It is well established that the most prevalent types of thyroid cancers are the papillary and follicular thyroid carcinomas (PTC and FTC, respectively) both in children and adults
. After the Chernobyl accident, almost all childhood thyroid cancers were PTCs
. In earlier cases, a large proportion of the PTCs were of the solid subtype, which was a unique characteristic observed after the Chernobyl accident
. Subsequently, the growth pattern was shifted to the classic subtype, which is less aggressive and metastatic, and importantly, it is quite common in a sporadic childhood PTC
. A recent comparative histological study in the Ukraine cases reported that a dominant papillary growth pattern was less frequent, and an aggressive tumor behavior was more frequent than the sporadic PTCs
.
Epidemiological studies in the Life Span Study (LSS) cohort of the A-bomb survivors in Hiroshima and Nagasaki, which include approximately 120,000 survivors in Hiroshima and Nagasaki, and the residents who were not in the cities at the time of bombing, have been conducted since 1950
[2]. Periodic reports from the Radiation Effects Research Foundation (RERF) have shown that the radiation exposure to the γ-rays and neutrons increases the risk of cancer mortality and incidence throughout the life. In the early years after the bombing, the risk of leukemia showed a significant increase, and then decreased but not to zero. Thereafter, the incidence and mortality risks for solid cancers started to increase. The excess relative risk for all solid cancer at age 70 years after the exposure at 30 years of age was estimated to be 0.42 per Gy [95% confidence interval: 0.32–0.53]
[1]. An increase in the mortality risk was confirmed for cancers of most of the tissues/organs, including the stomach, lung, liver, colon, breast, gallbladder, esophagus, bladder, and ovary, and its dose-response relationship has been reported to be linear. For several cancer types, the risks were higher in the survivors exposed as children
[1].
Molecular analyses in the adult-onset PTC cases have demonstrated that more than half of the exposed patients exhibited the
BRAF point mutation (56%), and the
RET/PTC rearrangement was observed in 22% of the exposed patients, while more than 80% of the non-exposed cases harbored the
BRAF gene point mutation
[25]. Of importance, there were the opposite trends for the oncogene frequency associated with the radiation dose: An uptrend for
RET/PTC and downtrend for the
BRAF mutation. Rearrangements of the
NTRK1 and the
ALK genes
[26], as well as the
ABCD5/RET rearrangement
[27], were also identified.
4. Radiation Signatures and Possible Mechanism of Radiation Carcinogenesis
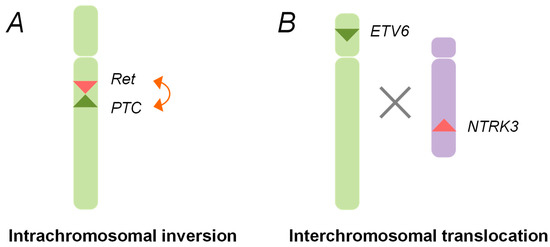
After the Chernobyl accident, chromosomal rearrangements, such as the
RET/PTC1 and
RET/PTC3, were identified in childhood thyroid cancers. These rearrangements are generated through the paracentric (intrachromosomal) inversion within the long arm of chromosome 10, where the
RET, the
CCDC6, and the
NCOA4 genes are located (
Figure 1)
[28][29][30]. The inter-chromosomal translocation is also involved in the formation of other types of rearrangements, such as
ETV6-NTRK3 (
Figure 1).
Figure 1. Schematic representation of oncogenic chromosomal rearrangements. (A) Intra-chromosomal inversion. The rearranged during transfection (RET) gene and the PTC1/3 gene (RET/PTC) rearrangements are generated by an intra-chromosomal inversion of chromosome 10, which gives rise to the fusion genes between the tyrosine kinase domain of the RET gene and the amino terminal region of the PTC gene; (B) Inter-chromosomal translocation. The chromosomal rearrangements, such as ETV6-NTRK3, are created through an illegitimate recombination between the different broken chromosomes.
Theoretically, rearrangements need at least two DNA double-strand breaks, so that exposure to the radiation, which is a well-known inducer for DNA double-strand breaks, has been assumed to cause such rearrangements through an illegitimate recombination of the broken DNA ends
[31]. Furthermore, it has been proposed that the folding of the chromosomal 10q11.2–21 region facilitates a spatial proximity of the
RET and
PTC genes, which could be a structural basis for the RET/PTC rearrangements
[32][33]. The close connection between the radiation exposure and the induction of chromosomal rearrangement was demonstrated experimentally. For example, the
RET/PTC rearrangements were detected in the X-irradiated primary thyroid cells and tissues
[34][35]. While the initial studies used a high-dose over 50 Gy, the generation of the
RET/PTC rearrangements were also identified in the thyroid epithelial cells receiving lower doses
[36]. The induction of other chromosomal rearrangements has also been demonstrated in vitro
[37].
