
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Caroline Billings | + 2626 word(s) | 2626 | 2021-07-22 12:33:39 | | | |
| 2 | Lindsay Dong | Meta information modification | 2626 | 2021-08-30 04:21:19 | | |
Video Upload Options
Magnetic nanoparticles (MNPs) have a wide range of applications; an area of particular interest is magnetic particle imaging (MPI). MPI is an imaging modality that utilizes superparamagnetic iron oxide particles (SPIONs) as tracer particles to produce highly sensitive and specific images in a broad range of applications, including cardiovascular, neuroimaging, tumor imaging, magnetic hyperthermia and cellular tracking.
1. Introduction
MPI is a tracer-based, functional and tomographic imaging modality, which determines the spatial distribution of MNPs [1]. MNPs are a widely diverse group of materials that have relevant and potentially ground-breaking applications in a wide variety of biomedical arenas including drug delivery, cell targeting, magnetic hyperthermia, and diagnostic imaging [2].
Magnetic particle imaging is an innovative imaging modality that is being developed to add strength and diversity to current imaging techniques such as MRI, CT, bioluminescence imaging (BLI), positron emission tomography (PET), and single photon emission computed tomography (SPECT) [3][4]. While each of these imaging techniques possess clinical utility, they are also accompanied by shortcomings. For example, MRI is a commonly utilized and powerful diagnostic tool in many clinical arenas, including cancer and cardiovascular imaging [5]. Recently, the value has come into question, as limitations to MRI are recognized. Limitations include long scan times, lack of standards for quantitative measures for clinical usage, and inefficient recognition of false positive results prior to initiating treatment [6]. Additionally, the various imaging techniques present a level of risk to patients, including radiation exposure during nuclear imaging procedures such as PET and CT scans [7], and gadolinium exposure during MRI, which can lead to systemic accumulation or nephrogenic systemic fibrosis in certain patients [8]. With these limitations in mind, the desire for a safe, efficient, ultra-sensitive and specific, multipurpose imaging system that will produce quantifiable 3D images is obvious—and leads us to MPI.
Considering the described and perceived strengths of MNPs and MPI, the question arises regarding the lack of clinical use of this system. While there are many advantages to MPI and it undoubtedly has the potential to improve biomedical imaging, there are also many obstacles and challenges. These challenges include ideal MNP development, safety concerns and practical implementation [9][1][10][11][12].
2. Magnetic Nanoparticle Synthesis Methods
As the utility of MNPs across various applications becomes more widely accepted, new methods for MNP synthesis are being developed. Specifically, as SPIONs hold such strong promise in the field of MPI, there are many synthesis methods reported. The most common methods include co-precipitation, thermal decomposition, hydrothermal synthesis, microemulsion, electrochemical synthesis and bacterial synthesis [13][14][15][16].
Presently, producing a colloidally stable batch of iron oxide nanoparticles is dependent on particle size, surface chemistry, density, and aqueous conditions [17][18]. Creating monodispersed iron oxide nanoparticles provides consistency, reproducible results, and predictable responses. This is critical, as limited reproducibility is a challenge in large-scale production and scalable production will be an important step to MPI becoming available for clinical use [19]. Research has demonstrated that thermal decomposition techniques using nonpolar solvents produce highly monodispersed MNPs with specific surface coatings [17].
3. MPI Applications
3.1. Cancer Imaging
MPI research is pushing the boundaries of conventional oncology imaging, especially sensitivity and resolution, by utilizing the intrinsic characteristics of MNPs and SPIONS. Song et al. [20] explored this approach by creating Janus iron oxide particles coated with a semiconducting polymer. Those MNPs demonstrated 7-fold the intensity of commercial MRI tracers and 3-fold the intensity of commercial MPI tracers. The Janus MNPs properly imaged as few as 250 cancer cells in vivo. Compared to the clinical imaging capability of 109 cancer cells, 250 cells indicate a substantial significant difference in resolution and sensitivity.
