
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Paolo Visconti | + 2069 word(s) | 2069 | 2021-09-17 10:01:42 | | | |
| 2 | Amina Yu | + 2069 word(s) | 2069 | 2021-09-17 10:14:33 | | |
Video Upload Options
The demand for wearable devices to measure respiratory activity is constantly growing, finding applications in a wide range of scenarios (e.g., clinical environments and workplaces, outdoors for monitoring sports activities, etc.). Particularly, the respiration rate (RR) is a vital parameter since it indicates serious illness (e.g., pneumonia, emphysema, pulmonary embolism, etc.). Therefore, several solutions have been presented in the scientific literature and on the market to make RR monitoring simple, accurate, reliable and noninvasive.
1. Introduction
A textile sensor was used in [1] to detect talk events based on changes in breathing patterns. Resistive strain sensors made of a conductive material and polymer mixture were used in the proposed solution. These sensors were applied to the upper chest, lower chest, and abdominal using elastic belts, changing their resistance due to thoracic or abdominal expansion and relaxation; movement artefacts are the main issue with this type of sensor. Considerable efforts are made to integrate these sensors into clothing to develop the next generation of wearable for healthcare applications, allowing monitoring biophysical parameters, for instance, during running and cycling [2][3][4].
Smart fabrics and layers are attracting great attention in the scientific community since pleasant and non-intrusive for developing wearable sensors in various application fields, including healthcare, sports, and military applications [5][6][7]. In particular, piezoresistive fabrics are well suited for integrating wearable devices for their easy-to-implement nature, low cost, and high sensitivity. Also, the strength of inertial sensors lies in their versatility and non-invasiveness, making them ideal for various applications, representing a reliable and inexpensive way for collecting user’s motion data; the most important field is undoubtedly health monitoring [8][9][10].
The main contributions of the proposed review work are: A comprehensive overview of the methodologies, materials, and techniques applied to piezoresistive breathing sensors. Specifically, novel IoT-based wearable devices for monitoring respiration activity are discussed and analyzed [11][12]. Also, innovative piezoresistive materials are introduced, analyzing their manufacturing processes and improvements enhancing their performances or reduce production costs or, last but not least, improve user’s experience by making the sensor more comfortable. Furthermore, we report a comparative analysis of discussed piezoresistive devices to define the features and functionalities of the next generation of RR sensors. An accurate survey of IoT-based wearable devices using inertial sensors (accelerometers, gyroscope, magnetometer, etc.) are analyzed for detecting the breathing movements and thus extracting the respiration rate [13][14][15]. Several embedded systems are proposed in the scientific literature, including one or more inertial sensors, a processing unit, and a communication module for wirelessly transmits the acquired data toward a host device or cloud platform, allowing remote monitoring of user’s conditions [16][17]. Furthermore, an overview of the main algorithms for extracting the respiratory rate from the raw inertial data is reported. Finally, a comparison of discussed devices based on inertial sensors is reported.
The proposed review paper is arranged as follows: Section 2 reports an overview of innovative devices, methodologies, and materials based on piezoresistive RR sensors; furthermore, a comparative analysis of discussed piezoresistive-based devices and materials are presented. Later, Section 3 presents a survey of wearable systems and algorithms for measuring breathing activity using inertial sensors. Finally, a critical analysis of the discussed inertial-based wearable sensors and algorithms are introduced.
2. Review of Innovative Piezoresistive System and Materials for Detecting the Respiration Rate
To measure the most common indicators in pneumography, like breath volume (BV), moment ventilation (MV), respiratory cycle (RC) and inspiratory duty cycle (DTCY), the authors used the KTC system. This last comprises an ARM Cortex-M3 STM32F401RCT6 microcontroller board for signal processing, two Li-Ion rechargeable batteries, a piezoresistive sensor knitted on a velcro strap, a signal acquisition circuit for converting the digital values from strain sensor signals, and an HC-08 Bluetooth 4.0 BLE (Bluetooth Low Energy) module for wirelessly transmitting the acquired data ( Figure 1 ).
