Iron-containing earth minerals of various hues were the earliest pigments of the prehistoric artists who dwelled in caves. Being a prominent part of human expression through art, nature-derived pigments have been used in continuum through ages until now. Studies reveal that the primitive artist stored or used his pigments as color cakes made out of skin or reeds. Although records to help understand the technical details of Indian painting in the early periodare scanty, there is a certain amount of material from which some idea may be gained regarding the methods used by the artists to obtain their results. Considering Indian wall paintings, the most widely used earth pigments include red, yellow, and green ochres, making it fairly easy for the modern era scientific conservators and researchers to study them.
1. Introduction
Since time immemorial, wall paintings have been done in almost all countries. Paintings of all kinds, encompassing all types of wall paintings as well, are a valuable part of many countries’ cultural heritage. In prehistoric paintings, the artistic style was primitive, and the techniques were also very basic, with the paint being applied directly onto the rock surface. As a result of this, the pigments seep through the pores and coarse surface of rocks. Later, more complex designs and figures were painted on walls, which were often prepared with several layers of plaster
[1].
The uses and evidence of earth pigments in the India begin with the hematite and quartz crystals found in the Acheulian deposits of the Lower Paleolithic period. One such hematite specimen was discovered in the exposed floor of Hunsgi (Karnataka), locality V, and has a worn facet with characteristic striation patterns, indicating that it was used as a crayon to color or mark a rock surface
[2]. Because this material does not occur in its natural state in the area surrounding the site, the site excavators believed that these little hematite nodules were brought-in from afar. Paddaya goes on to say that these red ochre nodules were probably also utilized for body ornamentation and other similar purposes
[3]. Six other tiny quartz crystals were discovered from the base of the lower Paleolithic deposit at Singi Talav (Rajasthan), which was interesting evidence. They were virtually fully unchanged and measured 7–25 mm in length, making them too small to have been employed as tools. They had been purposefully transported to the site, as had the Hunsgi hematite nodules, and were collected for their visual attributes
[4]. Although these are only inferences, it is impossible to rule out the possibility of a functioning reality involving an aesthetic sense among our forefathers.
According to Wakankar, some of the earliest depictions in Mesolithic rock paintings painted in green color could be those belonging to the upper Paleolithic era of Indian antiquity. The discovery of green earth (what he refers to as terra verte) in upper Paleolithic deposits in one of the excavated rock shelters (III A-28) at Bhimbetka
[5] could be the basis for this hypothesis. The chronological position in time of the green paintings, however, is a point of contention. Green pigments were discovered first, followed by red paintings, according to some scholars
[6]. Wakankar also discovered yellow ochre, manganese, and terra verte in Bhimbetka’s shelter III A-28 in 1975, where these pigments were observed to be smeared on multiple areas in the shelter III F-23
[2][7][8]. These early paintings represent the beginning of known or surviving rock art in India because of the quality of pigment applications in the form of fine and controlled lines. According to Mishra, for the paintings at Bhimbetka, apart from being executed in red or white, bluish-green and yellowish colors had also been used on occasions. Since they were only preserved in fragments and were found at the bottom of the paint layer; green painted layers tend to be the oldest. Many shades of red were visible, ranging from scarlet to pale red to dark chocolate. Natural minerals were used to make these pigments
[2][9]The largest concentration of prehistoric sites and rock paintings are found in Central India’s sandstone regions, which span three distinct mountain systems: the Vindhyachal and Satpura in Madhya Pradesh, Chhattisgarh, and a portion of Uttar Pradesh and the Aravalli in Rajasthan. In all more than a thousand rock-shelters with paintings have been explored and studied by archeologists. It is interesting to note that prehistoric paintings in India were first noticed 12 years earlier than the famous discovery of the cave paintings of Altamira (Spain). This was done by Archibald Carllyle, a superintendent of the Archaeological Survey of India (ASI) in 1867, in the Mirzapur area of the then United Provinces. Like Marcelino de Santuola, through discovery of Altamira in 1879, Carllyle was the first scholar to attribute these paintings to the Stone-Age period. Some of the prominent sites in India with shelters containing prehistoric paintings are Panchmari in the Mahadeo Hills of Madhya Pradesh, Adamgarph, Raisen, Mirzapur, etc. Mirzapur has evidence dating from the Lower Palaeolithic to the Mesolithic periods, and was a major center of rock painted shelters in Uttar Pradesh
[2][10][11][12][13].
