Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nilakshi Barua | + 2127 word(s) | 2127 | 2021-08-31 10:13:11 | | | |
| 2 | Vivi Li | Meta information modification | 2127 | 2021-09-10 09:33:07 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Barua, N. Curcumin as an Antimycobacterial Agent. Encyclopedia. Available online: https://encyclopedia.pub/entry/14051 (accessed on 08 February 2026).
Barua N. Curcumin as an Antimycobacterial Agent. Encyclopedia. Available at: https://encyclopedia.pub/entry/14051. Accessed February 08, 2026.
Barua, Nilakshi. "Curcumin as an Antimycobacterial Agent" Encyclopedia, https://encyclopedia.pub/entry/14051 (accessed February 08, 2026).
Barua, N. (2021, September 10). Curcumin as an Antimycobacterial Agent. In Encyclopedia. https://encyclopedia.pub/entry/14051
Barua, Nilakshi. "Curcumin as an Antimycobacterial Agent." Encyclopedia. Web. 10 September, 2021.
Copy Citation
Curcumin is the principal curcuminoid obtained from the plant Curcuma longa and has been extensively studied for its biological and chemical properties. Curcumin displays a vast range of pharmacological properties, including antimicrobial, anti-inflammatory, antioxidant, and antitumor activity. Specifically, curcumin has been linked to the improvement of the outcome of tuberculosis. There are many reviews on the pharmacological effects of curcumin; however, reviews of the antitubercular activity are comparatively scarcer.
tuberculosis
curcumin
Mycobacterium tuberculosis
antimycobacterial activity
1. Introduction
Tuberculosis (TB), a communicable disease caused by the bacillus Mycobacterium tuberculosis (MTB), is one of the top ten causes of death worldwide. Currently, about a quarter of the world’s population is infected with MTB [1]. TB is a major international health problem and is a leading cause of death from a single infectious agent (ranking above HIV/AIDS).
The emergence of multidrug-resistant strains of Mycobacterium tuberculosis (MTB) and the adverse effects of antituberculosis drugs have renewed the interest in natural products for the discovery of new antitubercular leads [2]. The use of new techniques for evaluation of the antimycobacterial activity has led to the identification of many natural products that demonstrate potent inhibition of MTB and, in some cases, the mechanism of action has also been determined [3][4]. Natural products that have demonstrated inhibitory effects on the growth of MTB include secondary metabolites derived from plants, marine organisms, algae bacteria, and fungi and are categorized as terpenes, steroids, alkaloids, aromatics, polyketides, peptides, [5] and their synthetic derivatives [6]. One such natural product is curcumin, a plant-derived lipophilic polyphenol that has been demonstrated to possess therapeutic benefits in multiple diseases, including arthritis [7], cancers [8], inflammation [9], liver disease [10], metabolic syndrome [11], neurodegenerative diseases [12], and obesity [13]. Recent studies present curcumin and its derivatives as promising antitubercular drugs that could be used alone or in combination with other drugs. In this review, we discuss how curcumin and its derivatives affect TB.
Curcumin is also known as turmeric yellow or diferuloylmethane, a beta-diketone [14] that constitutes 2–5% of turmeric powder [15]. Turmeric is acquired from the rhizome of a tuberous herbaceous perennial plant, Curcuma longa L., a member of the Zingiberaceae family. The turmeric plant is native to the Indian subcontinent, and its use can be found abundantly in the traditional medical literature of India, Pakistan, Bangladesh, Bhutan, and China [16][17].
Pharmacological studies of curcumin suggest that it has a potent protective capacity against TB [15]. Therefore, the explanation of the hypothesis that curcumin and its derivatives have an antitubercular role is no longer in doubt. In this manuscript, we attempt to update the knowledge and review a short historical overview of the past eight year’s findings on the antitubercular activity of curcumin and its derivatives. The review summarizes and discusses the studies that provide evidence of the potential development of curcumin-based antitubercular drugs. Figure 1 depicts an overview of our review.
