
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | NIKHEEL BHOJRAJ RATHOD | + 2264 word(s) | 2264 | 2021-09-01 10:28:31 | | | |
| 2 | Amina Yu | Meta information modification | 2264 | 2021-09-14 08:36:38 | | |
Video Upload Options
The use of fish by-products is an important production opportunity for the fishing and seafood processing industries since it can possibly create additional profits while also lowering disposal costs. Benefits of utilizing secondary raw materials from fish processing are depicted in. One of the potential possibilities for greater benefit is to use these industrial wastes and low-value fish for the recovery and hydrolysis of proteins rich in bioactive peptides.
1. Introduction
Proteins and natural peptides occurring in the food system and living organisms play a vital role in the functioning of various systems including the cardiovascular, nervous, gastrointestinal, and immune systems. Peptides with biological or nutritional qualities help an organism’s body function better and increase the quality of its diet. These peptides are known as “bioactive peptides” [1], and these are formed of short sequences of amino acids including peptides having two or more amino acids which are inactive within the parent protein’s sequence and can be released through proteolytic hydrolysis with commercially available enzymes or proteolytic microorganisms as well as fermentation [2]. Enzymatic hydrolysis is a useful method for recovering essential components such as bioactive peptides from fish by-products, as well as improving the functional and nutritional qualities of protein without compromising its nutritional value [3]. The Hydrolytic breakdown of high molecular weight proteins to low molecular weight proteins is the basis of protein hydrolysate production [4]. Using different enzymes, different fish as substrates, and variable proteolytic factors such as pH, temperature, enzyme to substrate ratio, and time, a large range of hydrolysates with different physical, chemical, and biological characteristics can be produced [5]. Fish protein hydrolysates, like other relevant protein hydrolysates, can be used in food systems.
In order to produce the FPH with different and desired properties, it is important to know the mechanism of protein hydrolysis. Enzymatic hydrolysis of fish proteins is a highly complex process, and its reaction mechanism has been poorly understood. Some proteases preferentially catalyze the hydrolysis of bonds adjacent to a particular amino acid residue, while some are less specific. The catalysis by proteases occurs primarily as three consecutive reactions [5]: (1) the formation of a complex between the original peptide chain and the enzyme referred to as the Michaelis complex; (2) cleavage of the peptide bond to liberate one of the two peptides; and (3) nucleophilic attack on the remains of the complex to split off the other peptide and to reconstitute the free enzyme. The generally accepted mechanism for proteases indicates that the dissociation of enzyme substrate complex into free enzyme and product is the rate-determining step, which in turn determines the overall rate of reaction. The hydrolysis of peptide bonds leads to an increase in the numbers of ionizable groups (NH3 + and COO − ), with a concomitant increase in hydrophobicity and net charge, decrease in molecular size of the polypeptide chain, and an alteration of the molecular structure leading to the exposure of the buried hydrophobic residues to the aqueous environment [6][7][8].
When the degree of hydrolysis or the products formed are plotted against duration of hydrolysis, the hydrolysis is characterized by an initial rapid hydrolysis phase followed by a rapid decrease in the reaction rate. From the studies, the observed phase changes in hydrolysis curve have been ascribed to the phenomena like decrease in the concentration of peptide bonds, enzyme inhibition by hydrolysis products, and inactivation of the enzyme. The inhibition of the reaction by hydrolysis products found to have a significant effect on the hydrolysis curve. This is responsible for the typical shape observed during the enzymatic hydrolysis of fish by-product proteins. Removing the products formed during the hydrolysis process may improve the hydrolysis efficiency by the proteases.