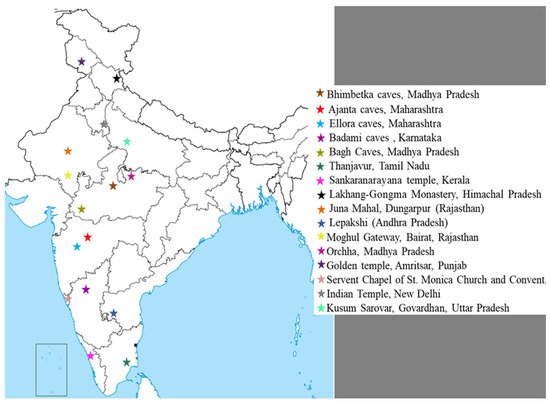
Figure 1 shows a list of most famous wall painting sites in India.
Figure 1. Location of most famous mural paintings in India.
Wall paintings are exposed to a wide range of climatic conditions due to their location in many large and small geographical regions throughout the country
[1]. They were executed on the rock surface, making use of the natural porosity of the rock exterior to secure the colors. The pigment was most likely powdered using a mortar and pestle or within natural depressions of rocks, combined with water, and applied with twig brushes. To remove the pulp and reveal the fibers, one end of the twig is pounded. It has been believed that palmetto twigs were most likely chosen since they are known to be the best among other materials. Considering sandstone shelters, when painting them, the pigment seeps into the porous rock surface and becomes permanently embedded in it, making it impossible to remove, even when washed. Only natural weathering of the granite surface can cause the paintings to deteriorate. It is believed that transparent and opaque color techniques were widely used to rock surfaces. It is a more relied upon technique than the stencil technique, which is traditionally used to make negative handprints. In the transparent color technique, the colors used were heavily diluted in water, except for the emerald green and white colors. For the first time, man-made mud brick structureswere observed during the Neolithic period around 6000 B.C.E where the structures were made by laying a smooth layer of fine mud plaster over a brick wall. Simultaneously, the need for a binding material, such as glue or gum, to set the color onto the smooth mud base became a necessity. As painting techniques developed, first of all a plaster layer was applied on the wall which was later painted upon
[14][15].
Tempera has been the mainstay of Indian wall painting for centuries. The basic feature of this technique is the use of a binding medium for emulsifying the pigment in gum Arabic, animal glue, egg yolk, etc., which achieve the adhesion between the pigments and the dry background
[14]. The paintings in southern India were mostly executed according to the tempera technique where walls were prepared with mud plaster or rock cuts as late as 6–7th C.E. century, such as Ajanta, cave no. 10. The Buddhist paintings in Bagh (Madhya Pradesh) executed in a similar technique immediately follow the Ajanta period. Some wall paintings in monuments and temples in South India were regarded be executed in lime medium (secco) by Paramasivan. The secco technique is done on dry lime plaster. The pigments are mixed with lime water, sometimes with the addition of a little skimmed milk. Some examples of secco paintings are Sitannavasal, Pallava paintings in Kaliasanatha temple, Kanchipuram, and Lepaskhi
[1]. The art of wall painting in Rajasthan evolved into what is known as Rajasthani style after the 17th century C.E. The Indian painters elaborately prepared lime plaster which was laid on walls in several thin layers, each well compacted to a total thickness of a quarter to a half inch to cover crevices and joints. This was overlaid with thin lime plaster and again a specially prepared milk of lime of was applied to the wet wall. Several coats were applied, followed by rubbing with a stone each time. The final application was done with the milk of lime followed by polishing the surface with an agate stone to produce a smooth glaze that later received the pigments prepared in water with a gum medium and occasionally glue in the case of certain pigments, especially black. There are several notable paintings which were executed using the Rajasthani style such as Badal Mahal (Nagaur Fort), Lal Baba Temple (Jodhpur), and Amer Palace (Jaipur)
[1][16].
The uses of earth pigments in India’s painted works has continued since prehistoric times. Because of their high coloring potential and stability under a variety of environmental conditions, light, oxidation, and corrosion, these painting materials have been widely used
[17]. Earth pigments can be used in almost any architectural background for the execution of wall paintings. Iron-rich ochres, green earth, wads (manganese-rich ochres), white earth (calcite, kaolinite, and gypsum), umbers, and vivianite-rich blue earth are the broad categories which are combinations of several chromophore minerals as well as accessory phases such as carbonates, clays, and quartz, rather than pure, single mineral colors. As deposits on the Earth’s surface, they would have been both evident and desired sources of pigment, and it is this category of pigments that is most essential in the evolution of prehistoric art
[18].
Table 1 listed earth pigments used in India’s wall paintings. A detailed explanation of earth pigments will be provided further as a separate section below.
