
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Natalia A. Shnayder | + 1826 word(s) | 1826 | 2021-08-30 05:08:26 | | | |
| 2 | Peter Tang | Meta information modification | 1826 | 2021-08-31 02:46:22 | | |
Video Upload Options
Intervertebral disc degeneration (IVDD) is a common pathology of the spine that significantly reduces the quality of life and performance of patients [1]. Neurologists, therapists, rheumatologists and orthopedists take part in the observation and treatment this disorder. The purpose of this entry is to analyze domestic and foreign studies on the role of collagen-encoding genes polymorphism in the development of intervertebral discs (IVDs) degeneration in humans.
1. Introduction
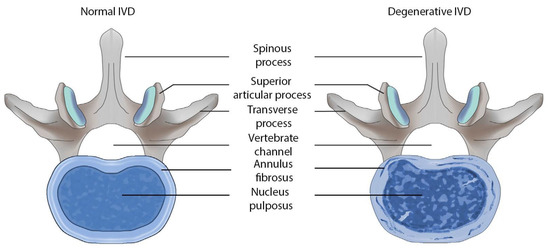
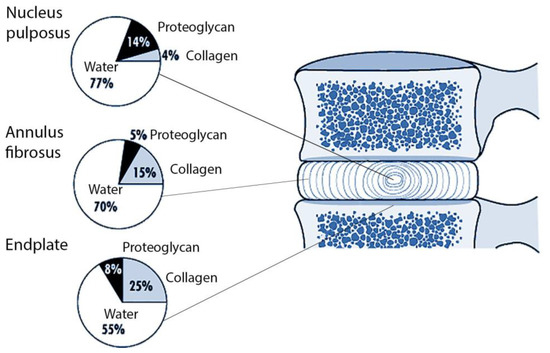
2. Current Insights on Polymorphisms in Collagen-Encoding Genes
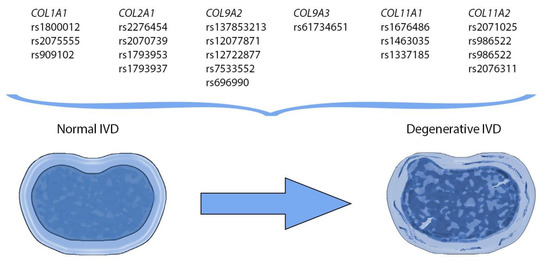
|
Gene, Chromosomal Locus |
Single Nucleotide Variants |
Protein |
Source |
|---|---|---|---|
|
COL1A1 17q21.33 |
rs1800012 rs2075555 rs909102 |
Alpha 1 chain of collagen type I |
|
| COL1A2
7q21.3 |
n/a * |
Alpha 2 chain of collagen type I |
|
|
COL2A1 12q13.11 |
rs2276454 rs2070739 rs1793953 rs1793937 |
Alpha 1 chain of collagen type II |
|
|
COL9A1 6q13 |
n/a * |
Alpha 1 chain of collagen type IX |
|
|
COL9A2 1p34.2 |
rs137853213 rs12077871 rs12722877 rs7533552 rs696990 |
Alpha 2 chain of collagen type IX |
|
|
COL9A3 20q13.33 |
rs61734651 |
Alpha 3 chain of collagen type IX |
|
|
COL11A1 1p21.1 |
rs1676486 rs1463035 rs1337185 |
Alpha 1 chain of collagen type XI |
|
|
COL11A2 6p21.32 |
rs2071025 rs986522 rs986522 rs2076311 |
Alpha 2 chain of collagen type XI |
3. Conclusions
References
- Munir, S.; Rade, M.; Määttä, J.H.; Freidin, M.B.; Williams, F.M.K. Intervertebral Disc Biology: Genetic Basis of Disc Degeneration. Curr. Mol. Biol. Rep. 2018, 4, 143–150.
- Motina, A.N.; Astaschenko, Y.A.; Masaleva, I.O.; Tretyakova, E.E. The social hygienic characteristic of patients with osteochondrosis of spine. Probl. Sotsial’noi Gig. Zdr. Istor. Meditsiny 2020, 28, 396–399. (In Russian)
- Dagenais, S.; Caro, J.; Haldeman, S.A. Systematic review of low back pain cost of illness studies in the United States and internationally. Spine J. 2008, 8, 8–20.
- Byvaltsev, V.A.; Kalinin, A.A.; Okoneshnikova, A.K.; Irintseev, A.A. Differentiated surgical tactics in degenerative diseases of lumbar spine department with the use of functional methods. Sib. Med. Rev. 2018, 5, 54–65.
- Melnikova, E.V.; Popov, A.P. Venlafaxine in the treatment of chronic pain syndromes. V.M. Bekhterev Rev. Psychiatry Med. Psychol. 2010, 4, 55–58. (In Russian)
- Schmidt, I.R. Solved and unsolved problems of vertebral neurology at the present stage of development of science. Med. Kuzbasse 2004, 3, 13–17. (In Russian)
- Feng, Y.; Egan, B.; Wang, J. Genetic Factors in Intervertebral Disc Degeneration. Genes Dis. 2016, 3, 178–185.