One of the most efficient approaches to recover powerful bioactive peptides is to use enzymes to hydrolyse fish waste proteins [9]. The ability of peptides to demonstrate bioactive qualities is determined by a number of factors. The protein supply, the specificity of the enzyme utilised in protein hydrolysis, the amino acid content, and the amino acid sequence in peptides sequence are important [2]. The reaction media’s physicochemical conditions including time, temperature, pH, and enzyme/substrate ratio have to be optimized by the enzyme’s activity. Proteases from a variety of sources are frequently utilised to achieve more selective hydrolysis since they are specific for peptide bonds next to specific amino acid residues. For the production of bioactive peptides from proteins, a variety of proteases has been utilised. Enzymatic protein hydrolysis has been achieved using proteases from animal, plant, and microbial sources. There are many scientific reports where different proteases from various sources have been used to produce bioactive peptides with desired health beneficial properties [10]. For the hydrolysis of tuna dark muscle [11], chymotrypsin, papain, neutrase, and trypsin at specific pH and temperature for each enzyme are used. The composition and sequence of amino acid residues in the peptides are considered to be directly linked to the biological activities [12]. The degree of hydrolysis (DH), as well as the size or molecular weight of the peptides, has been suggested as a contributing factor to the manifestation of bioactive properties [12]. The degree of hydrolysis is often used to evaluate the progress of hydrolysis reaction. However, for the given enzyme, substrate, and hydrolysis conditions, the composition, sequence, size/molecular weight of derived peptides, and, in turn, the bio-functionalities of protein hydrolysates derived from fish processing waste are all dictated by DH.
2. Fish Waste Protein Hydrolysates (FWPH)
Fish waste protein hydrolysate (FWPH) is a mixture of amino acids and peptides of varying sizes produced from secondary raw materials from fish processing, often by biological processes, that cleaves peptide bonds. Hydrolysis of proteins converts the secondary raw materials into a high value product which is rich in amino acids and biologically active peptides [13]. FWPHs have attracted significant attention due to their antioxidative, ACE-inhibitory, antibacterial, anticancer, and immunomodulatory activities, along with offering balanced essential amino acids as a source of bioactive components in food/feed. Proteases derived from plants, bacteria, fungi, and the viscera of fish can be exploited for making FWPH have good functionality and bioactive properties. Preparation steps involved in production of fish waste protein hydrolysate are given in Figure 1 .
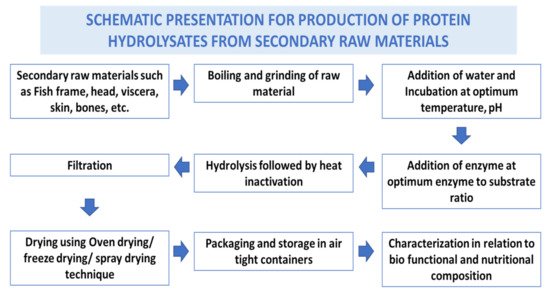
Protein hydrolysates and bioactive peptides were derived from various organs of aquatic organisms ( Table 1 ) and have shown bioactive properties. Several authors have reported in vitro and in vivo bioactivities of peptides/protein hydrolysate derived from fish processing waste. Protein hydrolysates have been derived from head waste from different fish species including sardine, herrings, and salmon. Protein hydrolysates from other secondary raw materials like dark muscle from tuna and frame waste from Japanese threadfin breams, tilapia and other species have been studied for their bio-functional potential [14]. There are numerous reports available on hydrolysed proteins and bioactive peptides from skin (gelatin and collagen peptides) from a variety of fish species including catfish, croaker, horse mackerel, tilapia, cobia, carps and many more.