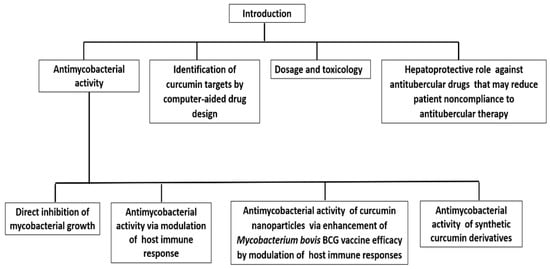
Figure 1. Scheme describing the overview of the review.
2. Antimycobacterial Activity of Curcumin Synthetic Derivatives
Although curcumin is a promising antimycobacterial agent, its use in clinical and pharmaceutical studies has been decelerated by its poor chemical stability [18][19]. In addition, curcumin has low oral bioavailability and is rapidly excreted due to its poor absorption and extensive intestinal and first-pass metabolism [20]. Therefore, efforts have been made to synthesize [21] and identify more stable analogues and potent antimycobacterial analogues. as listed in Table 1.
Table 1. Minimum inhibitory concentration of curcumin, demethoxycurcumin, and their respective synthetic analogues against various mycobacterial strains (μg/mL).
| Compound | Mycobacterial Strains | MIC (μg/mL) | Reference |
|---|---|---|---|
| Curcumin | MTB H37Rv | 16 | [22] |
| Demethoxycurcumin (DM) | MTB H37Rv | 200 | [23] |
| DM6 | MTB H37Rv | 7.8 | |
| DM7 | MTB H37Rv | 125 | |
| Mono-O-methylcurcumin- isoxazole | MTB H37Ra | 0.09 | [24] |
| a INH, RIF, STM-resistant MTB (clinical isolate M3) | 0.195 | ||
| b INH, RIF, EMB, STM-resistant MTB (clinical isolate M4) | 1.56 | ||
| INH, RIF, STM-resistant MTB (clinical isolate M5) | 3.125 | ||
| INH, RIF-resistant MTB (clinical isolate M6) | 0.39 | ||
| INH, RIF, EMB, STM-resistant MTB (clinical isolate M8) | 3.125 | ||
| INH, RIF-resistant MTB (clinical isolate M11) | 3.125 | ||
| INH, RIF, EMB-resistant MTB (clinical isolate M16) | 1.56 | ||
| INH, RIF, EMB, STM-resistant MTB (clinical isolate M21) | 3.125 | ||
| INH, RIF-resistant MTB (clinical isolate M22) | 0.39 | ||
| INH, RIF-resistant MTB (clinical isolate M27) | 0.195 | ||
| c INH, RIF, STM, OFX, CIP-resistant MTB (clinical isolate M46) | 1.56 | ||
| INH, RIF, STM, OFX, CIP-resistant MTB (clinical isolate M48) | 1.56 | ||
| INH, RIF, STM, OFX, CIP-resistant MTB (clinical isolate M53) | 3.125 | ||
| UBS-109 | M. marinum | 10 mM * | [25] |
| MTB H37Rv | ~10 μM * | ||
| EF-24 | MTB Beijing F2 | 20 μM * | |
| M. marinum | 25 mM * | ||
| CPMD-6- dihydrochloride | MTB H37R | 2 | [22] |
| INH-resistant MTB ATCC 35822 | 2 | ||
| RIF-resistant MTB ATCC 35838 | 2 | ||
| STM-resistant MTB ATCC 35820 | 2 | ||
| ETB-resistant MTB ATCC 35837 | 2 | ||
| M. fortuitum ATCC 6841 | 16 | ||
| M.abscessus ATCC 19977 | 16 | ||
| 3,3′-Dihydroxycurcumin | MTB | 156 | [26] |
| Quinolidene based monocarbonyl curcumin analogue 3e | MTB | >30 # | [27] |
| M. bovis BCG | 2.7 # | ||
| Quinolidene based monocarbonyl curcumin analogue 3h | MTB | >30 # | |
| M. bovis BCG | 9.2 # | ||
| Quinolidene based monocarbonyl curcumin analogue 4a | MTB | 26.5 # | |
| M. bovis BCG | 7.3 # | ||
| Quinolidene based monocarbonyl curcumin analogue 4e | MTB | 7.8 # | |
| M. bovis BCG | 9.4 # |
* IC50 of UBS-109 EF-24 against the mycobacterial strains; a INH, isoniazid; RIF, rifampicinn; STM, streptomycin; b EMB, ethambutol; c OFX, ofloxacin; CIP, ciprofloxacin; # MIC90.
