The continuous increase of the world’s population results in an increased demand for energy drastically from the industrial and domestic sectors as well. Moreover, the current public awareness regarding issues such as pollution and overuse of petroleum fuel has resulted in the development of research approaches concerning alternative renewable energy sources. Amongst the various options for renewable energies used in transportation systems, biodiesel is considered the most suitable replacement for fossil-based diesel. In what concerns the industrial application for biodiesel production, homogeneous catalysts such as sodium hydroxide, potassium hydroxide, sulfuric acid, and hydrochloric acid are usually selected, but their removal after reaction could prove to be rather complex and sometimes polluting, resulting in increases on the production costs. Therefore, there is an open field for research on new catalysts regarding biodiesel production, which can comprise heterogeneous catalysts. Apart from that, there are other alternatives to these chemical catalysts. Enzymatic catalysts have also been used in biodiesel production by employing lipases as biocatalysts. For economic reasons, and reusability and recycling, the lipases urged to be immobilized on suitable supports, thus the concept of heterogeneous biocatalysis comes in existence. Just like other heterogeneous catalytic materials, this one also presents similar issues with inefficiency and mass-transfer limitations. A solution to overcome the said limitations can be to consider the use of nanostructures to support enzyme immobilization, thus obtaining new heterogeneous biocatalysts.
1. Introduction
Biocatalysts based on enzymes have been investigated for decades due to their catalytic power, their high degree of specificity, and stereoselectivity
[1][2][3]. These properties allow enzymes to catalyze reactions undergoing in milder conditions, such as low temperature and pressure, turning them into interesting candidates for several industrial applications
[1][4].
Considering biodiesel production using biocatalysts, enzymatic transesterification using lipases has recently got the attention of researchers. Lipases are triacylglycerol acylhydrolases that exist on animals, plant, and microorganisms
[5][6].
According to the literature, lipases used in the production of biodiesel come from microorganisms, such as
Aspergillus niger [7],
Burkholderia cepacian (
[8][9],
Candida Antarctica [10][11][12],
Candida rugose [13],
Mucor mihei [14][15],
Pseudomonas cepacian [16],
Pseudomonas fluorescens [17][18],
Rhizopus oryzae [19],
Rhizomucor miehei [20][21], and
Thermomyces lanuginosus [22][23], to name a few. It is known that, in nature, they catalyze the hydrolysis of triglycerides to glycerol and free fatty acids as being present in the oil-water interface. Furthermore, lipases can also serve as a catalyst in esterification and transesterification reactions
[24].
Usually, lipases can achieve fatty acid alkyl esters (FAAE) conversion yields of over 90% after long reaction times (sometimes over 70 h), while reaction temperatures can range from 30 °C to 50 °C
[25].
In comparison with the transesterification of triglycerides using chemical catalysts, biodiesel production by lipase-catalysis offers some benefits, such as ease of product separation (and consequently, ease of glycerol separation), wastewater treatment requirement is reduced to a minimum and the complete lack of side reactions
[26][27][28].
Another major benefit lays in the possibility to process raw materials such as waste cooking oils (WCO), thus having high free fatty acid (FFA) content. As conventional homogenous alkaline catalyzed transesterification cannot achieve acceptable yields of FAAE due to the saponification reaction, the use of lipases to transform WCO into biodiesel may be a viable alternative by being able to process FFA and FAAE, thus offering a green route for biodiesel production at mild reaction conditions
[25][28][29].
Still, there are some technical drawbacks with using biocatalysts in biodiesel production, such as slower reaction rate than the alkaline catalyst (resulting in lower process productivity) and the risk of enzyme inactivation due to its sensitivity to methanol concentration
[30].
Nevertheless, lipases used in biodiesel production have to be non-stereospecific, so that all compounds of different molecular lengths, such as tri-, di-, monoglycerides, and FFA of different lengths, can be efficiently converted into FAAE
[28][31][32][33].
2. Immobilization of Lipases
Nevertheless, these biocatalysts present some disadvantages. The use of free enzymes as catalysts is not economically viable as these substances are not always as stable as required under operational conditions and restricted to one-time usage. Moreover, enzyme separation from the reaction mixture is very complicated, making catalyst recycling very much impossible
[4][34][35]. This is the main limitation of the large-scale application of enzymatic methods
[27].