| Fish | Secondary Raw Material | Reference |
|---|---|---|
| Sea bream | Fish Scales | [15] |
| Alaska pollack (Theragra chalcogramma) | Frame | [16] |
| Hoki (Johnius belengerii) | Frame | [17] |
| Catla catla | Visceral organs | [18] |
| Sturgeon | Visceral organs | [19] |
| Tuna | Liver by-products | [20] |
| Alaska pollock | Frames/backbones | [21] |
| Skate | Skin | [22] |
| Cod (Gadus macrocephalus) | Skin | [23] |
| Channa striatus | Roe | [24] |
| Bluefin tuna | Head | [25] |
| Salmon | Pectoral fin | [26] |
| Seabass | Skin | [27] |
| Leatherjacket | Head waste | [28] |
| Japanese threadfin bream | Frame waste | [29] |
| Rainbow trout | By-products | [30] |
| Squid | By-products | [31] |
| Leiognathus splendens | By-catch | [32] |
| Salmon | By-products | [33] |
| Monkfish | By-products like head and viscera | [34] |
| Turbot | By-products | [35] |
| Fish | Solid and liquid waste generated from processing operations | [36] |
3. Bioactivities of Fish Waste Protein Hydrolysates
Antimicrobials in food are used to prevent the growth of microorganisms that cause food spoilage. The most frequently used antimicrobial drugs are organic acids (sorbic acid, acetic acid, citric acid etc.), modifying cell membrane permeability to substrates and establishing inhospitable pH conditions for bacteria growth [37]. When exposed to water, organic acids such as sorbic acid, despite being a highly good preservative, are known to degrade and release potentially hazardous chemicals such as acetaldehyde. As a result, using antimicrobial peptides is a possibly safer approach for preventing similar incidents. Peptides with varied amino acid sequences and varying sizes have been found to have antibacterial action after enzymatic hydrolysis of proteins. Anti-microbial peptides have been found to have a wide spectrum of activity against bacteria, viruses, fungus, and protozoa, among other microbes. They have a broad spectrum of antibacterial and antifungal activities. Antimicrobial peptides that have antimicrobial properties are a class of bioactive peptides and are produced naturally or from protein hydrolysis.
The majority of antimicrobial peptides have been shown to be cationic, which means they have a net positive charge because of positively charged amino acid groups like lysine and arginine with hydrophobic properties and amphipathicity [38][39][40], which facilitate the bonding with the host and solubility in aqueous (lipid) membranes. Antimicrobial peptides are thought to work by forming membrane pores and then penetrating the cell, allowing microbial biological components to be released and the cell to be destroyed [38]. Antimicrobial activity is found in peptides which are released by appropriate hydrolysis conditions [38][41], usually with less than 50 amino acids and low molecular weight of <10 kDa [42].
Cancer has had a direct and indirect impact on the global population as a leading cause of death. While the number of cancer cases is increasing, some of them could be prevented or even treated with natural chemicals. It has been claimed that bioactive peptides found on land and in the sea can minimise the risk of chronic diseases and maintain good health. Since fish by-products as source of bioactive peptides have anticarcinogenic potential [43]. Anticarcinogenic peptides fight cancer cells in a variety of ways, including (1) in the cytoplasm, (2) through stimulation of membrane disruption by micellization, and (3) in the interaction of peptides with cells during apoptosis gangliosides on the surface [44]. Peptides derived from secondary raw material from fish processing demonstrated anticancer activities [45][46]. However, there is paucity of literature available on in vivo studies and cell line studies to evaluate the anticarcinogenic effect of peptides derived from waste of seafood processing. Table 2 shows the anticarcinogenic activity of peptides derived from secondary raw materials of aquatic origin.