Table 1. Chronological development of pigment used in Indian mural painting with techniques.
| Earth Pigment |
Color |
Name of Pigment |
Chemical Formula |
| Ochre |
Red
Purplish red |
Red Ochre
Indian Red |
α-Fe2O3
Fe2O3 |
| |
Yellow |
Yellow ochre |
Fe2O3·H2O or α-FeO(OH) |
| |
Brown or Reddish brown |
Umber |
Fe2O3·(H2O)+MnO2·(nH2O)+Al2O3 |
| |
Yellow-brown |
Sienna |
Fe2O3+MnO2 |
| Green earth |
Green |
Celadonite
Glauconite |
K[(Al,Fe3+),(Fe2+,Mg)](AlSi3,Si4)O10(OH)2
(K,Na)(Fe3+,Al,Mg)2(Si,Al)4O10(OH)2 |
| White earth |
White |
Calcite
Gypsum
Kaolinite |
CaCO3
CaSO4·2H2O
Al2[Si2O5][OH] |
| Wade |
Black |
Manganese ochre (Pyrolusite) |
MnO2 |
| Blue Earths |
Blue |
Vivianite |
Fe32+ [PO4]2·8H2O |
2. Earth Pigments
The term “earth pigments” refers to inorganic, naturally occurring mineral pigments that are employed as colorants. Earth pigments can be separated into non-clay pigments and pigments with the chromophore element contained in the clay’s crystal structure based on its coloring agent. The color is determined by the presence of non-clay pigments, such as those found in ochres, or by the presence of any chromogenic ingredient in the crystal structure. The natural color hue can be influenced by drying intensity and even more so by persistent high-temperature heating
[19].
2.1. Ochres
According to geology, ochres are oxides or oxide hydroxides of metal which form in a near surface setting. Iron-rich ochres are the most common ones, although copper and cobalt ochres have also been used as pigments
[20]. Ochres are a form of inorganic pigment that comes from natural minerals. The word “ochre” is a common term that refers to substances that range in color from yellow to deep purple and have an iron oxide chromophore
[21]. Types of ochres include red ochre, yellow ochre, purple ochre, brown ochre, sienna, umber, and a variety of other names depending on their hue.
Natural iron oxide pigments, unlike manufactured pigments, vary in color depending on the composition of the region of the earth from which they are mined. The color tends to be influenced by three primary factors where the existence of the iron oxide chromophore is the first consideration (principal coloring ingredient). The darker red ochres are more likely to contain hematite (Fe
2O
3), whereas the paler yellow ochres are more likely to contain goethite (FeOOH or Fe
2O
3·H
2O, hydrated iron oxide). The existence of other metal oxides or clay minerals (secondary coloring ingredients) is the second factor to consider while the particle size distribution within the material is the third factor to consider. This last element is thought to be responsible for the color spectrum of brick red to violet found in the pigment known as caput mortuum, where Fe
2O
3 is the chromophore
[22]. The earth’s color is determined by a combination of these variables. The wide variety of potential yellow, red, and brown is due to the numerous ways and combinations in which these ingredients can mix
[23].
When the colors are different, distinguishing between pigments is easy. When color tones are identical, however, the distinction becomes more complicated, and classification may be incorrect. This is one of the most serious issues with red ochres such as hematite, caput mortuum, red or warm ochres, umbers, and raw or burnt sienna which come in a range of red color tones. Aluminosillicates or lower amounts of other oxides may occur with natural mixtures which causes a minor difference in color tones. Their recognition becomes more difficult since they were almost always mixed with other pigments rather than used in their pure form
[24]. According to Bednarik
[25], the states of hydration, reduction, hydrolysis, oxidation, adsorption, grain form, and size distribution of the several components within the pigments are all factors that influence the final tone of the pigment in question. The degree of hydration of the pigment which is equivalent to the amounts of the two main ochre-coloring minerals present (hematite to goethite ratio) is widely recognized as the most important factor that affects color
[22]. As a result, anhydrous oxides (hematite) are more prevalent in purple and red shades of pigment. The lighter tones suggest hydration with goethite serving as the primary ferrous colourant whereas darker ochres contain several phases of iron hydroxides/oxides and manganese oxides.
Brown earth and umbers, which are sometimes represented as ochre variants
[26], are pigments in which the manganese oxide content exceeds 5% of the total colorant matter within the pigment. However, their overall features and structure indicate otherwise. The umbers, in particular, contain between 5–20% manganese oxides which have a substantial impact on the pigment’s physical properties. Besides color, accessory minerals may have affected a negative impact on ochre properties. Calcite, feldspars, quartz, dolomite, and other carbonate minerals, as well as clay minerals and probably gypsum, are examples of accessory minerals found in ochres
[27].
