
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mikiko Kudo | + 1523 word(s) | 1523 | 2021-08-05 12:01:06 | | | |
| 2 | Dean Liu | Meta information modification | 1523 | 2021-09-22 08:06:56 | | |
Video Upload Options
The structure of AKH comprises two von Willebrand factor-A (vWF-A) domains and one Limulus factor C, Coch-5b2 and Lgl1 (LCCL) domain. The chick AKH has an open reading frame of 748 amino acid residues, and the mouse AKH has an open reading frame of 650 amino acid residues (A). AKH has relatively high homology to vitrinand cochlin.
1. Introduction
Several studies have identified various cellular sources of NSCs in the adult vertebrate eye [1]. Retinal NSCs are present in the ciliary body epithelium [2][3] , iris pigment epithelium [4], peripheral margin of the retina [5], and Müller cells [6][7]. Müller cells are radial glial cells, with morphology and expression of glial markers similar to those of embryonic radial cells, which are used as progenitor cells in the CNS. To date, it is believed that Müller cells are the endogenous NSCs in the retina.
In the spinal cord, several cell types have been identified in the central canal, including cuboidal, tanycytic, and radial classes of lumen-contacted ciliated ependymal cells [8]. Numerous studies have indicated that ependymal cells localized in the dorsal central canal, originated from radial glial cells, show NSC activity [9]. Ependymal cells also contribute to the regeneration of oligodendrocytes and remyelination after spinal cord injury [10].
In the adult mouse brain, several studies have indicated two major neurogenic niches: the subventricular zone (SVZ) lining the lateral ventricle and the subgranular zone (SGZ) of the dentate gyrus (DG) of the hippocampus in the CNS [11][12]. In the SVZ, type B stem cells give rise to type C transit-amplifying cells which, in turn, produce type A neuroblasts [13]. Type B and C cells form a tubular network through which type A neuroblasts migrate into the rostral migratory stream toward the olfactory bulbs. In the SGZ, proliferating radial and nonradial precursors give rise to intermediate progenitors which, in turn, generate neuroblasts. Immature neurons migrate into the inner granule cell layer and differentiate into dentate granule cells in the hippocampus [13].
NSCs/NPCs are constantly maintained in a specific microenvironment (niche) since the time of development processes and throughout adulthood [14]. Typically, equilibrium between cell proliferation and differentiation between the two cell populations, NSCs and NPCs, is important for CNS development. Neurons emerge from a pool of NSCs/NPCs through neurogenesis, which is regulated by many extrinsic and intrinsic factors. The niche in which NSCs are maintained consists of a complex array of other neurons, blood vessels, and other glial cells. The division and self-renewal of NSCs are regulated by specialized niche regulators secreted by these cells. Despite the relevance of the fate of NSCs/NPCs, which is ultimately reflected in the final number of newly generated neurons, the timing and number of divisions of NSCs and their differentiation into neurons are flexible processes; moreover, in several cases, not all types of intermediate progenitors are generated in a clonal lineage [15][16]. Thus, the mechanisms that control their development processes are poorly understood.
2. AKH Localizes in the Niche of the Spinal Cord
The spinal cord is the caudal portion of the CNS and transduces information between the brain and the body. NSCs have been isolated from the ependymal zone surrounding the central canal of the spinal cord [17]. A recent study showed that NSCs are the most dorsally located glial fibrillary acid protein (GFAP)-positive cells lying ependymally [18]. AKH expression was observed in the spinal cord of mice on embryonic day 9.5 (E9.5), which disappeared by postnatal day 30 (P30). AKH -/- mice showed reduced spinal cord size compared to that in wild-type mice ( AKH+/+ ). The expression patterns of ependymal niche molecules (nestin and GFAP) in AKH -/- mice were changed when compared with those of AKH +/+ mice in vivo [19]. In vitro culture of the spinal cord neurospheres showed significant reduction in the size of the neurospheres of AKH -/- mice compared with those of AKH+/+ mice [19]. Interestingly, the distribution of ependymal proliferation factors (Cyclin D2 and vimentin) and proliferation markers (Ki67) in the neurospheres derived from AKH -/- was disturbed, indicating the involvement of AKH in NSCs/NPCs regulation.