| By-Product Source for Peptide | Peptide Sequence/Molecular Weight | Anticancer Effect | Researchers |
|---|---|---|---|
| Sepia Ink oligopeptides due to presence of lysine and proline in sequence | N Gln-Pro-Lys with a molecular mass of 343.4 Da |
Inhibition of proliferation of human prostate cancer (DU-145) cells |
[47] |
| Tuna dark muscle peptides | Leu-Pro-His-Val-Leu-Thr-Pro-Glu-Ala-Gly-Ala-Thr and Pro-Thr-Ala-Glu-Gly-Gly-Val-Tyr-Met-Val-Thr) |
Anticarcinogenic activity against breast cancer cell line | [11] |
| Snow crab by-product peptides | Two anionic peptides with MW of 537 and 216 Da and three cationic peptides with MW of 228, 241 and 291 Da | anticancer activity on colon, breast, prostate and lung cancer cell lines |
[48] |
| Shrimp shell peptide | Peptides with fractionation size < 10 and 10–30 kDa | Anticancer activity on colon and liver cancer cell lines | [49] |
| Flathead by-product peptides | <3 kDa | Anticancer activity against HT-29 colon cancer cells up to 91.04% | [50] |
| Lates calcarifer skin peptides | - | Anti-proliferative activity on human colon and liver cancer cell lines | [46] |
| Flying fish frame peptides | - | Anti-proliferative activity against Hep G2 cells |
[51] |
| Grouper roe peptides | - | Reduced cell viability of oral cancer cells & induced apoptosis of Ca9–22 cells |
[52] |
| Rohu roe peptides | - | Anti-proliferative activity on Human colon cancer cell line |
[53] |
| Threadfin bream (Nemipterus japonicus) Back bone peptides |
- | Anti-proliferative activity against HepG2 cell lines |
[11] |
| Cuttlefish mantle protein hydrolysates | - | MDA-231 and T47D cancer cell lines with growth inhibition of 78.2 and 66.2% | [54] |
| Gilthead seabream byproduct peptides | - | Antiproliferative activity on human colon and breast cancer cell lines | [55] |
Proliferation and cell migration with new extracellular matrix formation are a few of the steps involved in the wound healing process [56]. Oral administration of peptides extracted from various fish species and their by-products, such as collagen hydrolysates, demonstrates retention of moisture over the face along with enhanced viscoelastic properties and reduced sebum levels [57]. Enzymatic protein hydrolysates derived from the bones of silver carp and isolated peptides showed higher efficiency in stimulating metabolism of keratinocytes and wound healing activities, demonstrating the promising nature of bone peptides in care of wounds in the cutaneous region [58].
4. Research Gaps, Opportunities, and Challenges for Fish Protein Hydrolysate
The current interest in fish hydrolysates containing large amounts of protein and thereby peptides and amino acids is of great importance for future research. Although the functional and antioxidant characteristics of fish processing waste protein hydrolysates have been thoroughly reported [59][29], more study is needed in order to commercialise such hydrolysates while also taking into account the economic factors. To maintain the appropriate balance between the functional and bioactive qualities of these hydrolysates, the extent to which fish waste proteins can be hydrolysed must be optimised. There has not been enough reporting on the usage of protein hydrolysates in food ingredients. It is necessary to conduct additional research into the safety of these hydrolysates. Microbiological standards for fish protein hydrolysate have not been prescribed. However, there are limited studies on safety aspects of hydrolysates produced from fish processing waste and further research of a similar nature is required. Due to the inherent nature of process flow employed in hydrolysate production, bacteria that are more hazardous are killed during the enzymatic hydrolysis and post-hydrolysis processes; however, there is a scarcity of scientific evidence on the safety of fish waste protein hydrolysates. Before commercialization, microbial and allergen analyses are required to complete the food safety profile of protein hydrolysate. In addition, because these hydrolysates come from seafood, histamine levels must be monitored. Although many in vitro investigations on the bioactivity of protein hydrolysate have been undertaken, the destiny of these functional molecules in the gastrointestinal tract, as well as their absorption and bioavailability, has yet to be fully explored.
References
- Hartmann, R.; Meisel, H. Food-Derived Peptides with Biological Activity: From Research to Food Applications. Curr. Opin. Biotechnol. 2007, 18, 163–169.
- Je, J.-Y.; Qian, Z.-J.; Byun, H.-G.; Kim, S.-K. Purification and Characterization of an Antioxidant Peptide Obtained from Tuna Backbone Protein by Enzymatic Hydrolysis. Process Biochem. 2007, 42, 840–846.
- Nalinanon, S.; Benjakul, S.; Kishimura, H.; Shahidi, F. Functionalities and Antioxidant Properties of Protein Hydrolysates from the Muscle of Ornate Threadfin Bream Treated with Pepsin from Skipjack Tuna. Food Chem. 2011, 124, 1354–1362.
- Shirai, K.; Ramírez-Ramírez, J.C. 10 Utilization of Fish Processing By-Products for Bioactive Compounds. Fish Process. Sustain. New Oppor. 2011, 236.