One way to overcome these drawbacks consists in the enzyme immobilization onto supports. The immobilization of enzymes in solid carriers results in increasing chemical and thermal chemical stability, as well, and, also, contributes to protect the enzyme molecules from denaturation
[32]. Furthermore, immobilized enzymes can be described as being enclosed physically or located in a specific space while retaining its catalytic properties, and with the advantage of possibly being used repeatedly and continuously
[36][37]. However, there are some concerns with lipase immobilization. One is the possibility of enzyme shape change within the support matrix or getting detached, resulting in a drop in its catalytic activity. Another concern is the support materials being limited by their cost
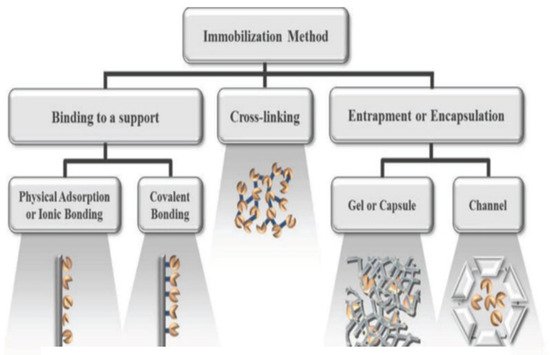
[38]. Thus far, some immobilization techniques were already developed, such as physical adsorption, entrapment, covalent attachment, and cross-linking (
Figure 1)
[1][39][40], which are the most used currently.
Figure 1. Overview of the different enzyme immobilization strategies
[41].
3. Physical Adsorption
The physical adsorption process of a protein onto a supporting material is regarded as very simple and one the most used method. In adsorption, the enzyme is immobilized through the existence of low energy bonds, e.g., van der Waals or hydrophobic interactions, hydrogen bonds, or ionic bonds within the exterior surface of the supporting material
[42][43].
De Paola et al.
[44] performed factor analysis concerning the enzymatic transesterification of waste oil aiming at biodiesel production. The chosen biocatalyst was a lipase extracted from
Rhizomocur miehei immobilized on a macroporous ion-exchange hydrophilic resin having an irregular shape, dimensions from 0.02 and 0.06 cm, 0.4 g/cm
3 in terms of bulk density of, and a porosity of 0.53 ± 0.02. In this case, the developed model predictions were in good agreement with experimental data, with yields of 90% for 8 h of reaction
[44].
Yagiz et al.
[45] studied hydrotalcite and four other types of zeolites (13-X, 5A, FM-8, and AW-300) as immobilization material for lipases by physical adsorption. The absorbed amount of lipase on hydrotalcite reached its highest at pH 8.5 and 4 °C. The obtained immobilized lipase was found to retain, after the seventh reuse cycle, 36% of its initial activity at 45 °C and 14% at 55 °C for the transesterification of WCO. Comparing the obtained results, for a reaction time of 105 h, the free lipase achieved a yield of 95%, and the immobilized lipase in hydrotalcite, a yield of 92.8%. However, considering the amount of lipase in the reaction, the immobilized lipase exhibited higher activity. The main reason for this could be that hydrotalcite; itself, is already an active site for transesterification reaction, and, thus, it has been used as a solid base heterogeneous catalyst in the transesterification reaction of edible oils. For the other types of support, the transesterification reaction showed no conversion of triglycerides to FAAE
[45].
Shah et al.
[46] produced biodiesel performing the alcoholysis of
Jatropha curcas (non-edible) oil using biocatalysts. The best yield achieved was 98% (
w/
w) for
Pseudomonas cepacia immobilized by adsorption on celite, at 50 °C with the presence of 4–5% (
w/
w) water in 8h of reaction. They also assessed that this catalyst could be recycled, at least, four times without any significant loss of activity
[46].
Katiyar and Ali
[47] studied the preparation of molecular sieve MCM-41 as effective support concerning the immobilization of
Candida rugosa lipase using the physical adsorption technique. They observed a maximum point for lipase immobilization (250 mg/g) on this support at pH 6 and the resulting immobilized lipase was used as biocatalysts for the transesterification of the cottonseed oil using methanol. Further optimization of all the reaction conditions (pH, temperature, and methanol/oil molar ratio), resulted in a maximum yield of FAME (98.3%)
[47].