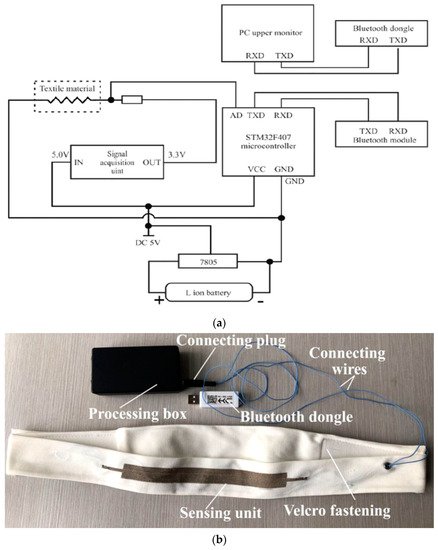
In [19], C. Massaroni et al. developed an innovative device for monitoring respiratory activities, including six piezoresistive sensors. These last were made of 89% silver and 11% spandex piezoresistive silver-plated knitted fabric. Intermeshed conductive zones were generated by knitting silver conductive yarns into the base structure. The resulting material was featured by 0.15 Ω zero-pressure resistance, reaching a maximum value of 0.5 Ω under pressure, and mass per unit area of approximately 346 g∙m −2 . The six sensors were arranged to monitor movements of the pulmonary rib cage (RCp), abdominal rib cage (RCa), and abdomen (AB), cut in different shapes with dimensions of 3 × 0.5, 3 × 1, 5 × 1, 7 × 0.5, and 7 × 1 cm 2 (length × width). The sensors were placed on three elastic bands, along with a custom electronic conditioning and acquisition section to convert their electrical resistances into voltages. In particular, the electronic section was composed of the sensing device, a Wheatstone bridge, an instrumentation amplifier, as well as a microcontroller and a Bluetooth module to wirelessly transmit the acquired data.
Wearable sensors are in high demand in various new application areas, including health screening, human-machine interfaces, soft robotics [20], and custom health monitoring [21][22][23][24]. Specifically, wearable strain sensors should be mechanically flexible for enabling conformal connection to a curved surface and high sensitivity, essential features for wearable electronics [25]. Piezoresistive sensors, which convert mechanical strains to resistance variations [6][26], are commonly used due to their simple read-out mechanism, high sensitivity, ease of design, and low fabrication costs. Traditional piezoresistive strain sensors, based on semiconductors and metal foils, are cost-effective, but their poor sensitivities prevent their usage as wearable strain sensors [26][27]. Therefore, innovative smart textiles are widely employed to develop enhanced healthcare monitoring systems, enhancing sensitivity, linearity, power consumption, and invasiveness [28][29]. Various nanoscale materials, such as metal nanowires nanoparticles [30][31], silicon nanoribbons [32], carbon black [33], carbon nanotubes (CNTs) [34], and graphene, have been investigated as alternative materials integrated into elastomeric polymers or fabric to build stretchable and responsive strain sensors. Because of its outstanding electrical conductivity, unique optical properties, and high flexibility, graphene is considered a suitable substrate for realizing strain sensors.
Active materials, versatile substrates, and conductive electrodes are the three critical components of piezoresistive pressure sensors. Metal nanoparticles and nanowires [35], conductive polymers [36], carbon nanotubes [37], graphene [38], transition metal compounds [39], and other conductive materials play a crucial role. The active material was included in the flexible substrate, constituted by a thin two-dimensional (2D) sheet or a three-dimensional (3D) pad in films and fabrics to withstand the strain. Due to their high compressibility and versatility, pressure sensors with 3D structures have become more popular, and sponges and foams are two common 3D flexible substrates.
3. State of the Art on Systems for Respiration Monitoring Based on Inertial Sensors
In [40], the authors presented a wearable device to continuously monitor RR in patients with Duchenne Muscular Dystrophy (DMD) and Limb-Girdle Muscular Dystrophy type R (LGMD2). The device was equipped with three IMUs, viz a three-axis accelerometer, gyroscope, and magnetometer. Two IMUs were located on the chest and abdomen to measure changes in orientation due to respiratory motion; the third IMU was installed in a place unaffected by respiratory activity (such as on the bed for bedridden patients) to serve as a reference. After removing non-respiratory movements, quarters derived from the thoracic and abdominal IMUs were calculated; then, the algorithm calculated their PSD (Power Spectrum Distrition) using Welch’s method and searched for peak frequency, representing the respiration component. Afterwards, the signal was damped with a third-order Savitzky-Golay filter to calculate the duration of exhalations and inspirations. In doing so, the expiratory time, inspiratory time, and total time (T TOT ) could be calculated to compute the respiratory rate as 60/T TOT .
