Wine fermentation is a specific and complex research subject and its control is essential to ensure full process completion while improving wine quality. It displays several specificities, in particular, (i) musts with a very high sugar content, low pH, and some limiting nutrients, as well as a great variability in must composition according to the year, grape variety, and so on; (ii) atypical fermentation conditions with non-isothermal temperature profiles, a quasi-anaerobiosis and legal constraints with a limited and predefined list of authorized operations. New challenges have emerged, related to the increasing diversity of commercially available yeast strains; the fluctuating composition of musts, particularly owing to climate change; and sustainability, which has become a key issue.
1. Introduction
Alcoholic fermentation is a key step in winemaking. During this process, hexoses and other grape must constituents are converted to ethanol, carbon dioxide, and many other secondary by-products that affect the sensorial properties of wine. Yeast strains have a major effect on the process, but must composition and the way the fermentation is controlled are also essential, so that the course of fermentation always depends on interactions between these three factors. This explains why alcoholic fermentation under oenological conditions is a specific and complex research subject.
The yeast strains used belong mainly to the S. cerevisiae species, but their number and diversity are constantly increasing, and increasingly more attention is being paid today to non-Saccharomyces species, mixed cultures, and natural microbial ecosystems.
Grape juice is characterized by a very high sugar content, a very low pH, a quasi-anaerobiosis, and low concentrations of certain nutrients, notably assimilable nitrogen. During fermentation, this leads to a succession of deficiencies and stresses that result, in particular, in (i) a long stationary phase during which a large part of the sugars is metabolized in the absence of yeast growth and (ii) significant risks of mortality by the end of fermentation.
Winemaking fermentation control is essential to ensure a successful completion of the process, but also to limit energy costs and to improve the wine organoleptic characteristics. Certain specificities are to be considered, in particular temperature profiles, which are often non-isothermal owing to the strong exothermic potential of fermentation, and oxygen additions, usually necessary for yeasts, but that must be very low (a few mg/L) to avoid detrimental effects on quality. Legal constraints must also be considered, as only a limited number of operations are authorized, mainly in terms of nutrient additions and temperature control.
Much progress has been made in the last few years, but, in parallel, new challenges have emerged. Indeed, available yeast strains are more and more numerous and diverse; the composition of musts has evolved considerably (in particular because of climate change); and winemaking sustainability has become an essential issue, with, for example, a reduction in input quantity and the prospect of vinifying new grape varieties, in particular those resistant to downy and powdery mildews.
2. Study of Metabolism
2.1. Online Fermentation Monitoring
2.1.1. Development of Online Fermentation Monitoring Devices
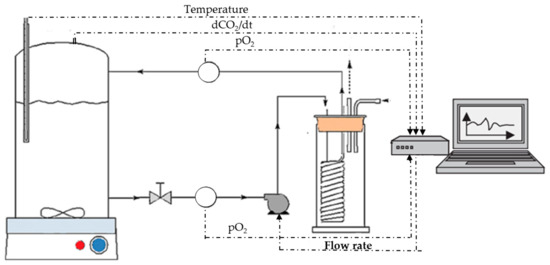
The study of yeast metabolism requires the monitoring of fermentation process dynamics. To improve this monitoring, we focused very early on the online monitoring of the main reaction, i.e., the production of ethanol and CO
2 [1]. The method we chose, also proposed in other laboratories
[2], was the measurement of CO
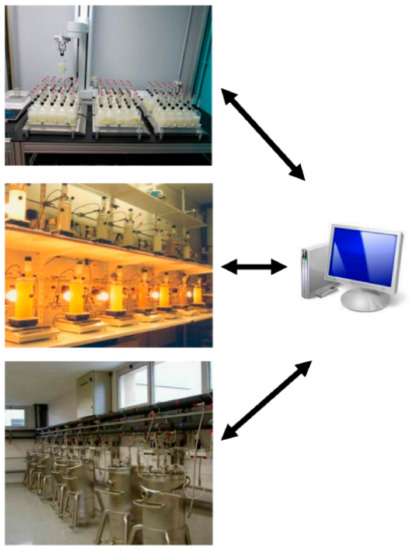
2 release, with adequate frequency and accuracy in order to calculate in real time the instantaneous rate of fermentation. This laboratory setup (36 × 1 L fermenters) was then extended to both pilot scale (16 × 100 L tanks) and small scale, with a fermentation robot (360 × 20 mL or 90 × 300 mL) (
Figure 1).