- Kristinsson, H.G.; Rasco, B.A. Fish Protein Hydrolysates: Production, Biochemical, and Functional Properties. Crit. Rev. Food Sci. Nutr. 2000, 40, 43–81.
- Mahmoud, M.I.; Malone, W.T.; Cordle, C.T. Enzymatic Hydrolysis of Casein: Effect of Degree of Hydrolysis on Antigenicity and Physical Properties. J. Food Sci. 1992, 57, 1223–1229.
- Kester, J.J.; Richardson, T. Modification of Whey Proteins to Improve Functionality. J. Dairy Sci. 1984, 67, 2757–2774.
- Phillips, R.D.; Beuchat, L.R. Enzyme Modification of Proteins; ACS Publications: Washington, DC, USA, 1981.
- Thiansilakul, Y.; Benjakul, S.; Shahidi, F. Antioxidative Activity of Protein Hydrolysate from Round Scad Muscle Using Alcalase and Flavourzyme. J. Food Biochem. 2007, 31, 266–287.
- Korhonen, H.; Pihlanto, A. Food-Derived Bioactive Peptides-Opportunities for Designing Future Foods. Curr. Pharm. Des. 2003, 9, 1297–1308.
- Hsu, K.-C.; Li-Chan, E.C.; Jao, C.-L. Antiproliferative Activity of Peptides Prepared from Enzymatic Hydrolysates of Tuna Dark Muscle on Human Breast Cancer Cell Line MCF-7. Food Chem. 2011, 126, 617–622.
- Ren, J.; Zhao, M.; Shi, J.; Wang, J.; Jiang, Y.; Cui, C.; Kakuda, Y.; Xue, S.J. Optimization of Antioxidant Peptide Production from Grass Carp Sarcoplasmic Protein Using Response Surface Methodology. LWT Food Sci. Technol. 2008, 41, 1624–1632.
- Wangkheirakpam, M.R.; Mahanand, S.S.; Majumdar, R.K.; Sharma, S.; Hidangmayum, D.D.; Netam, S. Fish Waste Utilization with Reference to Fish Protein Hydrolisate—A Review. Fish Technol. 2019, 56, 169–178.
- Dhanabalan, V.; Vinothkumar, L.; Manivannan, M.; KA, M.X. Fish Processing Byproducts Derived Protein Hydrolysates and It’s Potential as Antioxidants. Biot. Res. Today 2021, 3, 276–278.
- Fahmi, A.; Morimura, S.; Guo, H.-C.; Shigematsu, T.; Kida, K.; Uemura, Y. Production of Angiotensin I Converting Enzyme Inhibitory Peptides from Sea Bream Scales. Process Biochem. 2004, 39, 1195–1200.
- Je, J.-Y.; Park, P.-J.; Kim, S.-K. Antioxidant Activity of a Peptide Isolated from Alaska Pollack (Theragra chalcogramma) Frame Protein Hydrolysate. Food Res. Int. 2005, 38, 45–50.
- Jung, W.-K.; Kim, S.-K. Calcium-Binding Peptide Derived from Pepsinolytic Hydrolysates of Hoki (Johnius belengerii) Frame. Eur. Food Res. Technol. 2007, 224, 763–767.
- Bhaskar, N.; Mahendrakar, N.S. Protein Hydrolysate from Visceral Waste Proteins of Catla (Catla catla): Optimization of Hydrolysis Conditions for a Commercial Neutral Protease. Bioresour. Technol. 2008, 99, 4105–4111.
- Ovissipour, M.; Abedian, A.; Motamedzadegan, A.; Rasco, B.; Safari, R.; Shahiri, H. The Effect of Enzymatic Hydrolysis Time and Temperature on the Properties of Protein Hydrolysates from Persian Sturgeon (Acipenser persicus) Viscera. Food Chem. 2009, 115, 238–242.
- Je, J.-Y.; Lee, K.-H.; Lee, M.H.; Ahn, C.-B. Antioxidant and Antihypertensive Protein Hydrolysates Produced from Tuna Liver by Enzymatic Hydrolysis. Food Res. Int. 2009, 42, 1266–1272.