Ochres also have excellent pigment properties, such as non-toxicity, excellent hiding ability, and tinting strength
[28]. The continued and broad use of ochres can be attributed to a number of factors, including their abundance in Nature, suitable optical and handling characteristics, and relatively simple methods of preparation. These pigments are also among the most long-lasting pigments in the industry today, with exceptional resistance to water, light, and changing environmental conditions and, dilute acid and alkali attack
[29]. Since ochres absorb ultraviolet light, they appear to shield their binding medium from degradation, in addition to extending the paint’s overall permanence
[28]. Manufacturers denote synthetic iron oxide pigments with names commonly reserved for earth pigments, such as yellow ochre for an artificial yellow iron oxide, which complicates the issue. The term “iron oxide pigment” is commonly used to describe both natural and man-made materials that contain mixes of iron oxides and iron oxide hydroxides while the nomenclature earth and ochre are acceptable when it is evident that the colour comes from a natural source
[15][30]. The use of heat to manipulate color dates back to 298,000 B.C.E., demonstrating the scientific temper of the prehistoric people for artistic purposes.
Synthetic ochre was prepared by the irreversible process of calcining or boiling raw yellow soil to produce a variety of hues such as red, orange, brown, and mid tones of these colors. The dehydroxylation of hydrated iron oxides to oxides is involved in this procedure, with the final colour variation based on the initial material, temperatures, and heat treatment duration
[31]. Solid-state transformation by heating iron ores or iron salts such as iron sulfates (FeSO
4), iron chlorides (FeCl
2/FeCl
3), iron hydroxides (FeOOH), iron carbonate (Fe
2CO
3), and magnetite (Fe
3O
4) in an oxidizing atmosphere is one of the most extensively used ways of synthetic ochre processing. Methods for making synthetic therapeutic iron oxides by burning iron filings with various salts have been documented in India since the Ayurvedic period (600 B.C.E. to 800 C.E.)
[32].
2.1.1. Red Ochre
The name is attributed to the reddish color from the mineral hematite, which is occasionally found as fossilsized ores, secular iron ores and micaceous iron ores. The name hematite, derived from the Greek word “haima” meaning blood, aptly describes its usual red hue. Hematite is typically formed as a result of the oxidation of biotite (K(Mg,Fe)
3AlSi
3O
10 (F,OH)
2),magnetite (Fe
3O
4), (FeTiO
3), goethite (α-FeOOH),and ilmenite (FeTiO
3)
[28]. The latter case tends to be diagenetic, with increasing temperature and burial depth favoring the transformation
[33]. The transformation of geological ochres to ochre pigments is simple and requires a chain of operations that includes removing larger impurities (such as organic contaminants and plant roots), grinding, sieving, and levigation before intermixing withaa medium to make paint. Hematite grain size influences color as well, with a general shift from red to purple beds as the crystal size increases
[28]. Goethite is also one of the most common iron-bearing minerals in red beds, and is more common in younger deposits with a distinct yellow-brown appearance
[34]. One of the biggest issues with red ochres is in that tracing them back to their source which is extremely difficult, particularly after they’ve been heavily processed. The presence of carbonate or quartz along with the crystalline nature, color, and particle size, can help distinguish broad sedimentary environments. The character and mineralogy of surviving ochre deposits in the area of an archaeological site can be used to determine the possible provenance of local use. Red ochres are a universal pigment that can be employed in works of art from many periods and cultures. From the Pleistocene period to the present day, it has been used as a pigment
[35]. Ajanta, Ellora, Badami, Bagh, and other sites have Buddhist paintings rendered with red and yellow ochre pigments
[36].
Red ochres are non-toxic and are used in the production of paints that not only dry easily but also completely cover surfaces. Ochre sightings have been recorded in many regions throughout the world. In India, red ochre deposits can be found primarily in Bharatpur, Bhilwara, Bikaner, Chittorgarh and Udaipur districts in Rajasthan; Gwalior, Katni and Rewa districts in Madhya Pradesh; Anantapur, Kadapa, Visakhapatnam districts in Andhra Pradesh; Bhavnagar, and Kachchh and Patan districts in Gujarat; Ballari and Bidar districts in Karnataka, and Chandrapur district in Maharashtra
[37].
Indian red (hirmize) is also a natural iron ore or hematite with a rich purplish-red color and a ferric oxide content of over 90%. Some of the reds exported from India come from roasting lighter-colored ores, but the majority of the specimens are genuine natural ores. It was assumed that the some of the hematite was imported from the Forest of Dean and some from Ormuz in the Persian Gulf. The faint purplish tint of this pigment is its most distinguishing characteristic in terms of color. Indian red is sometimes marketed as an admixture of iron oxide with other materials, most likely the result of judicious mixing of purple-brown, red ochre, or “light red” and cheap white substances
[38].