Yu et al. [21] explored the possibility of in vivo cancer MPI imaging in a xenograft breast tumor rat model utilizing long circulating SPIO tracer (LS-008). Their work relied on the enhanced permeability and retention (EPR) effect that is understood to allow nanoparticles to preferentially accumulate in tumors due to the leaky vasculature of the tumor [21][22]. They were able to demonstrate preferential accumulation of the particles within the tumors, with particles present within the tumor up to six days post injection, and a dose dependent increase in concentration of the tracer in blood. These findings provide confidence in the EPR effect and the quantitative abilities of MPI in vivo. This work led Yu et al. [21] to pose the question of MPI’s efficacy in less vascular tumors as well as metastatic tumors, given the heterogeneity of neoplasia, prior to suggesting clinical use of MPI for cancer imaging.
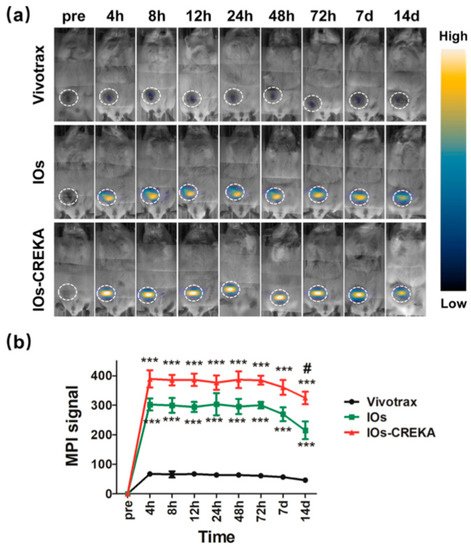
3.2. Cardiovascular Imaging
MPI offers benefits that are increasingly attractive for use in cardiovascular imaging [24]. Magnetic nanoparticles can act as nano-probes and deliver significant information including anatomic and physiologic details of cardiovascular diseases [25]. This behavior is largely a result of the size and physical characteristics of MNPs, as they are well suited for cellular imaging of myocardial and atherosclerotic anatomy and abnormalities [26]. A main application of MPI for cardiovascular imaging is direct administration of MNPs into blood vessels to visualize blood flow. This is feasible due to the ability of MPI to image any depth of tissue [27] and is quite impactful, as visualizing blood flow can identify abnormalities in velocity or pattern of blood flow [28], which can indicate structural changes. Vaalma et al. [29] described the ability of MPI to investigate degree of stenosis within vasculature. It was found that at an appropriate clinical MNP concentration, an area of stenosis as small as 2 mm could be successfully imaged. MPI can also be used during cardiovascular interventions, such as catheter guidance in minimally invasive procedures [4].
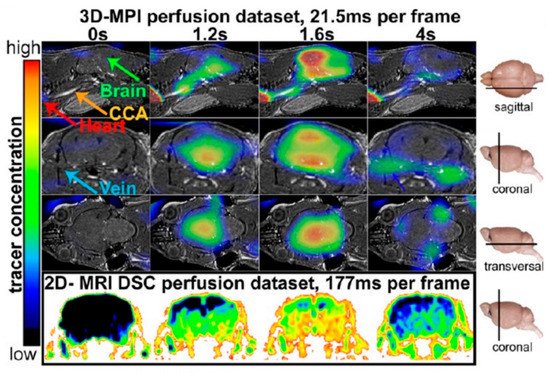
3.3. Neuroimaging
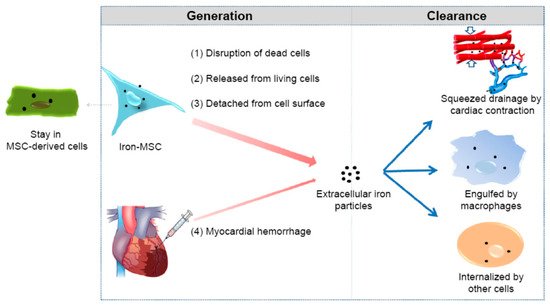
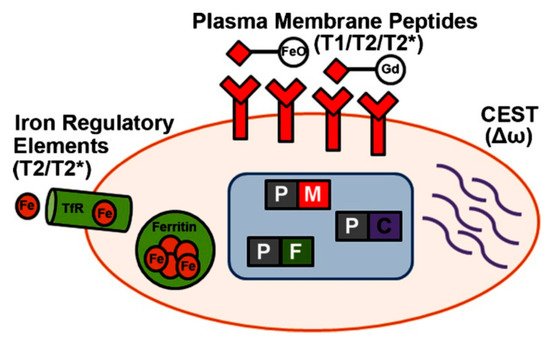
3.4. Cell Tracking
3.5. Magnetic Hyperthermia
4. Safety and Toxicity
4.1. Background
4.2. Toxicity Mechanisms
Accumulation and distribution of MNPs are a main cause for in vivo toxicity. It is well understood that the biodistribution of MNPs depends on the particle size, particle coating, and mass of particles administered [15][56][57][58][59].