- Hanaei, S.; Abdollahzade, S.; Khoshnevisan, A.; Kepler, C.K.; Rezaei, N. Genetic aspects of intervertebral disc degeneration. Rev. Neurosci. 2015, 26, 581–606.
- Kitis, S.; Coskun, Z.M.; Tasdemir, P.; Tuncez, E.; Zamani, A.G.; Acar, A. Analysis of genetic polymorphisms associated with intervertebral disc degeneration. Cell. Mol. Biol. 2018, 64, 61–65.
- Vieira, L.A.; Dos Santos, A.A.; Peluso, C.; Barbosa, C.P.; Bianco, B.; Rodrigues, L.M.R. Influence of lifestyle characteristics and VDR polymorphisms as risk factors for intervertebral disc degeneration: A case-control study. Eur. J. Med. Res. 2018, 23, 11.
- Buckwalter, J.A. Aging and degeneration of the human intervertebral disc. Spine 1995, 20, 1307–1314.
- Eskola, P.J.; Kjaer, P.; Daavittila, I.M.; Solovieva, S.; Okuloff, A.; Sorensen, J.S.; Karppinen, J.I. Genetic risk factors of disc degeneration among 12-14-year-old Danish children: A population study. Int. J. Mol. Epidemiol. Genet. 2010, 1, 158–165.
- Zheng, C.J.; Chen, J. Disc degeneration implies low back pain. Theor. Biol. Med. Model. 2015, 12, 24.
- Brinjikji, W.; Diehn, F.E.; Jarvik, J.G.; Carr, C.M.; Kallmes, D.F.; Murad, M.H.; Luetmer, P.H. MRI Findings of Disc Degeneration are More Prevalent in Adults with Low Back Pain than in Asymptomatic Controls: A Systematic Review and Meta-Analysis. AJNR Am. J. Neuroradiol. 2015, 36, 2394–2399.
- Janeczko, Ł.; Janeczko, M.; Chrzanowski, R.; Zieliński, G. The role of polymorphisms of genes encoding collagen IX and XI in lumbar disc disease. Neurol. Neurochir. Polska 2014, 48, 60–62.
- Kadow, T.; Sowa, G.; Vo, N.; Kang, J.D. Molecular basis of intervertebral disc degeneration and herniations: What are the important translational questions? Clin. Orthop. Relat. Res. 2015, 473, 1903–1912.
- Kalb, S.; Martirosyan, N.L.; Kalani, M.Y.; Broc, G.G.; Theodore, N. Genetics of the degenerated intervertebral disc. World Neurosurg. 2012, 77, 491–501.
- Antoniou, J.; Steffen, T.; Nelson, F.; Winterbottom, N.; Hollander, A.P.; Poole, R.A.; Aebi, M.; Alini, M. The human lumbar intervertebral disc. Evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J. Clin. Investig. 1996, 98, 996–1003.
- Hemanta, D.; Jiang, X.X.; Feng, Z.Z.; Chen, Z.X.; Cao, Y.W. Etiology for Degenerative Disc Disease. Chin. Med. Sci. J. 2016, 31, 185–191.
- Anjankar, S.D.; Poornima, S.; Raju, S.; Jaleel, M.A.; Bhiladvala, D.; Hasan, Q. Degenerated intervertebral disc prolapse and its association of collagen I alpha 1 Spl gene polymorphism: A preliminary case control study of Indian population. Indian J. Orthop. 2015, 49, 589–594.
- Genetic Home Reference, Your Guide to Undersatanding Genetic Conditions COL1A1. 2012. Available online: http://www.ghr.nlm.nih.gov/gene/COL1A1 (accessed on 14 April 2021).
- Pluijm, S.M.; van Essen, H.W.; Bravenboer, N.; Uitterlinden, A.G.; Smit, J.H.; Pols, H.A.; Lips, P. Collagen type I alpha1 Sp1 polymorphism, osteoporosis, and intervertebral disc degeneration in older men and women. Ann. Rheum Dis. 2004, 63, 71–77.
- Tilkeridis, C.; Bei, T.; Garantziotis, S.; Stratakis, C.A. Association of a COL1A1 polymorphism with lumbar disc disease in young military recruits. J. Med. Genet. 2005, 42, 44.
- Videman, T.; Saarela, J.; Kaprio, J.; Näkki, A.; Levälahti, E.; Gill, K.; Peltonen, L.; Battié, M.C. Associations of 25 structural, degradative, and inflammatory candidate genes with lumbar disc desiccation, bulging, and height narrowing. Arthritis Rheum. 2009, 60, 470–481.