Nevertheless, enzyme leaching is a real problem, in what concerns physical adsorption, as this greatly limits the use of the immobilized enzyme for different reaction conditions
[48][49]. One solution for this problem might be employing entrapment to restrict the enzyme inside the support framework
[50][51].
4. Entrapment
Entrapment is another physical immobilization method that refers to the capture of a lipase typically in a polymeric network such as an organic polymer or microcapsules of polymers allowing that the substrate and other products could pass through but still retaining the lipase. Usually, lipase entrapment is performed in situ, e.g., the lipase is placed into the monomeric phase solution, and as polymerization reaction develops, results in its entrapment
[18][42][43]. Compared with physical adsorption, lipase immobilization by entrapment is much more stable and protects the enzyme from the surrounding environment (hydrophobic solvents, mechanical sheer, and gas bubbles)
[52].
Moreno-Pirajàn and Giraldo
[53] studied the enzymatic transesterification of palm oil both with methanol and ethanol. The lipase
Candida rugosa was investigated in immobilized form entrapped within the support of activated carbon. This lipase, of the four that were tested, resulted in the highest yield of FAAE. Under optimal reaction conditions, FAME, and FAEE formation, after 1 h of reaction, was of 70 and 85% mol, respectively
[53].
Noureddini et al.
[54] studied the transesterification of soybean oil with methanol and ethanol using enzymes. They tested nine lipases and found that a lipase from
Pseudomonas cepacian resulted in the highest yield of FAAE. This lipase was thus investigated in terms of its immobilization within a chemically inert and hydrophobic sol-gel support. Under optimal reaction conditions, FAME and FAEE yield, after 1 h of reaction, were 67 and 65% mol, respectively
[54].
Kuan et al.
[55] entrapped lipase from
Pseudomonas cepacian in polyallylamine-mediated biometric silica. This biocatalyst was applied to the obtention of biodiesel with soybean oil and WCO as feedstock. For WCO transesterification, the optimal conditions were found to be 43.3 °C, substrate molar ratio of 5:1 and 38% (
w/
w)
n-hexane. The experimentally obtained conversion was 68%, while the predicted conversion was 67%
[55].
Hsu et al.
[56] investigated the lipase-catalyzed synthesis of alkyl esterified from tallow and grease using
Pseudomonas cepacian lipase immobilized within a phyllosilicate sol-gel matrix. This lipase effectively converted the raw materials into ethyl esters by more than 95% when ethanol is used. The matrix-immobilized lipase was also recovered easily and could be reused for, at least, five times without losing any catalytic activity
[56].
Unlike covalent bonding, a relatively simple procedure is followed, while, at the same time, the immobilized lipase can maintain its catalytic activity and stability also
[57].
5. Covalent Bonding
Lipase immobilization obtained by covalent bonding to solid support consists of an irreversible bonding of the lipase to the support matrix
[27]. This bond consists of a chemical reaction between the residue of active amino acid in the exterior of the active catalytic site of the lipase (usually thiol and amine groups) towards the active groups of the support matrix
[58][59]. This method results in strong interactions between the lipase and the support, making enzyme leaching to the reaction moiety during the catalytic process very rare, as the activity and stability of the immobilized enzyme are directly related to the main characteristics of the support itself (pore size, chemical stability, and binding affinity to the enzyme)
[27][39][43]. Multipoint covalent bonding seems to be the most efficient immobilization technique when compared to other methods in terms of thermal and operational lipase stability
[60]. Tang et al.
[61] assessed the immobilization of lipase from
Candida cylindracea performed by covalent bonding with alkaline Ca-bentonite, modified with glutamic acid. The immobilized lipase exhibited a considerably higher catalytic activity. At 50 °C, free lipase only retained 6% of its initial activity after 6 h of reaction. The Glu–Ca–Bent-lipase manages to retain 50% of its activity after 8 h. The prepared biocatalyst for biodiesel production showed an improvement in achieving a transesterification reaction conversion of 99% when free lipase only achieved 52.8%. Reusability wise, the said biocatalyst kept the reaction conversion at 56.2% after being reused five times, while the free lipase showed inactivity upon 2 uses
[61].
Rodrigues et al.