In general, ependymal cells of the spinal cord are normally quiescent in adult mice. However, when the spinal cord is damaged, ependymal cells are rapidly activated and undergo differentiation to form astrocytes at the injured site [8]. Although the expression of AKH in the central canal ependymal cells is very low or not observed in the central canal at P30, AKH expression is rapidly upregulated in ependymal cells after spinal cord injury, suggesting that AKH is involved in post-injury neuronal neogenesis [19]. These observations suggest that AKH plays a crucial role in spinal cord formation in mice by regulating the ependymal niche in the central canal [18].
3. AKH Is Exclusively Localized in Brain Neurogenic Niches
The adult mammalian brain contains billions of neurons assembled in defined neural circuits, which are the essential components for mediating the higher functions of the CNS. During the development of the mouse brain, NSCs exist around the ventricles, and the newborn neurons migrate to their destination through various pathways. In the niches, neurons and glia cells, such as microglia, astrocytes, and oligodendrocytes, emerge; they create a feedback interaction system via numerous secreted and contact-mediated signals for the regulation between quiescence and cell division of NSCs/NPCs. Although NSCs disappear from most parts of the brain after birth, they are still localized at the SVZ on the lateral wall of the LV and the SGZ of the hippocampal DG where they continue to produce neurons throughout life [18][20].
The expression of AKH at the SVZ was already observed at E17.5, which then disappeared by P20 ( Figure 1 B,D). In the hippocampal DG, AKH expression was observed in the entire hippocampal region immediately after birth, followed by a specific expression in the hippocampal CA2 region at P20 ( Figure 1 C,E). Thus, from the embryonic stage, AKH is expressed in the brain niche areas and disappears in tandem with the cessation of neurogenesis around P20 when brain formation is approximately complete; this observation suggests the involvement of AKH in neurogenesis and neuronal differentiation during early development but not in adult neurogenesis [21].
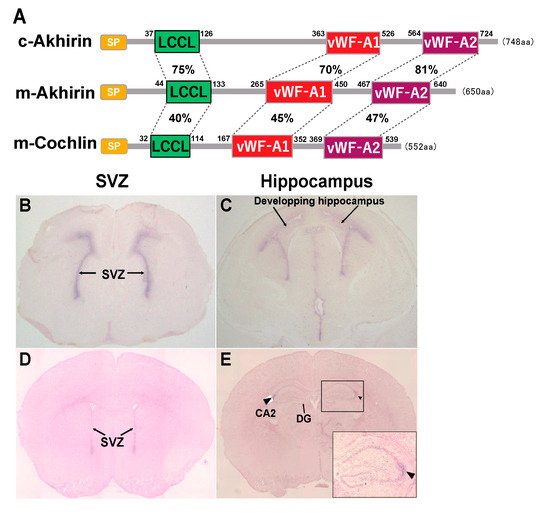
Newborn neurons migrate to other regions in the brain by forming special chain-like structures, which suggests that the interaction between newborn neurons and the extracellular matrix, such as AKH, is important in this process. NSCs are localized in the periventricular region, and nascent neurons migrate to the hippocampal region for hippocampal area formation. One possibility is that AKH might be involved in the migration of newborn neurons to the CA2 region because AKH secreted by NSCs is supposed to attach to the surface and interacts with the substrate during migration. The CA2 region of the hippocampus is a less well-characterized region than the CA1 and CA3 regions. In recent years, studies have reported the importance of the CA2 region in memory updating—a timeline of memory—and social memory [22][23][24]. When neuronal migration to the CA2 region is impaired due to the loss of AKH, psychiatric disorders, such as autism spectrum disorder, may occur. To examine the effect of AKH loss in the CA2 region, we are in the process of preparing a behavioral test battery with AKH -/- mice.