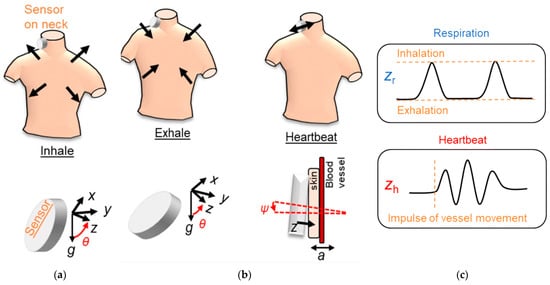
Specifically, in [41], S. Kano and H. Mekaru conducted a preliminary study to compare the RR obtained by two accelerometers (Bosh BM1160) placed on the neck with that derived by a humidity sensor. First, the authors installed an accelerometer on the neck, near the carotid artery, where the greatest acceleration is present during breathing. This position was considered favourable because it also allowed estimating the heart rate using the same setup ( Figure 2 ). The FFT was calculated to the signal obtained by applying a 0.5 Hz low-pass filter to estimate the respiratory rate and a band-pass filter between 20 and 40 Hz to evaluate the heart rate. Then, the respiratory signal was compared with that obtained by a nanoparticle humidity sensor to verify their agreement. The correlation coefficient between the two measurements was 0.61, the bias 0.41, and the standard deviation 2.5 BrPM.
In [42], the authors developed two algorithms for determining the breathing rate; the first derived the RR from the photoplethysmography (PPG) signals, the latter from acceleration data acquired by a 3-axis accelerometer. Both algorithms were suitable for implementation inside a low-cost intensive care unit (ICU) or in post-ICU. The accelerometry-based method relied on applying an adaptative line enhancer (ALE) on each axis to separate the information signal from the noise ( Figure 3 then, the axis with the highest signal-to-noise ratio was selected for the following spectral fusion process. In particular, the singular spectral analysis (SSA) was applied to the chosen ALE output. This analysis decomposed the ALE signal obtaining an elementary matrix constituted by two sub-bands, including the three higher signal eigenvalues (sub-band 1) and second and third eigenvectors (sub-band 2). After diagonal averaging, the FFT was applied to each sub-band signal, selecting the higher narrow peak, representing the RR.
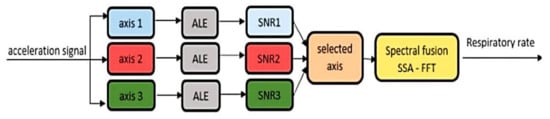
Table 1 reports a comparative analysis of the innovative algorithms for detecting the patient’s respiratory activity based on inertial data, from the point of view of the number of used IMUs, the main processing methods, the used additional information, the algorithm’s performances and the computational complexity.
| Work | Number of Used of IMUs | Processing Methods | Additional Information | Performances | Complexity |
|---|---|---|---|---|---|
| C.-C. Huang et al. [43] | 1 (LSM6DS3) |
Peak detection | No | 95% | Low |
| A. Raj [44] | 1 (LIS2HH12) |
Peak detection | No | 97.4% | Low |
| E.P. Doheny et al. [45] | 1 (MC10) |
Peak detection | PSG signal | 1.58 ± 0.54 BrPM 1 | Low |
| G. Dan et al. [46] | 1 (MPU6050) |
Peak detection | CO2 analysis | 99.8% | Low |
| A. Manoni et al. [47] | 1 (LSM6DSM) |
PSD PWA |
PPG signal | 93% | Medium |
| M. Jafari Tadi [48] | 1 (MMA8451Q) |
FFT Peak detection |
SCG signal | 99.41 ÷ 99.81% | Medium |
| G.-Z. Liu et al. [49] | 1 | Kalman filter PCA FFT |
EDR signal RIP signal CO2 analysis |
95.63% | Medium |
| D. Jarchi et al. [42] | 1 | SSA FFT |
PPG signal | 2.56 BrPM 1 | High |
| J. Warnecke et al. [50] | 3 (Shimmer3) |
PCA FFT |
ECG signal | 3.04 BrPM 1 | Medium |
| A. Cesareo et al. [51] | 1 | PCA FFT |
- | 2 BrPM 1 | Medium |
| J. Lee et al. [52] | 1 | ICA | - | 0.47 BrPM 1 | Medium |
| S. Wang et al. [53] | 1 | Kalman filter VCS |
RIP signal | 1.58 ± 0.54 BrPM 1 (MAE) |
High |
4. Conclusions
Wearable devices are revolutionizing how we treat, manage, and prevent diseases, enabling integrated, capillary and accurate monitoring of the patients’ health, lower management costs, better diagnosis, early prevention, continuous tracking, and quicker intervention. Monitoring respiratory activity is crucial to determine the user’s physical status, preventing diseases like pneumonia, emphysema, pulmonary embolism, etc.