Figure 1. Online monitoring of CO2 production rate at laboratory and pilot scale. Fermentation robot (360 × 20 mL or 90 × 300 mL), laboratory setup (16 × 1 L fermenters), and pilot scale (16 × 100 L tanks).
The next step was to monitor online the synthesis of some molecules of interest, mainly the fermentative aromas (higher alcohols and esters) and main sulfur compounds
[3][4]. The chosen technique was the use of online GC, with automatic sampling in the gas phase (sampling frequency = 1 h). Today, two devices are operational: (i) at the 10 L and 100 L scales, a GC-MS-FPD to analyze several dozens of (mainly carbonaceous) compounds; and (ii) at laboratory scale (2 L), a compact GC-FID-PFPD particularly well adapted to the analysis of the main sulfur compounds. In additional studies on gas–liquid equilibrium, we modeled the partition coefficient (k
i) for the main compounds
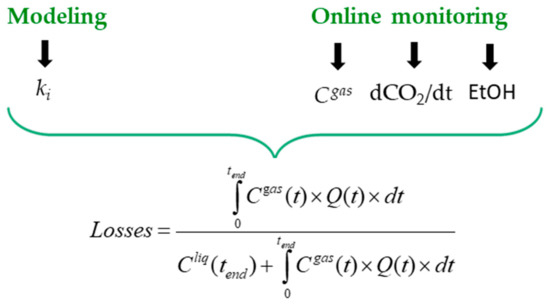
[5][6][7]. For each of these compounds, it then became possible to compute real-time balances between the quantity accumulated in the liquid and that lost in the gas (
Figure 2). The sum of both fractions corresponds to the actual quantity produced by yeast, which is the parameter with the highest metabolic interest.
Figure 2. Calculation of the overall losses of a volatile compound in the exhaust gas from (i) online monitoring of the fermentation kinetics (dCO
2/dt and EtOH) and concentration of the volatile compound in the gas phase (C
gas)) and (ii) the modelling of the gas–liquid equilibrium coefficient (k
i). Q(t)is the CO
2 production rate (per liter of must) and C
liq(t) the concentration of the volatile compound in the liquid phase
[6].
2.1.2. Interest in Online Fermentation Monitoring
Thanks to online monitoring, with dozens or hundreds of measurements during fermentation, new information of direct interest for the study of metabolism is now available:
-
The dynamics of the synthesis of different compounds and their timing, with, sometimes, the highlighting of peaks that would have gone undetected with a manual sampling;
-
The calculation of the production rates of CO2 and various metabolites. These rates, which can be transformed into specific production rates if the cell population is also monitored, are directly proportional to metabolic fluxes. By combining the monitoring of several compounds, it is also possible to calculate the evolution of production yields, which represent further information of metabolic interest.
It is then possible to couple these data with approaches such as metabolic flux analysis or transcriptomics. For example, this was achieved
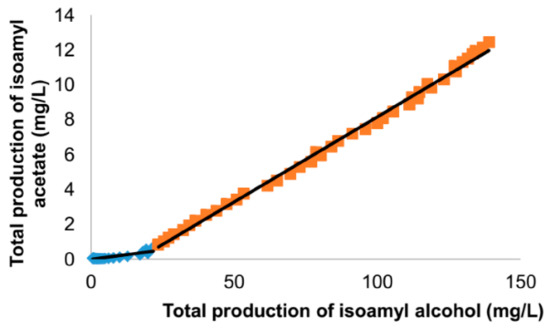
[8] when studying the effect of sterol additions during fermentation. These authors highlighted an increase in production yield between isoamyl alcohol and isoamyl acetate (
Figure 3), in such a way that they could precisely define the sampling times before and after this yield change (slope change) to perform transcriptomic analyses on yeast cells in two distinct, well-defined physiological states.
Figure 3. Online GC monitoring of fermentation aromas in 100 L tanks. Focus on the bioconversion of isoamyl alcohol to isoamyl acetate. Changes in total isoamyl acetate production as a function of total isoamyl alcohol production. The total productions represent the sum of the concentration in the liquid and the amount lost in the gas phase.