Biodistribution of MNPs has been tracked via opsonization, a process where antibodies mark foreign pathogens (in this case MNPs) for elimination by phagocytes [60]. It was determined that opsonized MNPs were removed from the bloodstream within a few minutes and were distributed as follows: 80–90% through the liver, 5–8% through the spleen, and 1–2% via the bone marrow [59]. Additionally, interaction and toxicity of MNPs is highly dependent upon the particle surface. Therefore, investigation into ideal coatings for functional, excretable MNPs is necessary.
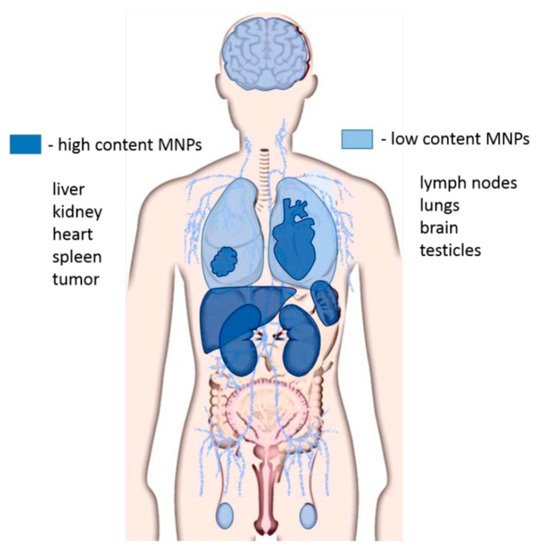
4.3. Methods for Limiting Toxicity
Desirable properties of MNPs to promote a healthy safety profile mainly include biocompatibility and an appropriate surface chemistry [61]. Of these characteristics, a major technique to effectively reduce toxic effects of these MNPs is surface modification. Surface modifications can: decrease aggregation of MNPs, prevent changes in magnetic properties to maintain efficacy of the MNPs, and inhibit adhesion with plasma proteins to prevent major inflammatory responses in vivo.
5. Conclusions and Perspectives
Overall, MPI offers great promise for a wide range of applications, including but not limited to cancer, cardiovascular, pulmonary and neuroimaging, cellular tracking, and therapeutics such as magnetic hyperthermia. Currently, MPI remains a pre-clinical imaging modality and requires significant upscaling and safety profile development prior to being utilized clinically. However, over time and with extensive research and development, the field of magnetic particle imaging will no doubt revolutionize the accuracy, precision, and methodology in which innumerable diseases are diagnosed and monitored, and the therapy that is administered.
References
- Knopp, T.; Gdaniec, N.; Moddel, M. Magnetic particle imaging: From proof of principle to preclinical applications. Phys. Med. Biol. 2017, 62, R124–R178.
- Williams, H. The application of magnetic nanoparticles in the treatment and monitoring of cancer and infectious diseases. Biosci. Horiz. Int. J. Stud. Res. 2017, 10, 1–10.
- Pablico-Lansigan, M.; Situ, S.; Samia, A. Magnetic particle imaging: Advancements and perspectives for real-time in vivo monitoring and image-guided therapy. Nanoscale 2013, 10, 4040–4055.
- Bakenecker, A.C.; Ahlborg, M.; Debbeler, C.; Kaethner, C.; Buzug, T.; Lüdtke-Buzug, K. Magnetic particle imaging in vascular medicine. Innov. Surg. Sci. 2018, 3, 179–192.
