Barba, F.J. Innovative processing techniques for altering the physicochemical properties of wholegrain brown rice (Oryza sativa L.)—opportunities for enhancing food quality and health attributes. Crit. Rev. Food Sci. Nutr. 2018, 1–22.
- Jin, Y.; Deng, Y.; Qian, B.; Zhang, Y.; Liu, Z.; Zhao, Y. Allergenic response to squid (Todarodes pacificus) tropomyosin Tod p1 structure modifications induced by high hydrostatic pressure. Food Chem. Toxicol. 2014, 76, 86–93.
- Peñas, E.; Préstamo, G.; Polo, F.; Gomez, R. Enzymatic proteolysis, under high pressure of soybean whey: Analysis of peptides and the allergen Gly m 1 in the hydrolysates. Food Chem. 2006, 99, 569–573.
- Barba, F.J.; Poojary, M.M.; Wang, J.; Olsen, K.; Orlien, V. Effect of high pressure processing and storage on the free amino acids in seedlings of Brussels sprouts. Innov. Food Sci. Emerg. Technol. 2017, 41, 188–192.
- Segat, A.; Misra, N.N.; Cullen, P.J.; Innocente, N. Atmospheric pressure cold plasma (ACP) treatment of whey protein isolate model solution. Innov. Food Sci. Emerg. Technol. 2015, 29, 247–254.
- Pal, P.; Kaur, P.; Singh, N.; Kaur, A.; Misra, N.N.; Tiwari, B.K.; Cullen, P.J.; Virdi, A.S. Effect of nonthermal plasma on physico-chemical, amino acid composition, pasting and protein characteristics of short and long grain rice flour. Food Res. Int. 2016, 81, 50–57.
- Meinlschmidt, P.; Ueberham, E.; Lehmann, J.; Reineke, K.; Schlüter, O.; Schweiggert-Weisz, U.; Eisner, P. The effects of pulsed ultraviolet light, cold atmospheric pressure plasma, and gamma-irradiation on the immunoreactivity of soy protein isolate. Innov. Food Sci. Emerg. Technol. 2016, 38, 374–383.
- Garde-Cerdán, T.; Arias-Gil, M.; Marsellés-Fontanet, A.R.; Ancín-Azpilicueta, C.; Martín-Belloso, O. Effects of thermal and non-thermal processing treatments on fatty acids and free amino acids of grape juice. Food Control 2007, 18, 473–479.
- Lee, J.W.; Kim, J.H.; Yook, H.S.; Kang, K.O.; Lee, S.Y.; Hwang, H.J.; Byun, M.W. Effects of gamma radiation on the allergenic and antigenic properties of milk proteins. J. Food Prot. 2001, 64, 272–276.
- Liu, C.; Xiong, Y.L. Oxidation-initiated myosin subfragment cross-linking and structural instability differences between white and red muscle fiber types. J. Food Sci. 2015, 80, C288–C297.
- Zhou, F.; Zhao, M.; Zhao, H.; Sun, W.; Cui, C. Effects of oxidative modification on gel properties of isolated porcine myofibrillar protein by peroxyl radicals. Meat Sci. 2014, 96, 1432–1439.
- McLachlan, A.D.; Karn, J. Periodic charge distributions in the myosin rod amino acid sequence match cross-bridge spacings in muscle. Nature 1982, 299, 226–231.
- Gülseren, İ.; Güzey, D.; Bruce, B.D.; Weiss, J. Structural and functional changes in ultrasonicated bovine serum albumin solutions. Ultrason. Sonochem. 2007, 14, 173–183.
- Galazka, V.B.; Dickinson, E.; Ledward, D.A. Influence of high pressure processing on protein solutions and emulsions. Curr. Opin. Colloid Interface Sci. 2000, 5, 182–187.
- Doi, E.; Shimizu, A.; Oe, H.; Kitabatake, N. Melting of heat-induced ovalbumin gel by pressure. Food Hydrocoll. 1991, 5, 409–425.
- Okamoto, M.; Kawamura, Y.; Hayashi, R. Application of high pressure to food processing: Textural comparison of pressure- and heat-induced gels of food proteins. Agric. Biol. Chem. 1990, 54, 183–189.
- Apichartsrangkoon, A.; Ledward, D.A.; Bell, A.E.; Brennan, J.G. Physicochemical properties of high pressure treated wheat gluten. Food Chem. 1998, 63, 215–220.
- Marcos, B.; Kerry, J.P.; Mullen, A.M. High pressure induced changes on sarcoplasmic protein fraction and quality indicators. Meat Sci. 2010, 85, 115–120.
- Laakkonen, E.; Sherbon, J.W.; Wellington, G.H. Low-temperature, longtime heating of bovine muscle 2. Changes in electrophoretic pattern. J. Food Sci. 1970, 35, 178–180.
- Fischer, C.; Hamm, R.; Honikel, K.O. Changes in solubility and enzymic activity of muscle glycogen phosphorylase in PSE-muscles. Meat Sci. 1979, 3, 11–19.
- Lopez-Bote, C.; Warriss, P.D.; Brown, S.N. The use of muscle protein solubility measurements to assess pig lean meat quality. Meat Sci. 1989, 26, 167–175.
- Joo, S.; Kauffman, R.; Kim, B.; Park, G. The relationship of sarcoplasmic and myofibrillar protein solubility to colour and water-holding capacity in porcine longissimus muscle. Meat Sci. 1999, 52, 291–297.
- Miyaguchi, Y.; Nagayama, K.; Tsutsumi, M. Thermal and functional properties of porcine sarcoplasmic proteins: A comparison with some water-soluble animal proteins. Nihon Chikusan Gakkaiho 2000, 71, 416–424.
- Galazka, V.B.; Dickinson, E.; Ledward, D.A. Emulsifying properties of ovalbumin in mixtures with sulphated polysaccharides: Effects of pH, ionic strength, heat and high-pressure treatment. J. Sci. Food Agric. 2000, 80, 1219–1229.
- Molina, E.; Papadopoulou, A.; Ledward, D.A. Emulsifying properties of high pressure treated soy protein isolate and 7S and 11S globulins. Food Hydrocoll. 2001, 15, 263–269.
- Dickinson, E.; James, J.D. Effect of high pressure processing on properties of emulsions made. In High Pressure Food Science, Bioscience and Chemistry; Isaacs, N.S., Ed.; Woodhead Publishing: Cambridge, UK, 1998; pp. 152–159.