[62] evaluated the feasibility of biodiesel production by transesterification of
Jatropha oil using methanol, catalyzed by non-commercial
sn-1,3-regioselective lipases. Heterologous
Rhizopus oryzae lipase (rROL) was immobilized by covalent bonding on different supports and was, then, tested. Transesterification reaction was tested at 30 °C, after seven stepwise methanol additions. All tested biocatalysts exhibited an average FAME conversion of 51–65% after 4 h of reaction
[62].
Yücel
[63] immobilized by covalent bonding microbial lipase form
Thermomynces lanuginosus onto olive pomace. This support was then used to obtain biodiesel from pomace oil using methanol. Considering the optimized conditions, the maximum biodiesel yield was found to be 93% at 25 °C for a 24 h reaction. The immobilized enzyme maintained its activity during 10 repeated batch reactions
[63].
Mendes et al.
[60] immobilized microbial lipases from
Thermomynces lanuginosus and
Pseudomonas fluorescens by multipoint covalent bonding on Toyopearl AF-amino-650M resin and the obtained most active and thermal stable biocatalyst was used to catalyze the transesterification reaction of babassu and palm oils using ethanol. Comparing to the free lipase,
Thermomynces lanuginosus immobilized biocatalyst presented the highest hydrolytic activity and thermal stability as well, being 27 to 31 times more stable. The highest conversion to biodiesel was found in the transesterification of palm oil catalyzed by both
Thermomynces lanuginosus and
Pseudomonas fluorescens biocatalysts, with transesterification yield exceeding 93.5%
[60].
6. Cross-Linking
The cross-linking technique is the basis to obtain the immobilization of lipase, which comprises the process to immobilize the enzyme through the formation of intermolecular cross-linkages using a cross-linker. Usually, cross-linkers are bi- or multi-functional reagents such as glutaraldehyde, bisdiazobenzidine, etc. Generally, this immobilization technique is support free and involves the aggregation of enzymes between themselves to obtain a 3-dimensional structure. This process starts with the precipitation of the enzyme aggregate using acetone. Then, the obtained aggregates are cross-linked by using a cross-linker so that to obtain a more robust structure
[35][64][65]. Cross-linking can also be regarded as an improvement to covalent bonding, as the enzyme can be cross-linked to the support through the cross-linker. The procedure usually includes a previous step involving the immobilization on an ion exchange resin, followed by treatment with a buffered solution of cross-linker to form a Schiff base
[43][44][45][46][47][48][49].
Abdulla and Ravindra
[66] studied the immobilization of lipase from
Burkholderia cepacian. In that study, the lipase was cross-linked with glutaraldehyde followed by entrapment into a hybrid matrix consisting of equal proportions of alginate and
κ-carrageenan. Then, this biocatalyst was applied to the transesterification reaction of
Jatropha curcas L. oil. For optimal conditions, a total conversion of fatty acid ethyl esters could be achieved. The immobilized lipase was found to be stable and able to retain 73% of the relative transesterification activity after 6 reuse cycles
[66].
Dizge and Keskinler
[67] developed a novel immobilization method of lipase from
Termomynces lanuginosus within hydrophilic polyurethane foams, using polygluturaldehyde as a cross-linking agent. The immobilized lipase was used as a biocatalyst to produce biodiesel from canola oil and methanol and the effects of enzyme loading, alcohol/oil molar ratio, water concentration, and temperature in the transesterification reaction were studied. The maximum FAME yield achieved was 90%, and it was found that enzymatic activity remained after 10 batches. The immobilized lipase exhibited stability and only lost little activity when subjected to repeated uses
[67].
Kumari et al.
[68] investigated the use of immobilizing lipase from
Pseudomonas cepacian as a catalyst in the transesterification reaction of
Madhuca indica oil. The best results were obtained when the cross-linked enzyme aggregates (CLEA) of the studied lipase are used. While free lipase powder could achieve a conversion yield of 98% in 6 h of reaction, after process optimization, CLEA of
Pseudomonas cepacian gave a conversion of 92% in 2.5 h of reaction. Both methods used around the same amount of lipase
[68].
Han and Kim
[69] explored the methanol tolerant lipase M37 from
Photobacterium lipolyticum and its immobilization by the cross-linked enzyme aggregate (CLEA) method. The cross-linked lipase can produce biodiesel from olive oil and alcohols such as methanol and ethanol. Regarding its physical stability and reusability, the cross-linked lipase can eventually be used as a catalyst for organic synthesis, including the biodiesel production reaction
[69].