- Hou, H.; Li, B.; Zhao, X.; Zhang, Z.; Li, P. Optimization of Enzymatic Hydrolysis of Alaska Pollock Frame for Preparing Protein Hydrolysates with Low-Bitterness. LWT Food Sci. Technol. 2011, 44, 421–428.
- Lee, J.K.; Jeon, J.-K.; Byun, H.-G. Effect of Angiotensin I Converting Enzyme Inhibitory Peptide Purified from Skate Skin Hydrolysate. Food Chem. 2011, 125, 495–499.
- Ngo, D.-H.; Ryu, B.; Vo, T.-S.; Himaya, S.W.A.; Wijesekara, I.; Kim, S.-K. Free Radical Scavenging and Angiotensin-I Converting Enzyme Inhibitory Peptides from Pacific Cod (Gadus macrocephalus) Skin Gelatin. Int. J. Biol. Macromol. 2011, 49, 1110–1116.
- Galla, N.R.; Pamidighantam, P.R.; Akula, S.; Karakala, B. Functional Properties and in Vitro Antioxidant Activity of Roe Protein Hydrolysates of Channa Striatus and Labeo Rohita. Food Chem. 2012, 135, 1479–1484.
- Bougatef, A.; Balti, R.; Haddar, A.; Jellouli, K.; Souissi, N.; Nasri, M. Protein Hydrolysates from Bluefin Tuna (Thunnus thynnus) Heads as Influenced by the Extent of Enzymatic Hydrolysis. Biotechnol. Bioprocess Eng. 2012, 17, 841–852.
- Ahn, C.-B.; Kim, J.-G.; Je, J.-Y. Purification and Antioxidant Properties of Octapeptide from Salmon Byproduct Protein Hydrolysate by Gastrointestinal Digestion. Food Chem. 2014, 147, 78–83.
- Senphan, T.; Benjakul, S. Antioxidative Activities of Hydrolysates from Seabass Skin Prepared Using Protease from Hepatopancreas of Pacific White Shrimp. J. Funct. Foods 2014, 6, 147–156.
- Chi, C.-F.; Wang, B.; Wang, Y.-M.; Zhang, B.; Deng, S.-G. Isolation and Characterization of Three Antioxidant Peptides from Protein Hydrolysate of Bluefin Leatherjacket (Navodon septentrionalis) Heads. J. Funct. Foods 2015, 12, 1–10.
- Gajanan, P.G.; Elavarasan, K.; Shamasundar, B.A. Bioactive and Functional Properties of Protein Hydrolysates from Fish Frame Processing Waste Using Plant Proteases. Environ. Sci. Pollut. Res. 2016, 23, 24901–24911.
- Wald, M.; Schwarz, K.; Rehbein, H.; Bußmann, B.; Beermann, C. Detection of Antibacterial Activity of an Enzymatic Hydrolysate Generated by Processing Rainbow Trout By-Products with Trout Pepsin. Food Chem. 2016, 205, 221–228.
- Apostolidis, E.; Karayannakidis, P.D.; Lee, C.M. Recovery of Bioactive Peptides and Omega-3 Fatty Acids-Containing Phospholipids from Squid Processing by-Product Hydrolysate. J. Aquat. Food Prod. Technol. 2016, 25, 496–506.
- Prabha, J.; Nithin, A.; Mariarose, L.; Vincent, S. Processing of Nutritive Fish Protein Hydrolysate from Leiognathus splendens. Int. J. Pept. Res. Ther. 2020, 26, 861–871.
- Vázquez, J.A.; Sotelo, C.G.; Sanz, N.; Pérez-Martín, R.I.; Rodríguez-Amado, I.; Valcarcel, J. Valorization of Aquaculture By-Products of Salmonids to Produce Enzymatic Hydrolysates: Process Optimization, Chemical Characterization and Evaluation of Bioactives. Mar. Drugs 2019, 17, 676.