- Zhong, B.; Huang, D.; Ma, K.; Deng, X.; Shi, D.; Wu, F.; Shao, Z. Association of COL1A1 rs1800012 polymorphism with musculoskeletal degenerative diseases: A meta-analysis. Oncotarget 2017, 8, 75488–75499.
- Hanaei, S.; Abdollahzade, S.; Sadr, M.; Fattahi, E.; Mirbolouk, M.H.; Khoshnevisan, A.; Rezaei, N. Lack of association between COL1A1 and COL9A2 single nucleotide polymorphisms and intervertebral disc degeneration. Br. J. Neurosurg. 2021, 35, 77–79.
- Li, Y.Z.; Li, J.; Zhang, J.; Lin, Q. Association of COL2A and Aggrecan polymorphisms with the susceptibility of intervertebral disc degeneration. Int. J. Clin. Exp. Med. 2016, 9, 3885–3892.
- Deng, Y.; Tan, X.T.; Wu, Q.; Wang, X. Correlations Between COL2A and Aggrecan Genetic Polymorphisms and the Risk and Clinicopathological Features of Intervertebral Disc Degeneration in a Chinese Han Population: A Case-Control Study. Genet. Test. Mol. Biomark. 2017, 21, 108–115.
- Zielinska, N.; Podgórski, M.; Haładaj, R.; Polguj, M.; Olewnik, L. Risk Factors of Intervertebral Disc Pathology—A Point of View Formerly and Today—A Review. J. Clin. Med. 2021, 10, 409.
- Wu, H.; Wang, S.; Chen, W.; Zhan, X.; Xiao, Z.; Jiang, H.; Wei, Q.; Wu, H.; Wang, S.; Chen, W.; et al. Collagen IX gene polymorphisms and lumbar disc degeneration: A systematic review and meta-analysis. J. Orthop. Surg. Res. 2018, 5, 47.
- Mio, F.; Chiba, K.; Hirose, Y.; Kawaguchi, Y.; Mikami, Y.; Oya, T.; Mori, M.; Kamata, M.; Matsumoto, M.; Ozaki, K.; et al. A functional polymorphism in COL11A1, which encodes the alpha 1 chain of type XI collagen, is associated with susceptibility to lumbar disc herniation. Am. J. Hum. Genet. 2007, 81, 1271–1277.
- Liu, W.; Sun, G.; Guo, L.; Wang, L.; Fan, W.; Lang, M.; Chen, D.; Yi, X. A genetic variant in COL11A1 is functionally associated with lumbar disc herniation in Chinese population. J. Genet. 2017, 96, 867–872.
- Solovieva, S.; Lohiniva, J.; Leino-Arjas, P.; Raininko, R.; Luoma, K.; Ala-Kokko, L.; Riihimäki, H. Intervertebral disc degeneration in relation to the COL9A3 and the IL-1ss gene polymorphisms. Eur. Spine J. 2006, 15, 613–619.
- Yang, X.; Jia, H.; Xing, W.; Li, F.; Li, M.; Sun, K.; Zhu, Y. Genetic variants in COL11A2 of lumbar disk degeneration among Chinese Han population. Mol. Genet. Genom. Med. 2019, 7, 00524.
- Zhang, Y.; Sun, Z.; Liu, J.; Guo, X. Advances in susceptibility genetics of intervertebral degenerative disc disease. Int. J. Biol. Sci. 2008, 4, 283–290.
- Chen, K.; Wu, D.; Zhu, X.; Ni, H.; Wei, X.; Mao, N.; Xie, Y.; Niu, Y.; Li, M. Gene expression profile analysis of human intervertebral disc degeneration. Genet. Mol. Biol. 2013, 36, 448–454.
- Huang, D.; Deng, X.; Ma, K.; Wu, F.; Shi, D.; Liang, H.; Chen, S.; Shao, Z. Association of COL9A3 trp3 polymorphism with intervertebral disk degeneration: A meta-analysis. BMC Musculoskelet. Disord. 2018, 19, 381.
- Annunen, S.; Paassilta, P.; Lohiniva, J.; Perälä, M.; Pihlajamaa, T.; Karppinen, J.; Tervonen, O.; Kröger, H.; Lähde, S.; Vanharanta, H.; et al. An allele of COL9A2 associated with intervertebral disc disease. Science 1999, 285, 409–412.
- Trefilova, V.V.; Shnayder, N.A.; Popova, T.E.; Balberova, O.V.; Nasyrova, R.F. The role of NO system in low back pain chronicity. Pers. Psychiatry Neurol. 2021, 1, 37–45.
- Cornetta, K.; Brown, C.G. Balancing personalized medicine and personalized care. Acad. Med. 2013, 88, 309–313.
- Neznanov, N.G. A paradigm shift to treat psychoneurological disorders. Pers. Psychiatry Neurol. 2021, 1, 1–2.