A summary of examples for lipase immobilization techniques is presented below in the form of a table (Table 1). For every example of biodiesel production using an immobilized lipase as biocatalyst, the respective name, technique for immobilization, lipidic feedstock, reaction parameters, and FAAE yields are displayed.
Table 1. Summary of examples of lipase immobilization techniques used in biodiesel production.
| Lipase |
Immobilization Technique |
Oil/Fat |
Alcohol |
Yield (%) |
Reaction Conditions |
Refs |
| Rhizomocur miehei |
Physical Adsorption |
Olive husk oil |
Ethanol |
90.0 |
8 h; EtOH/Oil molar ratio of 2:1; 37 °C |
[44] |
| Lipozyme-TL IM |
Physical Adsorption |
Waste cooking oil |
Methanol |
95.0 |
4% immob. lipase (wt%); 24 °C; MeOH/Oil molar ratio 4:1; 105 h |
[45] |
| Pseudomonas cepacian |
Physical Adsorption |
Jatropha curcas oil |
Ethanol |
98.0 |
10% immob. lipase; 40 °C; EtOH/Oil 4:1; 8 h |
[46] |
| Candida rugosa |
Physical Adsorption |
Cotton seed oil |
Methanol |
98.3 |
5% immob. lipase; 40 °C; MeOH/Oil 12:1; 48 h |
[47] |
| Candida rugosa |
Entrapment |
Palm oil |
Methanol/Ethanol |
70/85 |
1% immob. lipase; 37 °C; MeOH/Oil molar ratio 14:1; 1 h/1% immob. lipase; 35 °C; EtOH/Oil molar ratio 15:1; 1 h |
[53] |
| Pseudomonas cepacian |
Entrapment |
Soybean oil |
Methanol/Ethanol |
67/65 |
4.75% immob. lipase; 35 °C; MeOH/Oil molar ratio 7.5:1, 1 h/4.75% immob. lipase; 35 °C; EtOH/Oil molar ratio 15.2:1; 1 h |
[54] |
| Pseudomonas cepacian |
Entrapment |
Soybean oil/Waste cooking oil |
Methanol |
68 a/67 b |
1.0% immob. lipase; 43.3 °C; MeOH/Oil molar ratio 5:1; 36 h |
[55] |
| Pseudomonas cepacian |
Entrapment |
Tallow and grease |
Ethanol |
95 |
50 °C; EtOH/Oil molar ratio 4:1; 24 h |
[56] |
| Candida cylindracea |
Covalent Bonding |
Camellia oil |
Methanol |
99 |
40% immob. lipase; 40 °C; 48 h |
[61] |
| Rhizopus oryzae |
Covalent Bonding |
Jatropha curcas oil |
Methanol |
51–65 |
30 °C; 4 h |
[62] |
| Thermomynces lanuginosus |
Covalent Bonding |
Olive pomace oil |
Methanol |
93 |
MeOH/Oil molar ratio 6:1;25 °C; 24 h |
[63] |
| Thermomynces lanuginosus/Pseudomonas flourescens |
Covalent Bonding |
Babassu/Palm oils |
Ethanol |
>93 |
45 °C; EtOH/Oil molar ratio 9:1; 48 h/45 °C; EtOH/Oil molar ratio 18:1; 48 h |
[60] |
| Burkholderia cepacian |
Cross-linking |
Jatropha curcas oil |
Ethanol |
100 |
52.5% immob. Lipase; 35 °C; EtOH/Oil molar ratio 10:1; 24 h |
[66] |
| Thermomynces lanuginosus |
Cross-linking |
Canola oil |
Methanol |
90 |
40 °C; MeOH/Oil molar ratio 6:1; 24 h |
[67] |
| Pseudomonas cepacian |
Cross-linking |
Madhuca indica oil |
Ethanol |
92 |
10% immob. lipase; 40 °C; EtOH/Oil molar ratio 4:1; 2.5 h |
[68] |
| Photobacterium lipolyticum |
Cross-linking |
Olive oil |
Methanol |
64 |
40 °C; MeOH/Oil molar ratio 4:1; 12 h |
[69] |