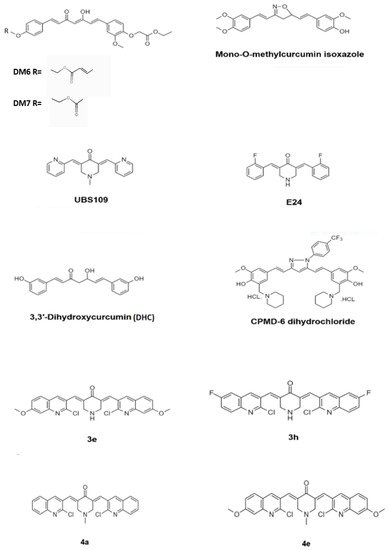
Demethoxycurcumin, one of the curcuminoids, exhibited potent antimycobacterial activity against MTB H37Rv strain at 200 μg/mL. Synthetic derivatives yielded potent antimycobacterial agents, which exhibited a considerable activity with the MIC value of 7.8 μg/mL. Four derivates of demethoxycurcumin, DM4–DM7, were synthesized by chemical modification at its phenolic hydroxy positions. The derivative DM6 exhibited potent antitubercular with a MIC of 7.812 μg/mL. Derivative DM7 showed moderate activity with a MIC of 125 μg/mL, whereas the derivatives DM1, DM3, DM4, and DM5 were inactive against MTB even at the concentration of 250 μg/mL. The derivatives DM6 and DM7 possessed increased lipophilicity due to the presence of fatty acid ester chains at the phenolic hydroxy groups. The increased lipophilicity may have endowed the two derivatives DM6 and DM7 with better antitubercular activity due to the increased interaction with the lipophilic MTB cell wall causing impairment of transport of polar compounds through the outer lipid layer of mycobacteria. The lead optimization of these two derivatives may lead to the identification of antitubercular drug candidates in the future [23]. Figure 4 depicts the chemical structure of the derivatives of curcumin.

Figure 4. Chemical structure of the curcumin derivatives that have exhibited potent antimycobacterial activity.
Out of 55 isoxazole synthetic analogues of the curcuminoids, mono-O-methylcurcumin isoxazole exhibited the potent antimycobacterial activity with the MIC 0.09 μg/mL, which is 1131-fold more active than the parent compound curcumin and approximately 18 and 2-fold more active than the antimycobacterial drugs kanamycin and isoniazid, respectively, against MTB H37Ra and the clinical isolates of MDR-TB obtained from Ramathibodi Hospital, Mahidol University, Bangkok, Thailand. Mono-O-methylcurcumin isoxazole also exhibited high activity against the multidrug-resistant MTB clinical isolates, with MICs of 0.195–3.125 μg/mL. The isoxazole ring and two unsaturated bonds on the heptyl chain of the curcuminoid analogue are responsible for the antimycobacterial activity. The biological activity was enhanced by the para-alkoxyl group on the aromatic ring, attached in close proximity to the nitrogen function of the isoxazole ring in addition to the free para-hydroxyl group on another aromatic ring [24].
A series of eight mono-carbonyl analogues of curcumin were synthesized to increase the bioavailability of curcumin and tested the antimycobacterial activity against MTB and M. marinum (MM). In the initial screening using the disk diffusion assay, out of the eight analogues, seven exhibited an antimycobacterial activity at a concentration of 100 mM. The analogue UBS-109 exhibited the highest activity against MM, with an inhibition zone of 5.7 ± 0.3 mm. The analogue U2-260 exhibited the lowest activity with 1.4 ± 0 mm. The analogue ECMN-951 did not exhibit antimycobacterial activity. Using liquid culture, the IC50 was reported to be 10 mM for UBS-109 and 25 mM for the analogue E-24. The analogue UBS-109 exhibited potent antimycobacterial activity against MTB H37Rv and Beijing F2 with an IC50 of ~10 μM and 20 μM, respectively. However, the inhibitory effect of the E-24 was much lower in comparison to UBS-109. However, these curcumin analogues did not exhibit synergistic effects between the monocarbonyl analogues and RIF on inhibiting mycobacterial growth. The structure–activity analysis showed that the Michael acceptor properties of the analogues are critical for antimycobacterial activity [25].