4. Effects of AKH Knockout on Neurogenesis and Neuronal Differentiation in the Brain NSC Niche
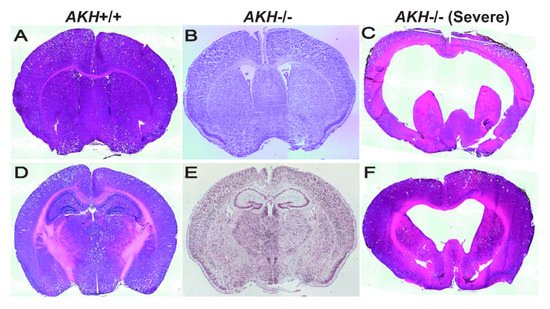
Self-renewal and differentiation potential of NSCs are dynamically regulated by various niche-derived factors, which relay signals in an autocrine or paracrine manner together with transcription factors that respond to those signals. To investigate whether AKH is a niche factor, we compared the brain morphology using AKH -/- and AKH +/+ mice and found that the ventricles of AKH -/- mice were widely expanded when compared with those of AKH +/+ mice ( Figure 2 A,B) [21]. The hippocampal DG region was reduced in AKH -/- mice when compared to that in AKH +/+ mice ( Figure 2 D,E) [21]. Furthermore, lower proportion of GFAP, SOX2, and Ki67 trip; e-positive (GFAP + /SOX2 + /Ki67 + ) cells was observed in AKH -/-, indicating reduced NSC proliferation, but higher population of GFAP and SOX2 double-positive (GFAP + /SOX2 + /Ki67 − ) cells was increased in AKH -/- mice, indicating increase of quiescent NSCs. Finally, AKH deficiency inhibited the differentiation of NSCs into mature neurons and reduced the length of their neurites [21]. These results suggest that the loss of AKH causes NSCs to lose their proliferative capacity and become quiescent, resulting in a decrease in neurogenesis from NSCs, leading to the enlargement of the ventricles and reduction of the DG area during early development.
References
- Ohta, K.; Ito, A.; Tanaka, H. Neuronal stem/progenitor cells in the vertebrate eye. Dev. Growth Differ. 2008, 50, 253–259.
- Ahmad, I.; Tang, L.; Pham, H. Identification of Neural Progenitors in the Adult Mammalian Eye. Biochem. Biophys. Res. Commun. 2000, 270, 517–521.
- Tropepe, V.; Coles, B.L.K.; Chiasson, B.J.; Horsford, D.J.; Elia, A.J.; McInnes, R.R.; van der Kooy, D. Retinal Stem Cells in the Adult Mammalian Eye. Science 2000, 287, 2032–2036.
- Haruta, M.; Kosaka, M.; Kanegae, Y.; Saito, I.; Inoue, T.; Kageyama, R.; Nishida, A.; Honda, Y.; Takahashi, M. Induction of photoreceptor-specific phenotypes in adult mammalian iris tissue. Nat. Neurosci. 2001, 4, 1163–1164.
- Moshiri, A.; Reh, T.A. Persistent Progenitors at the Retinal Margin of ptc+/− Mice. J. Neurosci. 2004, 24, 229–237.
- Fischer, A.J.; Reh, T.A. Müller glia are a potential source of neural regeneration in the postnatal chicken retina. Nat. Neurosci. 2001, 4, 247–252.
- Osakada, F.; Takahashi, M. Neurogenic potential of Mueller glia in the adult mammalian retina. Inflamm. Regen. 2007, 27, 499–505.
- Hamilton, L.; Truong, M.; Bednarczyk, M.; Aumont, A.; Fernandes, K. Cellular organization of the central canal ependymal zone, a niche of latent neural stem cells in the adult mammalian spinal cord. Neuroscience 2009, 164, 1044–1056.
- Namiki, J.; Tator, C.H. Cell proliferation and nestin expression in the ependyma of the adult rat spinal cord after injury. J. Neuropathol. Exp. Neurol. 1999, 58, 489–498.
- Meletis, K.; Barnabé-Heider, F.; Carlen, M.; Evergren, E.; Tomilin, N.; Shupliakov, O.; Frisén, J. Spinal Cord Injury Reveals Multilineage Differentiation of Ependymal Cells. PLoS Biol. 2008, 6, e182.