2.1.2. Yellow Ochre
Natural yellow ochre (yellow earth) also known as limonite (hydrated iron hydroxide, FeO(OH)·nH
2O which consists of a mixture of several iron-containing minerals including goethite (iron oxyhydroxide, FeO[OH]), akageneite, lepidocrocite, and jarosite, with goethite being the main component. Goethite is a type of pedogenic crystalline iron oxide hydroxides that can be found in rocks, soils, and ochre deposits
[28]. Because of this, goethite-based earths are the most widely used yellow pigments in antiquity. However, yellow ochre became a regular artists’ material as the palette grew beyond red and black, and the usage of goethite-rich yellow ochres, in particular, became temporally and globally widespread
[34]. The second main pigment is potassium jarosite. The finding of jarosite in Mars meteorites has spurred renewed interest in these minerals and their petrogenes is occurs in supergene settings, as well as their relationship with mine waste
[39][40][41][42].
Yellow ochre is prepared by washing the natural mineral to remove sand and other impurities, then drying, grinding, and sieving it
[43]. Some pigments can be safely combined with yellow ochre. Yellow ochre is non-toxic and stable, with excellent hiding ability and permanence in all media. Yellow earth is converted to burnt yellow earth by heating below 800 °C for several hours, where its constituent goethite is thermally converted to red iron oxide having hematite crystal structure. It has been known that heating yellow earth produces a red pigment. This technology seems to have been used in the Paleolithic era, and it is observed that the method had entered written history by the time of the Assyrian cuneiform tablets
[44]. The transition from goethite to hematite when burnt can be easily identified using X-ray diffraction
[44][45][46][47]. Depending on the temperature at which the goethite is heated, the product hematite is formed. Using X-ray diffraction, it is possible to separate burnt yellow earth pigments from the natural red earth
[48].
In early 20th C.E. India, yellow ochre deposits were found near Katni, and several central Indian states such as Sohawal (Uttar Pradesh), Gwalior and Panna (Madhya Pradesh), and Baraunda (Haryana). Yellow ochre was also found as inclusion within laterite soil of various localities such as Cretaceous rocks (Trichinopoly, Tamil Nadu) and Tertiary beds (Myingyan district, Burma)
[38].
Today’s, yellow ochre deposits can be found in Andhra Pradesh’s Guntur and Kurnool districts, Madhya Pradesh’s Jabalpur, Mandla, Satna, and Shahdol districts, and Maharashtra’s Nagpur district. According to Indian Minerals Yearbook (2015) data based on the United Nations Framework Classification for Resources (UNFC) scheme, the total reserves/resources of ochre as of 1.4.2015 are estimated to be 167.79 million tones. Around 36.93 million tons of these resources fall into the ‘Reserves’ category, while 130.86 million tons fall into the ‘Remaining Resources’ category. Around 87% of the total resources are red ochre, 11 percent are yellow ochre, and the remaining 2% are grade “Unknown.” Rajasthan has about 78% of the resources, followed by Madhya Pradesh with 11%, Andhra Pradesh with 7%, and Gujarat with 2%. Karnataka, Maharashtra, Jharkhand, and Uttar Pradesh have the remaining 2% of the total
[37].
2.1.3. Umber
Umber is a naturally occurring iron oxide and manganese oxide-containing ore which is used as brown and reddish-brown earth pigment
[21]. It has been used for thousands of years and has earth tones ranging from cream to brown, depending on the amount of iron and manganese compounds present. It is also completely stable and darker than ochre and sienna, which are similar earth pigments and can be, safely mixed with other pigments. The best variety comes from Cyprus which is distinguished by the presence of manganese dioxide
[48][49]. Umber is made by mining the ore followed by grinding and washing it which leaves a mixture of rust-stained clay. In its natural state, it is known as raw umber. The brown red color of umber intensifies when calcined and is known as burnt umber due to the loss of water (dehydration). Raw earth umber (raw sienna earth) consisting of iron oxide, aluminum oxide, and manganese oxide while burnt umber (burnt sienna earth) consisting of iron oxide, clay, and manganese oxide
[21].
Being a mineral pigment with yellowish-brown to greenish brown color, derived from natural earth colored iron and manganese oxides, umber is normally used in tempera, paint and water color paintings. Because of their high oil adsorption, umbers need about 18% oil to coalesce with oil paints. As a result, the later oil films darken over time. Umber’s chemical composition is closely linked to its iron oxide content, much like sienna’s. The increased manganese content distinguishes umber from other colors. Clay, talc, and calcium carbonate are other naturally occurring substances in umber that do not effect its color. The particles of umbers are heterogeneous and rounded in appearance. Burnt umber particles are almost similar to raw umber particles. Umbers with high tinting strength are the ones which have high content of iron and manganese within them. Alkalis and dilute acids have no effect on umbers
[21].