This review paper presents a comprehensive overview of piezoresistive wearable devices and smart materials for monitoring breathing activity. Specifically, innovative wearable sensors applicable to different body areas (e.g., chest, abdomen, nose, ear, etc.) are discussed, exploiting the resistance change of a piezoresistive transducer induced by respiratory movements. Also, a survey of novel smart textiles applied to the detection of breathing movements is presented, featured by low weight and cost and high flexibility and sensitivity. Besides, comparative analysis of discussed wearable piezoresistive devices and smart materials are presented, providing useful insights to define the future generation of health monitoring sensors. Later, a detailed survey on wearable devices and algorithms based on inertial sensors for monitoring breathing activity is introduced. Different embedded systems are presented, equipped with one or more IMUs to detect respiratory movements. Also, an overview of algorithms and processing tools to extract the RR from the respiratory signal is reported. Finally, comparative analysis of the discussed inertial-based wearable devices and algorithms are presented.
From carried out work, we can state that the inertial-based wearable devices represent the future trend for monitoring respiratory activity, given the recent advances in developing MEMS inertial sensors, making them compact, reliable and sensitive, fundamental requirements for their integration into smart clothes [54][55]. Also, frequency-domain processing and advanced data fusion techniques can be easily implemented on embedded devices, given the high computational power, wide memory and reduced sizes of modern processing platforms (microcontrollers, FPGA, etc.) [56][57].
References
- Ejupi, A.; Menon, C. Detection of talking in respiratory signals: A feasibility study using machine learning and wearable textile-based sensors. Sensors 2018, 18, 2474.
- Molinaro, N.; Massaroni, C.; Lo Presti, D.; Saccomandi, P.; di Tomaso, G.; Zollo, L.; Perego, P.; Andreoni, G.; Schena, E. Wearable textile based on silver plated knitted sensor for respiratory rate monitoring. In Proceedings of the 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 2865–2868.
- Mitchell, E.; Coyle, S.; O’Connor, N.E.; Diamond, D.; Ward, T. Breathing feedback system with wearable textile sensors. In Proceedings of the 2010 International Conference on Body Sensor Networks, Singapore, 7–9 June 2010; pp. 56–61.
- Zięba, J.; Frydrysiak, M. Textronics—Electrical and electronic textiles. Sensors for breathing frequency measurement. Fibres Text. East. Eur. 2006, 14, 7.
- Capineri, L. Resistive sensors with smart textiles for wearable technology: From fabrication processes to integration with electronics. Procedia Eng. 2014, 87, 724–727.
- Calvert, P.; Patra, P.; Lo, T.-C.; Chen, C.H.; Sawhney, A.; Agrawal, A. Piezoresistive sensors for smart textiles. In Proceedings of the Electroactive Polymer Actuators and Devices, Portland, OR, USA, 17 April 2007; Volume 6524, pp. 1–5.
- Tan, Y.; Ivanov, Z.M.; Li, H.; Lubich, L.; Wang, C.; Wang, C. A soft wearable and full textile piezoresistive sensor for plantar pressure capturing. Res. Sq. Nano Express 2020, 1, 110.
- Kos, A.; Umek, A. Wearable sensor devices for prevention and rehabilitation in healthcare: Swimming exercise with real-time therapist feedback. IEEE Internet Things J. 2019, 6, 1331–1341.
- Tarannum, S.; Farheen, S. Wireless sensor networks for healthcare monitoring: A review. In Inventive Computation Technologies; Smys, S., Bestak, R., Rocha, Á., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 669–676.