- Doetsch, F.; Caillé, I.; Lim, D.; García-Verdugo, J.M.; Alvarez-Buylla, A. Subventricular Zone Astrocytes Are Neural Stem Cells in the Adult Mammalian Brain. Cell 1999, 97, 703–716.
- Furutachi, S.; Miya, H.; Watanabe, T.; Kawai, H.; Yamasaki, N.; Harada, Y.; Imayoshi, I.; Nelson, M.; I Nakayama, K.; Hirabayashi, Y.; et al. Slowly dividing neural progenitors are an embryonic origin of adult neural stem cells. Nat. Neurosci. 2015, 18, 657–665.
- Ming, G.-L.; Song, H. Adult Neurogenesis in the Mammalian Brain: Significant Answers and Significant Questions. Neuron 2011, 70, 687–702.
- Eriksson, P.S.; Perfilieva, E.; Björk-Eriksson, T.; Alborn, A.M.; Nordborg, C.; Peterson, D.A.; Gage, F.H. Neurogenesis in the adult human hippocampus. Nat. Med. 1998, 4, 1313–1317.
- Falk, S.; Bugeon, S.; Ninkovic, J.; Pilz, G.-A.; Postiglione, M.P.; Cremer, H.; Knoblich, J.A.; Götz, M. Time-Specific Effects of Spindle Positioning on Embryonic Progenitor Pool Composition and Adult Neural Stem Cell Seeding. Neuron 2017, 93, 777–791.e3.
- Pilz, G.-A.; Shitamukai, A.; Reillo, I.; Pacary, E.; Schwausch, J.; Stahl, R.; Ninkovic, J.; Snippert, H.J.; Clevers, H.; Godinho, L.; et al. Amplification of progenitors in the mammalian telencephalon includes a new radial glial cell type. Nat. Commun. 2013, 4, 2125.
- Weiss, S.; Dunne, C.; Hewson, J.; Wohl, C.; Wheatley, M.; Peterson, A.C.; Reynolds, B.A. Multipotent CNS Stem Cells Are Present in the Adult Mammalian Spinal Cord and Ventricular Neuroaxis. J. Neurosci. 1996, 16, 7599–7609.
- Sabourin, J.-C.; Ackema, K.B.; Ohayon, D.; Guichet, P.-O.; Perrin, F.E.; Garcès, A.; Ripoll, C.; Charitã, J.; Simonneau, L.; Kettenmann, H.; et al. A Mesenchymal-Like ZEB1+Niche Harbors Dorsal Radial Glial Fibrillary Acidic Protein-Positive Stem Cells in the Spinal Cord. Stem Cells 2009, 27, 2722–2733.
- Abdulhaleem, M.F.A.; Song, X.; Kawano, R.; Uezono, N.; Ito, A.; Ahmed, G.; Hossain, M.; Nakashima, K.; Tanaka, H.; Ohta, K. Akhirin regulates the proliferation and differentiation of neural stem cells in intact and injured mouse spinal cord. Dev. Neurobiol. 2014, 75, 494–504.
- Acharjee, U.K.; Felemban, A.A.; Riyadh, A.M.; Ohta, K. Regulation of the neural niche by the soluble molecule Akhirin. Dev. Growth Differ. 2016, 58, 463–468.
- Anam, M.B. Akhirin regulates the proliferation and differentiation of neural stem cells/progenitor cells at neurogenic niches in mouse brain. Dev. Growth Differ. 2020, 62, 97–107.
- Wintzer, M.E.; Boehringer, R.; Polygalov, D.; McHugh, T.J. The Hippocampal CA2 Ensemble Is Sensitive to Contextual Change. J. Neurosci. 2014, 34, 3056–3066.
- Hitti, F.L.; Siegelbaum, S.A. The hippocampal CA2 region is essential for social memory. Nature 2014, 508, 88–92.
- MacDonald, C.J.; Tonegawa, S. Crucial role for CA2 inputs in the sequential organization of CA1 time cells supporting memory. Proc. Natl. Acad. Sci. USA 2021, 118.