A synthetic molecule CPMD-6-dihydrochloride, exhibiting potent antimycobacterial activity, was identified from a series of 21 curcumin–pyrazole–mannich derivatives. The bacteriostatic, bactericidal synergy with first-line antituberculosis drugs against MTB H37Rv, drug-resistant MTB strains, M. forutitum and M. abscessus was investigated. The in vivo efficacy of the derivatives was evaluated in a BALB/c mice model of MTB infection. The compound CPMD-6-dihydrochloride exhibited promising antimycobacterial activity with a MIC 2 μg/mL against MTB H37Rv, drug-sensitive as well as drug-resistant mycobacterial strains compared to curcumin which exhibits a MIC of 16 μg/mL against MTB H37Rv. While curcumin did not exhibit activity against M. forutitum and M. abscessus (MIC > 64 μg/mL), CPMD-6 dihydrochloride exhibited potent activity with a MIC of 16 μg/mL. Interestingly, CPMD-6 dihydrochloride antimicrobial activity was specific to Mycobacterium sp. and did not show any antimicrobial activity against the Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp. (ESKAPE) panel. CPMD-6-dihydrochloride also exhibited strong synergy with the current first-line antimycobacterial drugs with a fractional inhibitory concentration (FIC) index of 0.5 for rifampin (RIF), INH, and 0.37 for ethambutol (EMB), but did not exhibit any interaction with streptomycin (STR) with a FIC index of 0.75 for STR. Three-week treatment with 25 mg/kg of CPMD-6-dihydrochloride showed a significant reduction in the bacterial load in the lung of infected six-week-old BALB/c mice by 1.06 log10 in comparison to 100 mg/kg of EMB treated group. CPMD-6-dihydrochloride also reduced bacterial counts by 0.63 log10 while EMB reduced by 0.51 log10 in the spleen, demonstrating that CPMD-6 dihydrochloride has superior efficacy with respect to a reduction in mycobacterial CFU at one-fourth of the dosage in comparison to EMB in the murine MTB infection model [22].
In a series of twelve diphenylheptanoid-derived synthetic curcuminoid analogues, 3,3′-dihydroxycurcumin (DHC) exhibited promising antimycobacterial activity against MTB with a MIC of 156 μg/mL. DHC is more stable than curcumin in phosphate buffer (pH 7.4) and acetate buffer (pH 5.0) for 24 h at 37 °C. The cell division protein FtsZ may be the target for DHC due to the fact that curcumin exhibits this mode of antibacterial action. DHC exhibited moderate toxicity in human cells from the liver (tumorigenic HepG2 cell line) and lung (tumorigenic A549 cell) and normal MCR-5 cell lines with the IC50 values ranging from 9.6 to 10.6 μg/mL, respectively, which is slightly more toxic then curcumin. Curcumin exhibited IC50 values ranging from 15.5 μg/mL to 32.3 μg/mL. No significant difference in IC50 in the lungs and liver cell lines indicated that the biotransformation capacity of hepatocytes did not affect the cytotoxicity of DHC. DHC toxicity in normal human fibroblasts (MCR-5) and adenocarcinoma cells (A549) showed no significant difference, indicating that tumorigenic genes and proteins did not affect its toxicity and alkaline comet assay revealed that DHC could not induce DNA damage in the A549 cell line [26].