- Marcu, C.B.; Beek, A.M.; van Rossum, A.C. Clinical applications of cardiovascular magnetic resonance imaging. CMAJ 2006, 175, 911–917.
- Van Beek, E.J.; Kuhl, C.; Anzai, Y.; Desmond, P.; Ehman, R.L.; Gong, Q.; Gold, G.; Gulani, V.; Hall-Craggs, M.; Leiner, T.; et al. Value of MRI in medicine: More than just another test? J. Magn. Reson. Imaging 2019, 49, e14–e25.
- Nievelstein, R.A.J.; Van Ufford, H.M.E.Q.; Kwee, T.C.; Bierings, M.B.; Ludwig, I.; Beek, F.J.A.; De Klerk, J.M.H.; Mali, W.P.T.M.; De Bruin, P.W.; Geleijns, J. Radiation exposure and mortality risk from CT and PET imaging of patients with malignant lymphoma. Eur. Radiol. 2012, 22, 1946–1954.
- Choi, J.W.; Moon, W.J. Gadolinium deposition in the brain: Current updates. Korean J. Radiol. 2019, 20, 134–147.
- Sun, C.; Lee, J.S.; Zhang, M. Magnetic nanoparticles in MR imaging and drug delivery. Adv. Drug. Deliv. Rev. 2008, 60, 1252–1265.
- Talebloo, N.; Gudi, M.; Robertson, N.; Wang, P. Magnetic particle imaging: Current applications in biomedical research. J. Magn. Reason. Imaging 2020, 51, 1659–1668.
- Ludwig, F.; Eberbeck, D.; Löwa, N.; Steinhoff, U.; Wawrzik, T.; Schilling, M.; Trahms, L. Characterization of magnetic nanoparticle systems with respect to their magnetic particle imaging performance. Biomed. Tech. 2013, 6, 535–545.
- Lee, J.-H.; Huh, Y.-M.; Jun, Y.-W.; Seo, J.-W.; Jang, J.-T.; Song, H.-T.; Kim, S.; Cho, E.-J.; Yoon, H.-G.Y.; Suh, J.-S.; et al. Artificially engineered magnetic nanoparticles for ultra-sensitive molecular imaging. Nat. Med. 2007, 13, 95–99.
- Sanz, B.; Calatayud, M.P.; Cassinelli, N.; Ibarra, M.R.; Goya, G.F. Long term stability and reproducibility of magnetic colloids are key issues for steady values of specific power through time. Eur. J. Inorg. Chem. 2015, 2015, 4524–4531.
- Wu, W.; He, Q.; Jiang, C. Magnetic iron oxide nanoparticles: Synthesis and surface functionalization strategies. Nanoscale Res. Lett. 2008, 3, 397–415.
- Malhotra, N.; Lee, J.-S.; Liman, R.A.D.; Ruallo, J.M.S.; Villaflores, O.B.; Ger, T.-R.; Hsiao, C.-D. Potential toxicity of iron oxide magnetic nanoparticles: A review. Molecules 2020, 25, 3159.
- Jang, J.H.; Lim, H.B. Characterization and analytical application of surface modified magnetic nanoparticles. Microchem. J. 2010, 94, 148–158.
- Clift, M.J.D.; Rothen-Rutishauser, B.; Brown, D.M.; Duffin, R.; Donaldson, K.; Proudfoot, L.; Guy, K.; Stone, V. Highly stable superparamagnetic iron oxide nanoparticles as functional draw solutes for osmotically driven water transport. npj Clean Water 2020, 3, 1–6.
- Ramimoghadam, D.; Bagheri, S.; Hamid, S.B.A. Stable monodisperse nanomagnetic colloidal suspensions: An overview. Coll. Surf. B Biointerfaces 2015, 133, 388–411.
- Rubia-Rodríguez, I.; Santana-Otero, A.; Spassov, S.; Tombácz, E.; Johansson, C.; De La Presa, P.; Teran, F.; Morales, M.D.P.; Veintemillas-Verdaguer, S.; Thanh, N.; et al. Whither magnetic hyperthermia? A tentative roadmap. Materials 2021, 14, 706.