- Villeneuve, E.; Harwin, W.; Holderbaum, W.; Janko, B.; Sherratt, R.S. Reconstruction of angular kinematics from wrist-worn inertial sensor data for smart home healthcare. IEEE Access 2017, 5, 2351–2363.
- Aliverti, A. Wearable technology: Role in respiratory health and disease. Breathe 2017, 13, 27–36.
- Liu, H.; Allen, J.; Zheng, D.; Chen, F. Recent development of respiratory rate measurement technologies. Physiol. Meas. 2019, 40, 07TR01.
- Buke, A.; Gaoli, F.; Yongcai, W.; Lei, S.; Zhiqi, Y. Healthcare algorithms by wearable inertial sensors: A survey. China Commun. 2015, 12, 1–12.
- Liu, G.-Z.; Guo, Y.-W.; Zhu, Q.-S.; Huang, B.-Y.; Wang, L. Estimation of respiration rate from three-dimensional acceleration data based on body sensor network. Telemed. E-Health 2011, 17, 705–711.
- Ponciano, V.; Pires, I.M.; Ribeiro, F.R.; Marques, G.; Villasana, M.V.; Garcia, N.M.; Zdravevski, E.; Spinsante, S. Identification of diseases based on the use of inertial sensors: A systematic review. Electronics 2020, 9, 778.
- Malasinghe, L.P.; Ramzan, N.; Dahal, K. Remote patient monitoring: A comprehensive study. J. Ambient Intell. Hum. Comput. 2019, 10, 57–76.
- Ali, M.; Elsayed, A.; Mendez, A.; Savaria, Y.; Sawan, M. Contact and remote breathing rate monitoring techniques: A review. IEEE Sens. J. 2021, 21, 14569–14586.
- Raji, R.K.; Miao, X.; Wan, A.; Niu, L.; Li, Y.; Boakye, A. Knitted Piezoresistive smart chest band and its application for respiration patterns assessment. J. Eng. Fibers Fabr. 2019, 14.
- Massaroni, C.; Di Tocco, J.; Lo Presti, D.; Longo, U.G.; Miccinilli, S.; Sterzi, S.; Formica, D.; Saccomandi, P.; Schena, E. Smart Textile based on piezoresistive sensing elements for respiratory monitoring. IEEE Sens. J. 2019, 19, 7718–7725.
- Bauer, S.; Bauer-Gogonea, S.; Graz, I.; Kaltenbrunner, M.; Keplinger, C.; Schwödiauer, R. 25th anniversary article: A soft future: From robots and sensor skin to energy harvesters. Adv. Mater. 2014, 26, 149–162.
- Zhang, C.; Zhang, J.; Chen, D.; Meng, X.; Liu, L.; Wang, K.; Jiao, Z.; Sun, T.; Wang, D.; Niu, S.; et al. Crack-based and hair-like sensors inspired from arthropods: A review. J. Bionic Eng. 2020, 17, 867–898.
- De Fazio, R.; Cafagna, D.; Marcuccio, G.; Minerba, A.; Visconti, P. A multi-source harvesting system applied to sensor-based smart garments for monitoring workers’ bio-physical parameters in harsh environments. Energies 2020, 13, 2161.
- Gaetani, F.; Primiceri, P.; Antonio Zappatore, G.; Visconti, P. Hardware design and software development of a motion control and driving system for transradial prosthesis based on a wireless myoelectric armband. IET Sci. Meas. Technol. 2019, 13, 354–362.
- Visconti, P.; de Fazio, R.; Costantini, P.; Miccoli, S.; Cafagna, D. Innovative complete solution for health safety of children unintentionally forgotten in a car: A smart arduino-based system with user app for remote control. IET Sci. Meas. Technol. 2020, 14, 665–675.
- Tang, Y.; Zhao, Z.; Hu, H.; Liu, Y.; Wang, X.; Zhou, S.; Qiu, J. Highly stretchable and ultrasensitive strain sensor based on reduced graphene oxide microtubes–elastomer composite. ACS Appl. Mater. Interfaces 2015, 7, 27432–27439.
- Pang, C.; Lee, G.-Y.; Kim, T.; Kim, S.M.; Kim, H.N.; Ahn, S.-H.; Suh, K.-Y. A flexible and highly sensitive strain-gauge sensor using reversible interlocking of nanofibres. Nat. Mater. 2012, 11, 795–801.