3. Identification of Curcumin Targets in Mycobacterium by Computer-Aided Drug Design
The systematic study of the targets and mechanisms of natural products through traditional assay-based methods is a time consuming and costly process because of the difficulty of extraction and activity testing. Virtual in silico screening is anticipated to be an alternative approach for low-cost and rapid analysis of natural products and efficient identification for their targets. Molecular docking is a potent virtual screening tool in rational drug design that could be utilized to investigate and identify ligands and the potential targets that could be extended to analyze the structural and molecular mechanics of the binding between the ligand and protein.
The structure-based drug design (SBDD) approach, a category of computer-aided drug design, has contributed to the introduction of lead compounds into clinical trials and to numerous drug approvals [28][29]. Molecular docking studies also revealed universal stress protein (USP), a novel therapeutic target of MTB, to be the target of curcumin. The docking of curcumin to the USP protein was done using AutoDock 4.2. Curcumin was shown to form a hydrogen bond ARG 136 (1.8 Å) and two ionic bonds with a carboxyl group of curcumin with LEU 130 (3.3 Å) and ASN 144 (3.4 Å) indicating possible new curcumin analogues for future therapy to downregulate USP [30]. The bottom-up systems biology approach revealed that aspartate-β-semialdehyde dehydrogenase (ASD), dihydrodipicolinate reductase and diaminopimelate decarboxylase are potential therapeutic targets of MTB infections. In silico molecular docking study using AutoDock 4.2.6 of these targets, which was prioritized based on flux and elementary mode analysis using direct mathematical modeling of the relevant metabolic pathways, identified curcumin as ASD inhibitors [31]. Computational models revealed that the synthetic derivative of curcumin, monoacetylcurcumin, binds to the specific BRCT domain of the essential enzyme MtuLigA for MTB. MtuLigA is unique to MTB, thus making it a promising drug target [32]. In vitro experiments to investigate the inhibitory activity of curcumin monoacetyl derivative against BRCT domain-containing DNA polymerase λ [33] proved to be nearly twice as effective (IC50 3.9) as curcumin (IC50 7.0 μM). These predicted targets are summarized in Table 2.
Table 2. Predicted targets of Curcumin and its analogues by computer-aided drug design.
| Compound | Predicted Mycobacterial Target | Reference |
|---|---|---|
| Curcumin | Universal stress protein (USP) | [30] |
| Aspartate-β-semialdehyde dehydrogenase (ASD) | [31] | |
| Dihydrodipicolinate reductase | ||
| Monoacetylcurcumin | M. tuberculosis NAD+-dependent DNA ligases (MtuLigA) | [32] |
| BRCT domain-containing DNA polymerase λ | [33] | |
| Quinolidene based monocarbonyl curcumin analogues 3e, 3h, 4a and 4e | Pantothenate synthetase (MTB PS) | [27] |
References
- Alipoor, S.D.; Adcock, I.M.; Tabarsi, P.; Folkerts, G.; Mortaz, E. MiRNAs in tuberculosis: Their decisive role in the fate of TB. Eur. J. Pharmacol. 2020, 886, 173529.
- Adnan, M.; Ali, S.; Sheikh, K.; Amber, R. Review on antibacterial activity of Himalayan medicinal plants traditionally used to treat pneumonia and tuberculosis. J. Pharm. Pharmacol. 2019, 71, 1599–1625.
- Singh, S.B.; Odingo, J.; Bailey, M.A.; Sunde, B.; Korkegian, A.; O’Malley, T.; Ovechkina, Y.; Ioerger, T.R.; Sacchettini, J.C.; Young, K.; et al. Identification of cyclic hexapeptides natural products with inhibitory potency against Mycobacterium tuberculosis. BMC Res. Notes 2018, 11, 416.
- Pires, C.T.; Scodro, R.B.; Cortez, D.A.; Brenzan, M.A.; Siqueira, V.L.; Caleffi-Ferracioli, K.R.; Vieira, L.C.; Monteiro, J.L.; Corrêa, A.G.; Cardoso, R.F. Structure–activity relationship of natural and synthetic coumarin derivatives against Mycobacterium tuberculosis. Future Med. Chem. 2020, 12, 1533–1546.