- Song, G.; Chen, M.; Zhang, Y.; Cui, L.; Qu, H.; Zheng, X.; Wintermark, M.; Liu, Z.; Rao, J. Janus iron oxides @ semiconducting polymer nanoparticle tracer for cell tracking by magnetic particle imaging. Nano Lett. 2018, 18, 182–189.
- Yu, E.Y.; Bishop, M.; Zheng, B.; Ferguson, R.M.; Khandhar, A.; Kemp, S.J.; Krishnan, K.M.; Goodwill, P.; Conolly, S.M. Magnetic particle imaging: A novel in vivo imaging platform for cancer detection. Nano Lett. 2017, 17, 1648–1654.
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284.
- Du, Y.; Liu, X.; Liang, Q.; Liang, X.J.; Tian, J. Optimization and design of magnetic ferrite nanoparticles with uniform tumor distribution for highly sensitive MRI/MPI performance and improved magnetic hyperthermia therapy. Nano Lett. 2019, 19, 3618–3626.
- Tong, W.; Hui, H.; Shang, W.; Zhang, Y.; Tian, F.; Ma, Q.; Yang, X.; Tian, J.; Chen, Y. Highly sensitive magnetic particle imaging of vulnerable atherosclerotic plaque with active myeloperoxidase-targeted nanoparticles. Theranostics 2021, 11, 506–521.
- Tu, Y.; Sun, Y.; Fan, Y.; Cheng, Z.; Yu, B. Multimodality molecular imaging of cardiovascular disease based on nanoprobes. Cell Physiol. Biochem. 2018, 48, 1401–1415.
- Sosnovik, D.E.; Nahrendorf, M.; Weissleder, R. Magnetic nanoparticles for MR imaging: Agents, techniques and cardiovascular applications. Basic Res. Cardiol 2008, 103, 122–130.
- Tay, Z.W.; Chandrasekharan, P.; Chiu-Lam, A.; Hensley, D.W.; Dhavalikar, R.; Zhou, X.Y.; Yu, E.Y.; Goodwill, P.; Zheng, B.; Rinaldi, C.; et al. Magnetic particle imaging–guided heating in vivo using gradient fields for arbitrary localization of magnetic hyperthermia therapy. ACS Nano 2018, 12, 3699–3713.
- Lima, R.; Joseyphus, R.J.; Ishikawa, T.; Imai, Y.; Yamaguchi, T. Micro-flow visualization of magnetic nanoparticles for biomedical applications. In Single and Two-Phase Flows on Chemical and Biomedical Engineering; Dias, R., Lima Portugal, R., Eds.; Bentham Science Publishers: Sharjah, United Arab Emirates, 2012; pp. 600–612.
- Vaalma, S.; Rahmer, J.; Panagiotopoulos, N.; Duschka, R.L.; Borgert, J.; Barkhausen, J.; Vogt, F.M.; Haegele, J. Magnetic particle imaging (MPI): Experimental quantification of vascular stenosis using stationary stenosis phantoms. PLoS ONE 2017, 12, e0168902.
- Yu, E.Y.; Chandrasekharan, P.; Berzon, R.; Tay, Z.W.; Zhou, X.Y.; Khandhar, A.; Ferguson, R.M.; Kemp, S.J.; Zheng, B.; Goodwill, P.; et al. Magnetic particle imaging for highly sensitive, quantitative, and safe in vivo gut bleed detection in a murine model. ACS Nano 2017, 11, 12067–12076.
- Ryken, T.C.; Aygun, N.; Morris, J.; Schweizer, M.; Nair, R.; Spracklen, C.; Kalkanis, S.N.; Olson, J.J. The role of imaging in the management of progressive glioblastoma. J. Neuro. Oncol. 2014, 118, 435–460.
- Wu, L.C.; Zhang, Y.; Steinberg, G.; Qu, H.; Huang, S.; Cheng, M.; Bliss, T.; Du, F.; Rao, J.; Song, G.; et al. A review of magnetic particle imaging and perspectives on neuroimaging. Am. J. Neuroradiol. 2019, 40, 206–212.