- Vázquez, J.A.; Menduíña, A.; Nogueira, M.; Durán, A.I.; Sanz, N.; Valcarcel, J. Optimal Production of Protein Hydrolysates from Monkfish By-Products: Chemical Features and Associated Biological Activities. Molecules 2020, 25, 4068.
- Vázquez, J.A.; Rodríguez-Amado, I.; Sotelo, C.G.; Sanz, N.; Pérez-Martín, R.I.; Valcárcel, J. Production, Characterization, and Bioactivity of Fish Protein Hydrolysates from Aquaculture Turbot (Scophthalmus maximus) Wastes. Biomolecules 2020, 10, 310.
- Navarro-Peraza, R.S.; Osuna-Ruiz, I.; Lugo-Sánchez, M.E.; Pacheco-Aguilar, R.; Ramírez-Suárez, J.C.; Burgos-Hernández, A.; Martínez-Montaño, E.; Salazar-Leyva, J.A. Structural and Biological Properties of Protein Hydrolysates from Seafood By-Products: A Review Focused on Fishery Effluents. Food Sci. Technol. 2020, 40, 1–5.
- Lassoued, I.; Mora, L.; Nasri, R.; Jridi, M.; Toldrá, F.; Aristoy, M.-C.; Barkia, A.; Nasri, M. Characterization and Comparative Assessment of Antioxidant and ACE Inhibitory Activities of Thornback Ray Gelatin Hydrolysates. J. Funct. Foods 2015, 13, 225–238.
- Song, R.; Wei, R.-B.; Luo, H.-Y.; Wang, D.-F. Isolation and Characterization of an Antibacterial Peptide Fraction from the Pepsin Hydrolysate of Half-Fin Anchovy (Setipinna taty). Molecules 2012, 17, 2980–2991.
- Song, R.; Wei, R.; Zhang, B.; Wang, D. Optimization of the Antibacterial Activity of Half-Fin Anchovy (Setipinna taty) Hydrolysates. Food Bioprocess Technol. 2012, 5, 1979–1989.
- Brogden, K.A. Antimicrobial Peptides: Pore Formers or Metabolic Inhibitors in Bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250.
- Himaya, S.W.A.; Ngo, D.-H.; Ryu, B.; Kim, S.-K. An Active Peptide Purified from Gastrointestinal Enzyme Hydrolysate of Pacific Cod Skin Gelatin Attenuates Angiotensin-1 Converting Enzyme (ACE) Activity and Cellular Oxidative Stress. Food Chem. 2012, 132, 1872–1882.
- Najafian, L.; Babji, A.S. A Review of Fish-Derived Antioxidant and Antimicrobial Peptides: Their Production, Assessment, and Applications. Peptides 2012, 33, 178–185.
- Nurdiani, R.; Vasiljevic, T.; Singh, T.K.; Donkor, O.N. Bioactive Peptides from Fish By-Products with Anticarcinogenic Potential. Int. Food Res. J. 2017, 24, 1840–1849.
- Abdelhedi, O.; Jridi, M.; Jemil, I.; Mora, L.; Toldrá, F.; Aristoy, M.-C.; Boualga, A.; Nasri, M.; Nasri, R. Combined Biocatalytic Conversion of Smooth Hound Viscera: Protein Hydrolysates Elaboration and Assessment of Their Antioxidant, Anti-ACE and Antibacterial Activities. Food Res. Int. 2016, 86, 9–23.
- Alemán, A.; Pérez-Santín, E.; Bordenave-Juchereau, S.; Arnaudin, I.; Gómez-Guillén, M.C.; Montero, P. Squid Gelatin Hydrolysates with Antihypertensive, Anticancer and Antioxidant Activity. Food Res. Int. 2011, 44, 1044–1051.
- Picot, L.; Bordenave, S.; Didelot, S.; Fruitier-Arnaudin, I.; Sannier, F.; Thorkelsson, G.; Bergé, J.P.; Guérard, F.; Chabeaud, A.; Piot, J.M. Antiproliferative Activity of Fish Protein Hydrolysates on Human Breast Cancer Cell Lines. Process Biochem. 2006, 41, 1217–1222.