- Wang, C.; Li, X.; Gao, E.; Jian, M.; Xia, K.; Wang, Q.; Xu, Z.; Ren, T.; Zhang, Y. Carbonized silk fabric for ultrastretchable, highly sensitive, and wearable strain sensors. Adv. Mater. 2016, 28, 6640–6648.
- Esfahani, M.I.M. Chapter 6—Smart textiles in healthcare: A summary of history, types, applications, challenges, and future trends. In Nanosensors and Nanodevices for Smart Multifunctional Textiles; Ehrmann, A., Nguyen, T.A., Nguyen Tri, P., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2021; pp. 93–107. ISBN 978-0-12-820777-2.
- Angelucci, A.; Cavicchioli, M.; Cintorrino, I.A.; Lauricella, G.; Rossi, C.; Strati, S.; Aliverti, A. Smart textiles and sensorized garments for physiological monitoring: A review of available solutions and techniques. Sensors 2021, 21, 814.
- Amjadi, M.; Pichitpajongkit, A.; Lee, S.; Ryu, S.; Park, I. Highly stretchable and sensitive strain sensor based on silver nanowire–elastomer nanocomposite. ACS Nano 2014, 8, 5154–5163.
- Hwang, B.-U.; Lee, J.-H.; Trung, T.Q.; Roh, E.; Kim, D.-I.; Kim, S.-W.; Lee, N.-E. Transparent stretchable self-powered patchable sensor platform with ultrasensitive recognition of human activities. ACS Nano 2015, 9, 8801–8810.
- Kim, J.; Lee, M.; Shim, H.J.; Ghaffari, R.; Cho, H.R.; Son, D.; Jung, Y.H.; Soh, M.; Choi, C.; Jung, S.; et al. Stretchable silicon nanoribbon electronics for skin prosthesis. Nat. Commun. 2014, 5, 5747.
- Lu, N.; Lu, C.; Yang, S.; Rogers, J. Highly sensitive skin-mountable strain gauges based entirely on elastomers. Adv. Funct. Mater. 2012, 22, 4044–4050.
- Park, J.; Lee, Y.; Hong, J.; Lee, Y.; Ha, M.; Jung, Y.; Lim, H.; Kim, S.Y.; Ko, H. Tactile-direction-sensitive and stretchable electronic skins based on human-skin-inspired interlocked microstructures. ACS Nano 2014, 8, 12020–12029.
- Wei, Y.; Chen, S.; Lin, Y.; Yuan, X.; Liu, L. Silver nanowires coated on cotton for flexible pressure sensors. J. Mater. Chem. C 2016, 4, 935–943.
- Zhou, R.; Li, P.; Fan, Z.; Du, D.; Ouyang, J. Stretchable heaters with composites of an intrinsically conductive polymer, reduced graphene oxide and an elastomer for wearable thermotherapy. J. Mater. Chem. C 2017, 5, 1544–1551.
- Luo, J.; Lu, H.; Zhang, Q.; Yao, Y.; Chen, M.; Li, Q. Flexible carbon nanotube/polyurethane electrothermal films. Carbon 2016, 110, 343–349.
- Pang, Y.; Tian, H.; Tao, L.; Li, Y.; Wang, X.; Deng, N.; Yang, Y.; Ren, T.-L. Flexible, highly sensitive, and wearable pressure and strain sensors with graphene porous network structure. ACS Appl. Mater. Interfaces 2016, 8, 26458–26462.
- Ma, Y.; Liu, N.; Li, L.; Hu, X.; Zou, Z.; Wang, J.; Luo, S.; Gao, Y. A highly flexible and sensitive piezoresistive sensor based on mxene with greatly changed interlayer distances. Nat. Commun. 2017, 8, 1207.
- Cesareo, A.; Nido, S.A.; Biffi, E.; Gandossini, S.; D’Angelo, M.G.; Aliverti, A. A wearable device for breathing frequency monitoring: A pilot study on patients with muscular dystrophy. Sensors 2020, 20, 5346.
- Kano, S.; Mekaru, H. Preliminary comparison of respiratory signals using acceleration on neck and humidity in exhaled air. Microsyst. Technol. 2021, 27, 1–9.