2.1.4. Sienna
Sienna is an iron oxide and manganese oxide-based earth pigment while raw sienna is a yellow-brown pigment which occurs only in its natural state. Raw sienna’s chemical breakdown is roughly 90% iron oxide (yellow) with minor amounts of manganese oxide, which distinguishes it from pure yellow ochre. Burnt sienna is produced by heating raw sienna to 537–1093 °C before the iron oxide is mainly converted to hematite. Sienna was one of the first pigments used for painting and can be found in prehistoric cave art. However, it was not until the 14th century C.E., at the dawn of the Renaissance period, that the pigment was further refined for artistic use. It gets its name from the Italian city-state of Siena, where it was created. During this time, the Italians improved the pigment’s variety of colors by roasting sienna, resulting in raw sienna and burnt sienna pigments. Earth colors were used extensively in Renaissance painting techniques. It is stable at high temperatures but not acid resistant, and is compatible with all other pigments; so it is often mixed with a variety of others
[18][50].
2.2. Wads: Manganese Ochres
Soot is the natural choice of black pigment for any society since it is widely available and is a high purity carbon-based black pigment. Alternatively, woody plant material (as well as bones and ivory) can be quickly burned to create high-quality black pigments. Mineral blacks, such as manganese ochre or wad, are among the first examples of black pigment for artistic applications.
Wad is an old English term for black soil used by miners which refers to manganese-rich earths, as well as graphite deposits. Manganese (Mn) is one of nature’s most abundant elements, accounting for one-tenth of all elements found in the Earth’s crust. According to geochemistry, Mn is found in minerals formed during the early stages of magmatic crystallization. Mn is easily oxidized near the Earth’s surface, forming several oxide-hydroxide minerals; however, it melts and can be abundant in late-stage deposits such as pegmatites; Mn is easily oxidized near the Earth’s surface, forming several oxide-hydroxide minerals; however, it melts and can be abundant in late-stage deposits such as pegmatites. Manganese ochres are made up of a complex mix of manganese oxide and hydroxide minerals, as well as iron oxides
[51]. Simple oxides like Mn
xO
y or mixed oxides like A
xMn
yO
z, where A is K or Ba, reveal their sedimentary origin. The black mineral pyrolusite (MnO
2) is the most prevalent one, while other minerals that contain manganese include rhodonite(MnSiO
3), rhodochrosite (MnCO
3), manganite (MnO(OH), cryptomelane (K
x(Mn
4+,Mn
3+)
8O
16), alabandite (MnS), and hollandite (Ba(Mn
4+6,Mn
3+2)O
16)
[52]. Identification of the provenance of wads has proven to be extremely difficult due to their lack of documentation in geological literature. This material also has properties that make it useful for lighting fire, but it wasn’t always used as a pigment in this sense.
2.3. Green Earths (Terreverte)
Green earth, a siliceous mineral with a dull grayish-green hue, is found all over the world and has long piqued artists’ interest
[53]. Green earth has been discovered in some early Bhimbetka rock paintings, but there appears to be a general lack of black, such as carbon black or soot, in the primitive Indian paintings. The pigment was used by American Indians as well as artists from Pompeii and Ajanta caves in India. Perhaps the most well-known use of the pigment for the green under painting and shadows in medieval era was for painting’s flesh colors. Green earth was perfect for the artist because it was lightfast and easy to prepare by crushing and grinding, even though the hue was not as strong in chroma as malachite and azurite. The pigment is still sold in modern times from high-quality deposits found on Cyprus
[54].
Green earths, also known as terreverte, are grey to blue green pigments made mainly from two closely related clay minerals, celadonite (K[Mg,Fe
2+]Fe
3+[Si
4O
10][OH]
2) and glauconite ([K,Na][Mg,Fe
2+,Fe
3+][Fe
3+,Al][Si,Al]
4 O
10[OH]
2). Despite their chemical similarities, glauconite and celadonite are distinguished by a significantly higher number of trivalent ions in the octahedrally coordinated layer and a partial substitution of Al
3+ for Si
4+ in the tetrahedrally coordinated layer
[55][56]. Celadonite is a relatively pure mineral that can be present in small amounts in vesicular cavities (amygdules) or volcanic rock fractures. Glauconite, a less pure but more commonly dispersed mineral, is often present in the form of small greenish pellets (green sand). Other greenish clay minerals may have been mixed into green earth pigments occasionally. One explanation for this is the morphological rather than the chemical characterization of the green pigment, and they may contain other clay minerals such as montmorillonite, chlorite, and kaolinite
[56].
