2. Analysis on Results
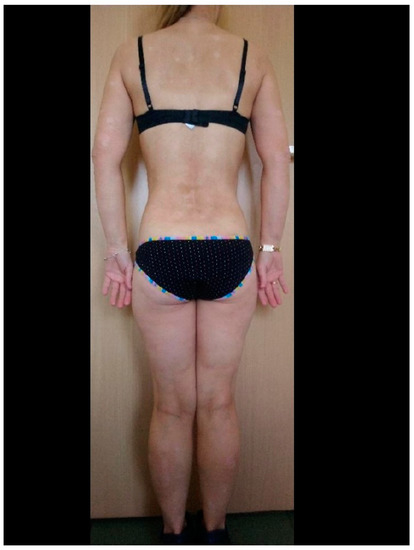
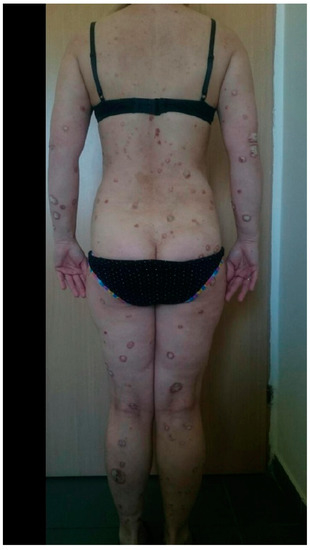
In the group of patients under biological treatment, SARS-CoV-2 infection was confirmed by a positive RT-PCR test in eight patients (14.0%). All of these patients had mild clinical symptoms, including anosmia, cough, mild breathing difficulties, and fever. Hospitalization due to COVID-19 did not occur in this group. Similar data were obtained in the control group: 11 (16%) patients were confirmed to be infected with SARS-CoV-2. In this group, only mild disease was observed, and hospitalization was not required for any patient. These results were statistically comparable (p > 0.05). In the group of patients undergoing biological treatment, six (75%) of eight patients developed an exacerbation of psoriasis during SARS-CoV-2 infection (example of a patient before and immediately after SARS-CoV-2 infection: Figure 1 and Figure 2). A similar relationship was observed in the control group, in which 8 (72%) of 11 patients with SARS-CoV-2 infection experienced an exacerbation of psoriasis. Detailed data are presented in Table 1. In any described patients, psoriasis therapy was not suspended in the case of SARS-CoV-2 infection. In all patients with an exacerbation of psoriasis, the condition resolved within 4–6 weeks from the diagnosis of COVID-19 and did not require intensification of systemic treatment (local treatment was increased), with the exception of two patients on cyclosporin for whom the dose was temporarily increased (4–6 weeks) by 50%. There was no situation in which the patients themselves modified their psoriasis treatment.
Figure 1. Patient before COVID-19 during biological therapy.
Figure 2. The same patient just after SARS-Co-V-2 infection.
Table 1. Characteristics of psoriasis patients who developed SARS-CoV-2 infection.
N
S/C |
Sex |
Age |
Type of Psoriasis According to ICD-10 |
Drug Name |
Exacerbation of Psoriasis
PASI Score
before vs. after |
Concomitant Diseases |
Time of Exacerbation (days) |
| 1S |
M |
72 |
L40.0 |
adalimumab |
No
32–28 |
Arterial
hypertension |
- |
| 2S |
M |
60 |
L40.0 |
secukinumab |
No
29–31 |
- |
- |
| 3S |
W |
50 |
L40.0 |
ustekinumab |
Yes
4–64 |
Obesity |
12 |
| 4S |
M |
44 |
L40.0 |
ustekinumab |
Yes
23–51 |
- |
24 |
| 5S |
W |
43 |
L40.5 |
adalimumab |
Yes
18–33 |
- |
28 |
| 6S |
M |
50 |
L40.0 |
risankizumab |
Yes
4–31 |
Arterial
hypertension |
28 |
| 7S |
W |
52 |
L40.0 |
ustekinumab |
Yes
8–44 |
- |
21 |
| 8S |
W |
52 |
L40.0 |
adalimumab |
Yes
15–25 |
- |
36 |
| 1C |
M |
51 |
L40.5 |
cyclosporin |
No
17–15 |
- |
- |
| 2C |
M |
42 |
L40.5 |
PUVA |
Yes
32–46 |
Arterial
hypertension |
24 |
| 3C |
M |
45 |
L40.0 |
cyclosporin |
Yes
28–39 |
- |
38 |
| 4C |
W |
62 |
L40.0 |
PUVA |
Yes
12–54 |
- |
42 |
| 5C |
W |
55 |
L40.0 |
cyclosporin |
No
19–42 |
- |
- |
| 6C |
M |
54 |
L40.5 |
cyclosporin |
Yes
33–63 |
Arterial
hypertension |
32 |
| 7C |
M |
46 |
L40.0 |
methotrexate |
No
8–7 |
- |
- |
| 8C |
W |
69 |
L40.0 |
methotrexate |
Yes
5–26 |
Diabetes
Arterial
hypertension |
25 |
| 9C |
M |
38 |
L40.0 |
cyclosporin |
Yes
9–52 |
- |
60 |
| 10C |
M |
44 |
L40.0 |
PUVA |
Yes
12–33 |
- |
24 |
| 11C |
W |
49 |
L40.0 |
methotrexate |
Yes
25–38 |
- |
32 |
3. Current Insights
With the onset of the COVID-19 pandemic, there were alarming case reports of exacerbation of psoriasis after SARS-CoV-2 infection in patients not being treated with biological therapy
[5][6][7].