- Jarchi, D.; Rodgers, S.J.; Tarassenko, L.; Clifton, D. Accelerometry-based estimation of respiratory rate for post-intensive care patient monitoring. IEEE Sens. J. 2018, 18, 4981–4989.
- Huang, C.-C.; Lin, W.-Y.; Lee, M.-Y. Development and verification of an accelerometer-based respiratory detection algorithm with wearable instrumented smart clothes. In Proceedings of the 2017 IEEE International Conference on Systems, Man, and Cybernetics (SMC), Banff, AB, Canada, 5–8 October 2017; IEEE: Banff, AB, Canada, 2017; pp. 578–581.
- Antony Raj, A.; Preejith, S.P.; Raja, V.S.; Joseph, J.; Sivaprakasam, M. Clinical validation of a wearable respiratory rate device for neonatal monitoring. In Proceedings of the 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; IEEE: Honolulu, HI, USA, 2018; pp. 1628–1631.
- Doheny, E.P.; Lowery, M.M.; Russell, A.; Ryan, S. Estimation of respiration rate and sleeping position using a wearable accelerometer. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine Biology Society (EMBC), Montral, QC, Canada, 20–24 July 2020; IEEE: Montral, QC, Canada, 2020; pp. 4668–4671.
- Dan, G.; Zhao, J.; Chen, Z.; Yang, H.; Zhu, Z. A novel signal acquisition system for wearable respiratory monitoring. IEEE Access 2018, 6, 34365–34371.
- Manoni, A.; Loreti, F.; Radicioni, V.; Pellegrino, D.; Della Torre, L.; Gumiero, A.; Halicki, D.; Palange, P.; Irrera, F. A new wearable system for home sleep apnea testing, screening, and classification. Sensors 2020, 20, 7014.
- Jafari Tadi, M.; Koivisto, T.; Pänkäälä, M.; Paasio, A. Accelerometer-based method for extracting respiratory and cardiac gating information for dual gating during nuclear medicine imaging. Int. J. Biomed. Imaging 2014, 2014, 690124.
- Liu, G.-Z.; Wu, D.; Mei, Z.; Zhu, Q.; Wang, L. Automatic detection of respiratory rate from electrocardiogram, respiration induced plethysmography and 3d acceleration signals. J. Cent. South Univ. 2013, 20, 2423–2431.
- Warnecke, J.M.; Wang, J.; Deserno, T.M. Noise reduction for efficient in-vehicle respiration monitoring with accelerometers. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; IEEE: Berlin, Germany, 2019; pp. 3257–3261.
- Cesareo, A.; Previtali, Y.; Biffi, E.; Aliverti, A. Assessment of breathing parameters using an inertial measurement unit (imu)-based system. Sensors 2019, 19, 88.
- Lee, J.; Yoo, S.K. Respiration rate estimation based on independent component analysis of accelerometer data: Pilot single-arm intervention study. JMIR Mhealth Uhealth 2020, 8.
- Wang, S.; Liu, M.; Pang, B.; Li, P.; Yao, Z.; Zhang, X.; Chen, H. A new physiological signal acquisition patch designed with advanced respiration monitoring algorithm based on 3-axis accelerator and gyroscope. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; IEEE: Honolulu, HI, USA, 2018; pp. 441–444.
- El-Sheimy, N.; Youssef, A. Inertial sensors technologies for navigation applications: State of the art and future trends. Satell. Navig. 2020, 1, 2.
- Rast, F.M.; Labruyère, R. Systematic review on the application of wearable inertial sensors to quantify everyday life motor activity in people with mobility impairments. J. Neuroeng. Rehabil. 2020, 17, 148.
- Karacocuk, G.; Höflinger, F.; Zhang, R.; Reindl, L.M.; Laufer, B.; Möller, K.; Röell, M.; Zdzieblik, D. Inertial sensor-based respiration analysis. IEEE Trans. Instrum. Meas. 2019, 68, 4268–4275.
- Balestrieri, E.; Boldi, F.; Colavita, A.R.; de Vito, L.; Laudato, G.; Oliveto, R.; Picariello, F.; Rivaldi, S.; Scalabrino, S.; Torchitti, P.; et al. The architecture of an innovative smart t-shirt based on the internet of medical things paradigm. In Proceedings of the 2019 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Istanbul, Turkey, 26–28 June 2019; pp. 1–6.