Although the chemical compositions of the two main source minerals, glauconite and, celadonite, are identical, traditionally the minerals were considered apart disparately, with glauconite attributedto a sedimentary origin and celadonite derived from altered basaltic rocks. Only recently has research allowed for an unmistakable distinction between the two minerals based on fractional differences in ionic substitution. Unfortunately, the name glauconite is used to characterize both a specific micaceous mineral and a specific morphological type (green sand). This arose from a long-standing inability to properly identify the components of glauconite pellets. Grissom
[54] examined the terminology of green earth pigments, their materials, and their appearance in works of art. Green earth exhibits slight light scattering ability in oil due to its low refractive index of about 1.62
[56], where the resulting paint is relatively translucent. Apart from this the tinting ability and hiding power of the pigment are both poor.
When the pigment is glazed over white or used in conventional water-dispersed mediums like glue or egg tempera, which usually produce films with high pigment volume concentration, the highest saturation (chroma) is achieved. Green earth is partly soluble in acids and alkalis, making it easy to obtain enough ferrous and ferric ions for micro-chemical testing. Burnt green earth is formed when the pigment becomes brownish when heated. Key elements of celadonite and glauconite (iron, silicon, aluminium, magnesium), as well as small amounts of potassium-replacement ions (calcium, manganese and sodium) along with trace amounts of titanium were identified in dozens of samples of green earth examined in India. The elements that were not present were beryllium, boron, arsenic, bismuth, cadmium, mercury, cobalt, nickel, molybdenum, tin, strontium, vanadium, zinc, and zirconium
[57][58].
Green earths were and continue to be the most widely available pure green pigments, and as a result, they are well-known in the art world. Green earth is commonly used in Indian wall paintings and has been discovered in large quantities. The color green is prevalent in the Buddhist frescoes painted on lime and mud plasters in the caves of Ajanta, Maharashtra, India, from the 2nd Century B.C.E. to the 6th Century C.E.
[59], and M. Singh and Arbad
[36] identified the green pigment as celadonite which was derived from altered basalt.
2.4. White Earths
Kaolinite, calcium carbonate and gypsum were used as white pigments since prehistoric times. M. Singh reported the kaoline, and calcium carbonate were used as white pigments, and often also with gypsum in Ajanta’s wall paintings
[60].
2.4.1. Calcite and Gypsum
In all cultures around the world, calcium carbonate (CaCO
3) and calcium sulfate {gypsum (CaSO
4 2H
2O), anhydrite (CaSO
4), and other hydration states of calcium sulfate} were invariably used for backgrounds and white pigments. They can be referred as simply as white or calcium-based white
[20]. Calcium carbonate in different forms has played a significant role in art since ancient times. Calcite is the most common natural source of calcium carbonate. It is mostly found in sedimentary rocks like limestone and chalk, but it’s also found in metamorphic rocks (marble) and igneous rocks in rare cases. Calcite and its dimorph form aragonite are the principal components of some mollusk shells, but aragonite (the less stable type) is more likely to be found in living or fossilized lower organisms, which is converted to calcite with climate changes, especially when heated
[54].
Since pure calcium carbonate is white, it has a wide range of applications in the arts and industries, both in powdered and large forms. Some modern paints also use powdered whites as the primary pigment white, as well as bulking agents and extenders for white hiding and colored paints. Calcium carbonate pigments such as chalk, calcite, precipitated chalk, shell white, lime white, and coral have all been employed in the history of painting materials. Aragonite and vaterite are two less common polymorphic forms while calcite is frequently found as single large transparent crystals. The varieties range from coarse granular to impalpable. Both of these types of calcite can be found in paintings, both in the support structures and pictorial layers, along with several quantities of common impurities like dolomite, quartz, magnesite, clays, and coloring agents like carbon and hematite. In fresco painting, marble dust or ground marble was used
[61]. Powdered forms of all kinds of calcium carbonate are white if relatively pure, but they lack the strong scattering and hiding power of heavy-metal whites when used in drying oil-based paints. Depending on the amount of impurities present, particularly iron oxide, natural chalk is typically a yellowish-white rock or grayish white. When exposed to light, all types of calcium carbonate become permanent. Hydrogen sulfide gas or contact with sulfide pigments does not darken them. Except for alkali-sensitive colors like Prussian blue, which may be discolored by lime white, which can maintain its alkalinity for a long time, they are compatible with most other pigments. However, acids found in modern industrial and urban environments are harmful to calcite (particularly the finely crystalline and porous varieties). Since calcium sulfate has a larger volume than carbonate when produced in the presence of sulfuric acid, it causes spalling and flaking of lime surfaces
[62].