For volatile compounds, real-time balances between the gas and liquid phases enable differentiation between microbiological and physical phenomena. Thus, for example, when comparing fermentations at 20 °C and 30 °C
[5], Morakul et al., 2011 calculated that the total production of ethyl hexanoate was 1.61 mg/L and 1.22 mg/L, respectively, whereas the final concentrations in the wines were 0.92 mg/L and 0.36 mg/L, respectively. This example shows that (i) the quantity of ester lost can be very high, in this case, 43% at 20 °C and 70% at 30 °C; and (ii) the temperature effect is very different depending on whether one considers the total production (which reflects yeast metabolic capability) or the concentration in the wine, with 30% and 150% increases between 30 °C and 20 °C, respectively. If considering only the liquid content of ester, the temperature impact on yeast metabolism is highly overestimated.
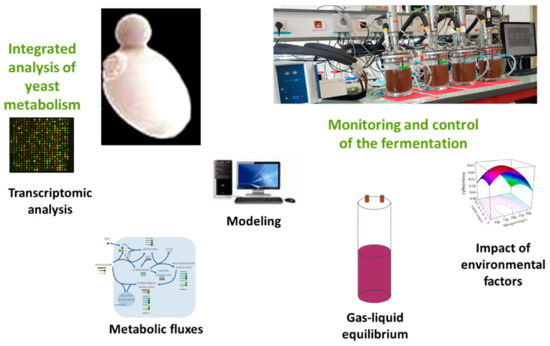
Thus, integrated approaches can be implemented, such as the one carried out on the production of fermentation aromas, which combines online monitoring with biochemical engineering and systems biology approaches (Figure 4).
Figure 4. Integrated approach combining biochemical engineering and system biology. Example of fermentative aroma synthesis. Online monitoring and fermentation control tools make it possible to (i) carry out cultures under perfectly controlled conditions; (ii) monitor parameters of metabolic interest: fermentation rate, and synthesis dynamics of the main fermentative aromas (with total production, i.e., concentration in the liquid + losses); (iii) cross-reference this information with system biology approaches: choice of key moments for post-genomic analyses and comparison of metabolic flux estimates (production rates vs. flux modelling).
2.1.3. Interest of Industrial Sensors
In addition to these specific devices, we considered whether using industrial sensors was relevant in the context of monitoring oenological fermentation.
The value of measuring medium conductivity has been demonstrated
[9]. Indeed, its evolution is very well correlated to the variation of assimilable nitrogen concentration. This measurement is thus very relevant to follow in a very fine way the dynamics of nitrogen assimilation at the beginning of fermentation and after additions of nitrogenous nutrients.
The measurement of dissolved CO
2 is very useful for monitoring very precisely the recovery of yeast activity at the very beginning of fermentation
[10]. It has also been used to study the rehydration phase of active dry yeasts
[11].
Monitoring dissolved oxygen has made it possible to calibrate the oxygenation system
[12]. Monitoring during fermentation is of little interest because the oxygen content is almost always equal to zero, even during the oxygenation phases, as the rate of consumption by yeast is generally higher than the supply rate. However, recent sensors allow more practical analyses and make this measurement potentially interesting to follow, for example, the heterogeneity within large volume tanks
[13].
The evolution of redox potential and pH have also been monitored. They highlighted the main phases of fermentation
[14]. However, it is difficult to obtain more precise information because these values are influenced by many compounds, and thus vary in relation to must composition.
The above results were obtained using fermentors or tanks. Other authors have also shown the benefit of combining different industrial sensors (temperature, humidity, level) to monitor the evolution of vinifications in barrels
[15].
2.2. Design of Dedicated Fermentation Tools
In addition to online monitoring systems, we developed several fermentation tools dedicated to the study of yeast metabolism under oenological conditions.
2.2.1. Controlled Rate Fermentations
The CO
2 production rate, which is proportional to yeast activity, can be regulated by real-time temperature control or the supply of nutrients, mainly assimilable nitrogen. This possibility has been very useful, especially for studying the nitrogen requirements of different yeast strains by comparing the nitrogen inputs necessary to achieve fermentations at identical rates
[16][17].