- Guo-Fang, D.; Huang, F.-F.; Zui-Su, Y.; Di, Y.U.; Yong-Fang, Y. Anticancer Activity of an Oligopeptide Isolated from Hydrolysates of Sepia Ink. Chin. J. Nat. Med. 2011, 9, 151–155.
- Doyen, A.; Beaulieu, L.; Saucier, L.; Pouliot, Y.; Bazinet, L. Demonstration of in Vitro Anticancer Properties of Peptide Fractions from a Snow Crab By-Products Hydrolysate after Separation by Electrodialysis with Ultrafiltration Membranes. Sep. Purif. Technol. 2011, 78, 321–329.
- Kannan, A.; Hettiarachchy, N.S.; Marshall, M.; Raghavan, S.; Kristinsson, H. Shrimp Shell Peptide Hydrolysates Inhibit Human Cancer Cell Proliferation. J. Sci. Food Agric. 2011, 91, 1920–1924.
- Nurdiani, R.; Vasiljevic, T.; Yeager, T.; Singh, T.K.; Donkor, O.N. Bioactive Peptides with Radical Scavenging and Cancer Cell Cytotoxic Activities Derived from Flathead (Platycephalus fuscus) by-Products. Eur. Food Res. Technol. 2017, 243, 627–637.
- Suarez-Jimenez, G.-M.; Burgos-Hernandez, A.; Ezquerra-Brauer, J.-M. Bioactive Peptides and Depsipeptides with Anticancer Potential: Sources from Marine Animals. Mar. Drugs 2012, 10, 963–986.
- Yang, J.-I.; Tang, J.-Y.; Liu, Y.-S.; Wang, H.-R.; Lee, S.-Y.; Yen, C.-Y.; Chang, H.-W. Roe Protein Hydrolysates of Giant Grouper (Epinephelus lanceolatus) Inhibit Cell Proliferation of Oral Cancer Cells Involving Apoptosis and Oxidative Stress. BioMed Res. Int. 2016, 2016, 8305073.
- Hung, C.-C.; Yang, Y.-H.; Kuo, P.-F.; Hsu, K.-C. Protein Hydrolysates from Tuna Cooking Juice Inhibit Cell Growth and Induce Apoptosis of Human Breast Cancer Cell Line MCF-7. J. Funct. Foods 2014, 11, 563–570.
- Hamzeh, A.; Rezaei, M.; Khodabandeh, S.; Motamedzadegan, A.; Noruzinia, M. Antiproliferative and Antioxidative Activities of Cuttlefish (Sepia pharaonis) Protein Hydrolysates as Affected by Degree of Hydrolysis. J. Food Meas. Charact. 2018, 12, 721–727.
- Kandyliari, A.; Golla, J.P.; Chen, Y.; Papandroulakis, N.; Kapsokefalou, M.; Vasiliou, V. Antiproliferative Activity of Protein Hydrolysates Derived from Fish By-Products on Human Colon and Breast Cancer Cells. Proc. Nutr. Soc. 2020, 79.
- Al Guo, S.; di Pietro, L.A. Factors Affecting Wound Healing. J. Dent. Res. 2010, 89, 219–229.
- Matsumoto, H. Clinical Effects of Fish Type I Collagen Hydrolysate on Skin Properties. ITE Lett. Batter New Technol. Med. 2006, 7, 386–390.
- Iosageanu, A.; Oancea, A.; Ilie, D.; Anton, E.D.; Craciunescu, O. The Effect of Fish Bone Bioactive Peptides on the Wound Healing Process: An in Vitro Study on Keratinocytes. Rom. Biotechnol. Lett. 2021, 26, 2692–2699.
- Chalamaiah, M.; Hemalatha, R.; Jyothirmayi, T. Fish Protein Hydrolysates: Proximate Composition, Amino Acid Composition, Antioxidant Activities and Applications: A Review. Food Chem. 2012, 135, 3020–3038.