The mineral gypsum is made up of hydrated calcium sulfate (CaSO
4 2H
2O). Selenite is the name given to the mineral when in well-developed crystals. Satin spar is a fibrous vast variety with a silky lustre that is transparent and opalescent. Gypsum comes in a variety of types and is extremely economically valuable. It is colorless or white, but due to the presence of impurities, it may be tinted light brown, grey, yellow, green, or orange. Anhydrite (CaSO
4) is a natural mineral that contains calcium sulfate but is devoid of all water of crystallization. This can be observed as large opaque white mineral underlying gypsum in several locations. The two layers are undergo net-mixing in an intermediate region, so it’s not unusual to find gypsum samples containing anhydrite, and vice versa. In terms of crystal habit and other physical properties, gypsum differs from anhydrite due to the two molecules of water used in crystallization. The former can be used in a variety of ways
[63].
Paramasivan reported that the calcium carbonate (lime plaster) levels found during analysis were also quite low and present in the form of impurities only. He chemically examined the fine plaster layers of several Indian wall paintings after separating the paint film from rough plasters. He treated rough plasters with dilute hydrochloric acid, which dissolved the fine plaster giving efflorescence with emission of carbon dioxide. The presence of calcium and traces of sulfate in the solution indicated that a lime-calcium sulfate mixture was used as lime plaster over the rough plaster. Calcium sulfate is an impurity in the lime, according to him. Calcite and gypsum both were used either as white pigments or ground in many rock and cave paintings of India
[64][65][66][67][68][69]. Through his researchSingh has identified calcite as the white pigment used at the Ajanta and Ellora paintings
[70][71].
2.4.2. Kaolinite
Kaolin is a type of natural clay rock that is mostly composed of the clay mineral kaolinite, which has the chemical composition (Al
2[Si
2O
5][OH]
4). The structural layer of kaolinite is made up of two sheets, one of which is made up of tetrahedra of [SiO
4]
4− (“T”) and the other of which is made up of octahedra of [AlO
3(OH)
3]
6−(“O”), with a T/O ratio of 1:1 and the net charge of a layer iszero
[72]. The lack of or random displacement of two-sheet layers along the b axis indicate whether the kaolinite structure is well or poorly organized
[73]. With a soft consistency and earthy texture, kaoline is abundant locally in combination with hydrothermally altered granites and volcanic clastic rocks, and it can be broken easily and molded or formed, particularly when wet. In spite of this, it is yet to be widely recognized as a pigment in its own right.
While kaolin is a dull and uninteresting mineral in and of itself, it does occasionally form interesting pseudomorphs, especially after feldspars. The word kaolin refers to both a group of closely related clay mineral and a particular member of that group. Many commercial kaolinite mines exist, where this mineral is extracted in large quantities for various industrial applications
[74]. Works bySingh have evinced the use of kaolin for executing floral designs in Ajanta. The Ajanta caves belong to the 2nd B.C.E. and painting work was ere executed from the 4th C.E. Singh has suggested that kaolin was used in combination with different pigments such as red and yellow ochres as well to change the tone and hue of the paintings
[75].
2.5. Blue Earths
The mineral vivianite (Fe
32+ [PO
4]
2·8H
2O) refers to the blue earth, an iron phosphate hydrate that forms naturally in peat bogs and is also known as “blue ochre”
[19]. It can also be found in organic-rich habitats, such as the insides of fossilized mollusk shells, and also is found in traces quantity within bones, rotting wood, and other organic matter. In combination with hematite, siderite, or anapaite, vivianite is formed as radiating clusters of acicular (needle-like), prismatic, or fibrous bluish-green crystals. Vivianite is a dark blue or green mineral that is normally stable, but it can be colorless when first exposed. This color transformation is unique to vivianite, which is found in peat bogs
[76].
Since vivianite has a low tinting intensity, it is rarely reliably opaque unless applied in thick or multiple coats, allowing the substrate to show through. Vivianite is not as reflective as other pigments when visually recognizing it. Unlike “brighter” ultramarine blue and darker, more concentrated Prussian blue, vivianite absorbs light rather than reflecting it, even though a lipid binder is used
[77].
Vivianite is seldom found in archaeological contexts in the ancient world, but further identifications of this peculiar pigment may be produced soon. Vivianite has been discovered in several easel paintings from the historical period and it has been found in medieval art in France. However, vivianite as a blue pigment could not be detected in Indian painting, but its existence cannot be denied since the absence of a discussion of these minerals in the literature is simply an absence of analysis rather than proof of a mineral’s non-existence or rarity in a specific place.