- Nguta, J.M.; Appiah-Opong, R.; Nyarko, A.K.; Yeboah-Manu, D.; Addo, P.G. Current perspectives in drug discovery against tuberculosis from natural products. Int. J. Mycobacteriol. 2015, 4, 165–183.
- Tizabi, Y.; Hurley, L.L.; Qualls, Z.; Akinfiresoye, L. Relevance of the anti-inflammatory properties of curcumin in neurodegenerative diseases and depression. Molecules 2014, 19, 20864–20879.
- Dragos, D.; Gilca, M.; Gaman, L.; Vlad, A.; Iosif, L.; Stoian, I.; Lupescu, O. Phytomedicine in joint disorders. Nutrients 2017, 9, 70.
- Ashrafizadeh, M.; Zarrabi, A.; Hashemi, F.; Moghadam, E.R.; Hashemi, F.; Entezari, M.; Hushmandi, K.; Mohammadinejad, R.; Najafi, M. Curcumin in cancer therapy: A novel adjunct for combination chemotherapy with paclitaxel and alleviation of its adverse effects. Life Sci. 2020, 256, 117984.
- He, Y.; Yue, Y.; Zheng, X.; Zhang, K.; Chen, S.; Du, Z. Curcumin, inflammation, and chronic diseases: How are they linked? Molecules 2015, 20, 9183–9213.
- Vera-Ramirez, L.; Pérez-Lopez, P.; Varela-Lopez, A.; Ramirez-Tortosa, M.; Battino, M.; Quiles, J.L. Curcumin and liver disease. Biofactors 2013, 39, 88–100.
- Panahi, Y.; Hosseini, M.S.; Khalili, N.; Naimi, E.; Simental-Mendía, L.E.; Majeed, M.; Sahebkar, A. Effects of curcumin on serum cytokine concentrations in subjects with metabolic syndrome: A post-hoc analysis of a randomized controlled trial. Biomed. Pharmacother. 2016, 82, 578–582.
- Wang, Q.; Ye, C.; Sun, S.; Li, R.; Shi, X.; Wang, S.; Zeng, X.; Kuang, N.; Liu, Y.; Shi, Q. Curcumin attenuates collagen-induced rat arthritis via anti-inflammatory and apoptotic effects. Int. Immunopharmacol. 2019, 72, 292–300.
- Neyrinck, A.M.; Sánchez, C.R.; Rodriguez, J.; Cani, P.D.; Bindels, L.B.; Delzenne, N.M. Prebiotic Effect of Berberine and Curcumin Is Associated with the Improvement of Obesity in Mice. Nutrients 2021, 13, 1436.
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, pharmaceutical, nutraceutical, and analytical aspects. Molecules 2019, 24, 2930.
- Mangwani, N.; Singh, P.K.; Kumar, V. Medicinal plants: Adjunct treatment to tuberculosis chemotherapy to prevent hepatic damage. J. Ayurveda Integr. Med. 2020, 11, 522–528.
- Alsarhan, A.; Sultana, N.; Al-Khatib, A.; Kadir, M.R.A. Review on some Malaysian traditional medicinal plants with therapeutic properties. J. Basic Appl. 2014, 10, 149–159.
- Deb, B.C.; Chakraborty, S. Evaluation of genetic variability and characterization of some elite turmeric genotypes in Terai Region in India. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 2357–2366.
- Heger, M.; van Golen, R.F.; Broekgaarden, M.; Michel, M.C. The Molecular Basis for the Pharmacokinetics and Pharmacodynamics of Curcumin and Its Metabolites in Relation to Cancer. Pharmacol. Rev. 2013, 66, 222–307.
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637.
- Sharma, R.A.; Gescher, A.J.; Steward, W.P. Curcumin: The story so far. Eur. J. Cancer 2005, 41, 1955–1968.
- Nasir Abbas Bukhari, S.; Franzblau, S.G.; Jantan, I.; Jasamai, M. Current prospects of synthetic curcumin anlogues and chalcone derivatives against Mycobacterium tuberculosis. Med. Chem. 2013, 9, 897–903.