2.2.2. Controlled Micro-Oxygenation
The addition of small amounts of oxygen (a few mg/L) is very useful for the completion of fermentation, but the management and quantification of this oxygenation are often poorly controlled, even at the laboratory scale. To address this issue, we developed a device to control micro-oxygenation during fermentation
[12]. Oxygen is transferred by diffusion through a silicone tube and the amount transferred can be controlled, via the supervision software, either as a function of time or as a function of the reaction progress (e.g., 1 mg/L per % of ethanol produced). This device is very convenient to study the impact of oxygen not only on the main reaction, but also on the production of compounds such as fermentative aromas
[18], sulfur compounds, acetaldehyde, diacetyl, and so on, as well as under rosé or red wine conditions, on color, and polyphenolic compounds (
Figure 5).
Figure 5. Schematic representation of the bubble-free micro-oxygenation system. Oxygen is added by diffusion across a silicone tubing membrane with a controlled oxygen transfer rate (OTR). The OTR is modulated by controlling the liquid flow rate inside the silicone tube.
It should be noted that this system dedicated to the control of oxygenation during fermentation (addition of a few mg/L per hour or per day) is not adapted as it is to micro-oxygenation during wine aging, which requires additions of a few mg/L per month
[19].
2.2.3. Multi-Stage Continuous Bioreactor
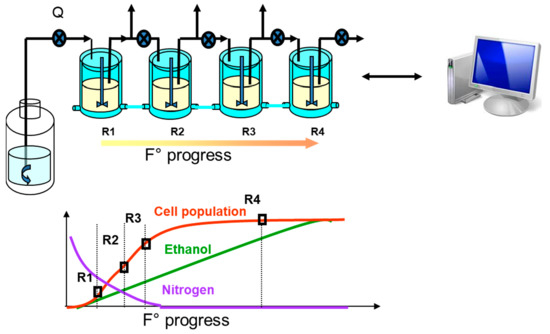
In winemaking conditions, yeasts go through a growth phase and then a stationary phase, during which more than half of the sugars is fermented. A comprehensive study of wine yeast physiology must thus include yeasts in a non-growing phase. To obtain non-growing yeasts in a steady state, we developed a four-stage continuous reactor that mimics, in a series of steady states, the conditions of batch fermentation (
Figure 6)
[20]. Despite its complexity, this fermentation device has shown its great interest for metabolic studies. For example, when studying valine uptake by yeast, it was shown that the corresponding transcriptomic data are of better quality than in a batch fermentation
[21]. It can also be noted that, in addition to its interest in the study of metabolism, this multistage fermenter has been used, in collaboration with automaticians, as a model to develop control strategies for non-linear and constrained systems
[22].
Figure 6. Schematic representation of the four-stage continuous fermentation device. The system is made up of four tanks (R1 to R4) linked in series. The five pumps are monitored independently by the control software, which also integrates the data coming from the mass flowmeters. Each bioreactor, except the first one, is fed with cells and partially fermented medium from the previous tank. The first stages are run with growing cells, while the last ones operate as resting cells. This device reproduces batch fermentation characteristics.
3. Development of New Strategies for Fermentation Control
Wineries are increasingly better equipped to control winemaking, especially alcoholic fermentation, with, for example, more and more precise temperature controls. On the other hand, control strategies still remain relatively empirical and are generally decided a priori, without taking into account must composition variability.
The rate of CO
2 release is proportional to yeast activity (cf. part 1.1.2). It is also directly correlated to the rate of heat production and, therefore, to the amount of energy required to regulate the temperature
[23]. Thus, this parameter is of major interest for the development of new fermentation control strategies optimized for each tank. The availability of online data on quality markers, mainly fruity aromas, also makes it possible to take into account quality-related parameters, even if sensory analysis cannot be replaced in the final validation steps.
Fermentation operations, mainly nutrient additions and temperature regulation, can be adapted to actual fermentation behavior, with the possibility of taking into account must composition variability, depending on the tank (cf. part 3.1). The huge amount of data on fermentation dynamics is also very useful to build and validate predictive models that can initially be used for simulation (cf. part 3.2) and later on for optimized control (cf. part 3.3).
3.1. Individual Tank Control
3.1.1. Nutrient Addition
The addition of nutrients is one of the main ways to control fermentation, but the challenge is to perform these additions only if they are necessary and in an optimized way.
With assimilable nitrogen generally being the limiting nutrient, Bely et al., 1990 proposed a method to (i) estimate its concentration in the must, based on the value of the maximum fermentation rate, and (ii) thus detect deficient musts to which nitrogen addition is recommended
[24]. Their work also highlighted the effectiveness of additions made during the stationary phase. To complement this work, we subsequently tested controlled continuous additions and compared different sources of nitrogen to evaluate their impact on fermentation kinetics and the aromatic characteristics of wines
[17][25]. The results could be compared to those obtained by other research groups, synthesized by Gobert et al., 2019
[26].