- Ludewig, P.; Gdaniec, N.; Sedlacik, J.; Forkert, N.D.; Szwargulski, P.; Gräser, M.; Adam, G.; Kaul, M.G.; Krishnan, K.M.; Ferguson, R.M.; et al. Magnetic particle imaging for real-time perfusion imaging in acute stroke. ACS Nano 2017, 11, 10480–10488.
- Nguyen, P.; Riegler, J.; Wu, J. Stem cell imaging: From bench to bedside. Stem Cell Stem 2014, 14, 431–444.
- Zheng, B.; Von See, M.P.; Yu, E.; Gunel, B.; Lu, K.; Vazin, T.; Schaffer, D.V.; Goodwill, P.; Conolly, S.M. Quantitative magnetic particle imaging monitors the transplantation, biodistribution, and clearance of stem cells in vivo. Theranostics 2016, 6, 291–301.
- Goradel, N.H.; Hour, F.G.; Negahdari, B.; Malekshahi, Z.V.; Hashemzehi, M.; Masoudifar, A.; Mirzaei, H. Stem cell therapy: A new therapeutic option for cardiovascular diseases. J. Cell. Biochem. 2018, 119, 95–104.
- Bagno, L.; Hatzistergos, K.E.; Balkan, W.; Hare, J.M. Mesenchymal stem cell-based therapy for cardiovascular disease: Progress and challenges. Mol. Ther. 2018, 26, 1610–1623.
- Müller, P.; Lemcke, H.; David, R. Stem cell therapy in heart diseases–cell types, mechanisms and improvement strategies. Cell. Physiol. Biochem. 2018, 48, 2607–2655.
- Wang, J.; Jokerst, J.V. Stem cell imaging: Tools to improve cell delivery and viability. Stem Cells Int. 2016, 2016, 1–16.
- Sehl, O.; Makela, A.; Hamilton, A.; Foster, P. Trimodal cell tracking in vivo: Combining iron- and fluorine-based magnetic resonance imaging with magnetic particle imaging to monitor the delivery of mesenchymal stem cells and the ensuing inflammation. Tomography 2019, 5, 367–376.
- Nejadnik, H.; Pandit, P.; Lenkov, O.; Lahiji, A.P.; Yerneni, K.; Daldrup-Link, H.E. Ferumoxytol can be used for quantitative magnetic particle imaging of transplanted stem cells. Mol. Imaging Biol. 2019, 21, 65–472.
- Zheng, B.; Vazin, T.; Goodwill, P.; Conway, A.; Verma, A.; Saritas, E.U.; Schaffer, D.; Conolly, S.M. Magnetic particle imaging tracks the long-term fate of in vivo neural cell implants with high image contrast. Sci. Rep. 2015, 5, 14055.
- Laurent, S.; Mahmoudi, M. Superparamagnetic iron oxide nanoparticles: Promises for diagnosis and treatment of cancer. Int. J. Mol. Epidemiol. Genet. 2011, 2, 367–390.
- Ahn, Y.J.; Kong, T.H.; Choi, J.S.; Yun, W.; Key, J.; Seo, Y.J. Strategies to enhance efficacy of SPION-labeled stem cell homing by magnetic attraction: A systemic review with meta-analysis. Int. J. Nanomed. 2019, 14, 4849–4866.
- Jasmin; de Souza, G.T.; Louzada, R.A.; Rosado-De-Castro, P.H.; Mendez-Otero, R.; de Carvalho, A.C.C. Tracking stem cells with superparamagnetic iron oxide nanoparticles: Perspectives and considerations. Int. J. Nanomed. 2017, 12, 779–793.
- Ge, J.; Huang, Z.; Li, C.; Yang, S.; Xu, J.; Shen, Y.; Xie, X.; Dai, Y.; Lu, H.; Gong, H.; et al. Magnetic resonance hypointensive signal primarily originates from extracellular iron particles in the long-term tracking of mesenchymal stem cells transplanted in the infarcted myocardium. Int. J. Nanomed. 2015, 10, 1679.