Although the experiments have proven that the
RET/PTC rearrangements are induced by the radiation exposure, in vitro studies are unable to evaluate a spontaneous incidence of the
RET/PTC rearrangements, as the frequency of those in the absence of the genotoxic stimuli is too low. Therefore, the information from human studies, which analyzed the
RET/PTC rearrangements in sporadic childhood thyroid cancers, is indispensable. While several independent groups have evaluated the prevalence of the
RET/PTC rearrangements in childhood thyroid cancer after the Chernobyl accident, only some studies have compared the results with the frequency of the
RET/PTC rearrangements in sporadic childhood PTCs
[38][39][40][41]. The compiled data demonstrated that except for the
RET/PTC3 in tumors developing within the first decade after the Chernobyl accident, the frequency of rearrangements, in particular that of the
RET/PTC1 rearrangement, was comparable between childhood thyroid cancers after the Chernobyl accident and those occurring irrespectively of the radiation exposure (
Table 1)
[42][43][44][45][46]. This suggests that the
RET/PTC rearrangements in radiation-related cases might not be the radiation signature. Rather, the radiation exposure could unveil the
RET/PTC rearrangements that occurred spontaneously
[31]. Considering that thyroid cancers in children began to manifest 4–5 years after the Chernobyl accident, it would be reasonable to hypothesize that thyroid follicular cells with the
RET/PTC rearrangements already existed, and the radiation exposure could provide a chance for the cells with such cancer signatures to proliferate
[31].
Table 1. Prevalence of oncogenic mutations in childhood papillary thyroid carcinomas.
| Studies |
Prevalence (Positive Cases/Total (%)) |
| RET/PTC1 Rearrangement |
Ref |
| Chernobyl-related |
Sporadic |
| Nikiforov et al. (1997) |
5/22 |
6/14 |
27 |
| Thomas et al. (1999) |
12/63 |
|
61 |
| Rabes et al. (2000) |
40/172 |
|
62 |
| Elisei et al. (2001) |
6/25 |
5/25 |
63 |
| Ricarte-Filho et al. (2013) |
3/18 |
1/18 |
38 |
| Leeman-Neill et al. (2013) |
14/62 |
|
76 |
| Total |
80/362 (22.1) |
12/57 (21.1) |
|
It is well-documented that
RET/PTC1 is the predominant type of gene rearrangements in the pediatric PTC
[47][48][49][50], and that the frequency of sporadic thyroid cancer cases harboring the
RET/PTC rearrangements decreases with age, while those with the
BRAF mutation becomes more common
[20]. These two genetic changes are mutually exclusive. Individuals born before the accident are now at least thirty-three years old, and recent reports demonstrate that the frequency of thyroid cancer driven by the
BRAF mutation tends to grow in the affected group
[51][52]. This is an important observation indicating that molecular changes in the radiation-related thyroid cancer mirror those occurring spontaneously, although we also need to bear in mind that there were studies reporting a decrease of the
RET/PTC rearrangements over the years in adult PTCs
[53][54].
Thus, the spectrum of genetic alterations identified in thyroid cancers related to the Chernobyl accident are not very different from that found in the sporadic cases. Since exposures to natural reactive oxygen species and environmental chemicals may occur any time during the life, including the in utero period, it cannot even be ruled out that Chernobyl childhood PTCs could originate from the thyroid follicular cells that had already carried spontaneous RET/PTC rearrangements before the exposure.
Undoubtedly, there is considerable evidence of a link between the chromosomal rearrangements and radiation dose. For example, a recent publication analyzed driver mutations in a series of 65 PTCs diagnosed after the Chernobyl accident, with the individual doses available
[55]. Chromosomal rearrangements, including
RET/PTC1,
ETV6-NTRK3,
STRN-ALK, and
RET/PTC3, and point mutations, such as BRAF
V600E, were found in 70.8% and 26.2% of the cases, respectively. A significant positive correlation between the
131I thyroid dose and the incidence of chromosomal rearrangements was found, and the study reasonably claimed these could be induced by the radiation exposure. The dose-dependent incidence of the gene rearrangement was also reported for the
ETV6-NTRK3 and
STRN-ALK rearrangements
[37][56]. By contrast, other reports concluded that the prevalence of the
RET/PTC rearrangements were not associated with the exposure
[57] or individual radiation doses
[58]. The dose-dependency of
RET/PTC and
PAX8/PPARγ, which is the fusion occurring in the follicular thyroid carcinoma, between PAX8, a transcription factor involved in the thyroid development and PPAR
γ, the master transcriptional regulator of adipogenesis
[59][60], was also determined in radiation-related Chernobyl cases but the decline in the rearrangement frequency at higher doses was modelled and the confidence interval was very wide
[61].
Recent studies have analyzed various molecular changes besides the chromosomal rearrangements
[62]. For example, certain differences in the gene expression profiles between radiation-related and sporadic cancers were reported, although there is a lack of consistency between the gene signatures. Possible confounding factors, including pathological features of the tumors, could cause discrepancies between the studies, and the gene signatures might reflect the results of the radiation exposure. In addition, there has been a gain of chromosome 7q11.23, where the
CLIP2 gene is located, associated with radiation-related cases
[63][64]. ClIP2 (CAP-Gly domain containing linker protein 2) is a member of the cytoplasmic linker protein families, which might link organelles with microtubules. The CLIP2 protein also contains a SMC (structural maintenance of chromosomes) domain involved in the chromosome segregation and cell division. Its overexpression could be a marker of the radiation etiology of thyroid cancer, however, an involvement into the molecular mechanisms of radiation-induced thyroid carcinogenesis needs to be established. Furthermore, genetic determinants connected to the individual predisposition to thyroid cancer were reported. Genome-wide association studies using Chernobyl cases have identified common single nucleotide polymorphism markers, such as rs965513, located in the
FOXE1 region, while there is no marker specific for the radiation-related cancers
[65]. Thus, analyses with advanced technologies are necessary to obtain more information on molecular structures, which should be needed for determining molecular radiation signatures in childhood thyroid cancers related to the radiation exposure.