The positive effect of oxygenation has also been widely described, notably to limit the risks of stuck fermentations
[27]. Online monitoring has allowed both (i) to determine the best timing of oxygen addition and (ii) to show that oxygenation has mainly an impact on the final fermentation kinetics. It thus dramatically differs from the effect of nitrogen additions.
More recently, special attention has been given to the management of lipid compounds, especially in highly clarified musts
[28][29][30]. In this specific case, turbidity has to be managed in the interaction with nitrogen and oxygen additions. Online kinetics monitoring is a very useful tool for differentiating the type of deficiency and to optimize corrections
[31].
3.1.2. Temperature Control
Temperature profiles based on the control of fermentation rate and optimizing both the duration of fermentation and the energy required to regulate temperature were first proposed
[32]. Later, the online monitoring of fermentative aromas provided a better understanding of the effect of temperature on both the synthesis of these molecules and their loss
[4][33]. The importance of temperature control to fine-tune wine quality was also pointed out by Molina et al., 2007
[34].
3.2. Modeling
Several models have been developed. They are both (i) mechanistic, to consider the main biological or physico-chemical mechanisms involved, and thus widen the validity domain; and (ii) dynamic, to describe the evolution of the process monitored online.
The fermentation kinetics model calculates the evolution of the fermentation rate based on the initial concentration of available nitrogen and the temperature profile
[35]. After being validated in a wide range of oenological situations, it was combined with the thermal model (cf. part 2.1) and introduced into a simulation and optimization software for alcoholic fermentation
[36].
A dynamic model has been developed to predict the synthesis kinetics for the main aroma compounds produced by yeast during fermentation
[37]. This model is a proof of concept and its coefficients’ values may change when using different natural musts and yeast strains. However, the general structure of the model should remain valid regardless of the medium.
In parallel, the gas–liquid equilibrium of the main aromas was modeled for real-time calculation of concentrations in the gas and liquid phases as well as cumulative losses during the process
[4][5].
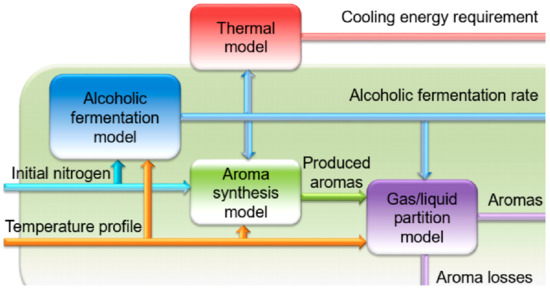
The four models can be combined (
Figure 8) into an overall model to search for new predictive control and fermentation optimization strategies (cf. part 3.3)
[38].
Figure 8. Structure of the global wine fermentation model. The global model integrates four models calculating the fermentation rate, the energy required for temperature regulation, the synthesis of the main fermentation aromas, and the distribution of these aromas between the gas and liquid phases.
3.3. Predictive Control and Optimization
A major challenge is to use modeling to anticipate and optimize the main fermentation parameters. The overall model takes into account both technological parameters and qualitative criteria and the optimization necessarily requires trade-offs. For example, low temperatures favor the synthesis of esters and limit their losses, but increase the fermentation duration. It is thus necessary to find a compromise in temperature management. The same holds true for the management of nutrient additions.
Obtaining wines with predefined aromatic profiles while optimizing tank use and energy expenses becomes a complex, but feasible issue in the framework of multidisciplinary collaborative projects including specialists in modeling, control, and optimization of bioprocesses.
In a first approach, Mouret et al., 2019 proposed a multi-criteria optimization in two phases: (i) generation of a Pareto front (set of optimal solutions) from the model, and then (ii) an interactive exploration (man–machine interface) of the Pareto front using a visualization software in order to progressively limit the number of possible solutions and ultimately define the optimal solution satisfying all the criteria
[39].
A current project consists of coupling optimization and online monitoring of kinetics and aromatic compounds in order to develop a real-time control strategy. This is still a proof of concept with synthetic media and a limited number of marker molecules, but its application in real conditions, for the production of wines with well-defined aromatic profiles, can already be considered.