- Amsalem, Y.; Mardor, Y.; Feinberg, M.S.; Landa, N.; Miller, L.; Daniels, D.; Ocherashvilli, A.; Holbova, R.; Yosef, O.; Barbash, I.M.; et al. Iron-oxide labeling and outcome of transplanted mesenchymal stem cells in the infarcted myocardium. Circulation 2007, 116, 38–45.
- Vandsburger, M. Cardiac cell tracking with MRI reporter genes: Welcoming a new field. Curr. Cardiovasc. Imaging Rep. 2014, 7, 9250.
- Jose, J.; Kumar, R.; Harilal, S.; Mathew, G.E.; Parambi, D.G.T.; Prabhu, A.; Uddin, S.; Aleya, L.; Kim, H.; Mathew, B. Magnetic nanoparticles for hyperthermia in cancer treatment: An emerging tool. Environ. Sci. Pollut. Res. 2020, 27, 19214–19225.
- Hensley, D.; Tay, Z.W.; Dhavalikar, R.; Zheng, B.; Goodwill, P.; Rinaldi, C.; Conolly, S. Combining magnetic particle imaging and magnetic fluid hyperthermia in a theranostic platform. Phys. Med. Biol. 2017, 62, 3483–3500.
- Sadhukha, T.; Wiedmann, T.S.; Panyam, J. Inhalable magnetic nanoparticles for targeted hyperthermia in lung cancer therapy. Biomaterials 2013, 34, 5163–5171.
- Gupta, R.; Sharma, D. Evoution of magnetic hyperthermia for glioblastoma multiforme therapy. ACS Chem. Neurosci. 2019, 10, 1157–1172.
- Lodi, M.B.; Fanti, A.; Muntoni, G.; Mazzarella, G. A multiphysic model for the hyperthermia treatment of residual osteosarcoma in upper limbs using magnetic scaffolds. IEEE J. Multiscale Multiphysics Comput. Tech. 2019, 4, 337–347.
- Mues, B.; Bauer, B.; Roeth, A.; Ortega, J.; Buhl, E.; Radon, P.; Wiekhorst, F.; Gries, T.; Schmitz-Rode, T.; Slabu, I. Nanomagnetic actuation of hybrid stents for hyperthermia treatment of hollow organ tumors. Nanomaterials 2021, 11, 618.
- Li, G.; Zhang, K.; Pei, Z.; Zhang, N.; Yu, Y.; Zhao, S.; Liang, G.; Zhou, J.; Xing, Y. A novel method to enhance magnetic property of bioactive glass-ceramics for hyperthermia. Ceram. Int. 2019, 45, 4945–4956.
- Zamay, G.S.; Zamay, T.N.; Lukyanenko, K.A.; Kichkailo, A.S. Aptamers increase biocompatibility and reduce the toxicity of magnetic nanoparticles used in biomedicine. Biomedicines 2020, 8, 59.
- Markides, H.; Rotherham, M.; El Haj, A.J. Biocompatibility and toxicity of magnetic nanoparticles in regenerative medicine. J. Nanomater. 2012, 2012, 1–11.
- Shubayev, V.I.; Pisanic, T.R.; Jin, S. Magnetic nanoparticles for theragnostics. Adv. Drug Deliv. Rev. 2009, 61, 467–477.
- Aggarwal, P.; Hall, J.B.; McLeland, C.B.; Dobrovolskaia, M.A.; McNeil, S.E. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv. Drug Deliv. Rev. 2009, 61, 428–437.
- Thau, L.; Asuka, E.; Mahajan, K. Physiology, opsonization. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK534215/ (accessed on 27 April 2021).
- Wáng, Y.X.J.; Idée, J.M. A comprehensive literatures update of clinical researches of superparamagnetic resonance iron oxide nanoparticles for magnetic resonance imaging. Quant. Imaging Med. Surg. 2017, 7, 88–122.
- Nosrati, H.; Salehiabar, M.; Fridoni, M.; Abdollahifar, M.-A.; Manjili, H.K.; Davaran, S.; Danafar, H. New insight about biocompatibility and biodegradability of iron oxide magnetic nanoparticles: Stereological and in vivo MRI monitor. Sci. Rep. 2019, 9, 7173.