- Singh, A.K.; Yadav, P.; Karaulia, P.; Singh, V.K.; Gupta, P.; Puttrevu, S.K.; Chauhan, S.; Bhatta, R.S.; Tadigoppula, N.; Gupta, U.D.; et al. Biological evaluation of novel curcumin-pyrazole-mannich derivative active against drug-resistant Mycobacterium tuberculosis. Future Microbiol. 2017, 12, 1349–1362.
- Agrawal, D.K.; Saikia, D.; Tiwari, R.; Ojha, S.; Shanker, K.; Kumar, J.K.; Gupta, A.K.; Tandon, S.; Negi, A.S.; Khanuja, S.P. Demethoxycurcumin and its Semisynthetic Analogues as Antitubercular Agents. Planta Med. 2008, 74, 1828–1831.
- Changtam, C.; Hongmanee, P.; Suksamrarn, A. Isoxazole anlogues of curcuminoids with highly potent multidrug-resistant antimycobacterial activity. Eur. J. Med. Chem. 2010, 45, 4446–4457.
- Baldwin, P.R.; Reeves, A.Z.; Powell, K.R.; Napier, R.J.; Swimm, A.I.; Sun, A.; Giesler, K.; Bommarius, B.; Shinnick, T.M.; Snyder, J.P.; et al. Monocarbonyl anlogues of curcumin inhibit growth of antibiotic sensitive and resistant strains of Mycobacterium tuberculosis. Eur. J. Med. Chem. 2015, 92, 693–699.
- Polaquini, C.R.; Morão, L.G.; Nazaré, A.C.; Torrezan, G.S.; Dilarri, G.; Cavalca, L.B.; Campos, D.L.; Silva, I.C.; Pereira, J.A.; Scheffers, D.; et al. Antibacterial activity of 3,3′-dihydroxycurcumin (DHC) is associated with membrane perturbation. Bioorg. Chem. 2019, 90, 103031.
- Subhedar, D.D.; Shaikh, M.H.; Nawale, L.; Sarkar, D.; Khedkar, V.M.; Shingate, B.B. Quinolidene based monocarbonyl curcumin analogues as promising antimycobacterial agents: Synthesis and molecular docking study. Bioorg. Med. Chem. Lett. 2017, 27, 922–928.
- Muegge, I.; Bergner, A.; Kriegl, J.M. Computer-aided drug design at Boehringer Ingelheim. J. Comput. Aided. 2017, 31, 275–285.
- Bruch, E.M.; Petrella, S.; Bellinzoni, M. Structure-Based Drug Design for Tuberculosis: Challenges Still Ahead. Appl. Sci. 2020, 10, 4248.
- Aanandhi, M.V.; Bhattacherjee, D.; George, P.S.G.; Ray, A. Natural polyphenols down-regulate universal stress protein in Mycobacterium tuberculosis: An in-silico approach. J. Adv. Pharm. Technol. Res. 2014, 5, 171–178.
- Khan, S.; Somvanshi, P.; Bhardwaj, T.; Mandal, R.K.; Dar, S.A.; Wahid, M.; Jawed, A.; Lohani, M.; Khan, M.; Areeshi, M.Y.; et al. Aspartate-β-semialdeyhyde dehydrogenase as a potential therapeutic target of Mycobacterium tuberculosis H37Rv: Evidence from in silico elementary mode analysis of biological network model. J. Cell. Biochem. 2018, 119, 2832–2842.
- Dube, D.; Kukshal, V.; Srivastava, S.K.; Tripathi, R.P.; Ramachandran, R. NAD+-dependent DNA ligase (Rv3014c) from M. tuberculosis: Strategies for inhibitor design. Med. Chem. Res. 2007, 17, 189–198.
- Takeuchi, T.; Ishidoh, T.; Iijima, H.; Kuriyama, I.; Shimazaki, N.; Koiwai, O.; Kuramochi, K.; Kobayashi, S.; Sugawara, F.; Sakaguchi, K.; et al. Structural relationship of curcumin derivatives binding to the BRCT domain of human DNA polymerase λ. Genes Cells 2006, 11, 223–235.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
10 Sep 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