The impact of SARS-CoV-2 infection on biologically treated patients with moderate to severe psoriasis is currently unclear. The primary immune response is satisfactory and leads to a decrease in viral load. Unfortunately, for reasons that remain unclear, the secondary immune response (the so-called “cytokine storm”) may be excessive and may be a factor leading to tissue integrity disorders, respiratory failure, or exacerbation of skin lesions. We cannot exclude the notion that biologics may reduce the production of pro-inflammatory cytokines, thereby reducing the negative impact of COVID-19 on the symptoms of psoriasis
[5][6][7][8][9][10].
The presented work documents that all patients undergoing biological treatment had a mild course of COVID-19 and did not require hospitalization. Earlier studies also confirmed that the use of biological drugs by patients with psoriasis was associated with a much lower risk of hospitalization, although other factors, such as a patient’s age or comorbidities, should not be overlooked
[11][12][13]. The current Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) guidelines recommend that biological treatments for psoriasis and psoriatic arthritis should not be discontinued or reduced during the pandemic, or when SARS-COV-2 virus infection is diagnosed. Research indicates that inappropriate treatment modification or discontinuation of treatment may worsen symptoms and reduce the response to re-treatment
[14][15].
There are no conclusive data on the safety of initiating biological therapy in patients with psoriasis. In addition, a joint decision by a patient and their physician on the initiation of treatment is recommended, given that delayed treatment of psoriasis can seriously affect a patient’s physical and mental health
[14][15][16].
The observed control group reacted in a similar manner to SARS-CoV-2 infection as patients on biological treatment. Specifically, no severe cases of COVID-19 and no cases requiring hospitalization were noted in either group. All patients were only symptomatically treated and did not require systemic steroids, heparin, or oxygen therapy. The patients only used antipyretic drugs.
None of our patients modified or suspended psoriasis treatment during SARS-Co-V-2 infection, and this excludes any additional impact on the course of psoriasis during COVID-19. This is different from other observations in which patients themselves decided to change or suspend such treatment
[17]. It should be emphasized that, in the studied group of patients, a smaller than expected effect was observed in several patients after one year of biological treatment, which was often due to poor adherence (limitations related to the COVID-19 pandemic). It seems that completely uncontrolled psoriasis in individual patients could lead to instability during SARS-Co-V-19 infection.
It can be assumed that the transient exacerbations of psoriasis are associated with viral infection as a non-specific factor, which is similar to what is often observed with other infections in patients with psoriasis. However, due to the small study group, it was difficult to assess the impact of drugs on the course of SARS-CoV-2 infection. This is a significant limitation of this study. However, a similar observational study in patients with atopic dermatitis and immunosuppressive therapy also found no effect on the severity of COVID-19
[18]. The possible negative effects of this type of therapy should be emphasized. In the case of biological treatment, there are indications of an increased risk of infection in adult psoriasis patients treated with biological drugs. An analysis of data from 11,466 adults with psoriasis in the Psoriasis Longitudinal Assessment and Registry revealed an increased risk of serious infections with adalimumab and infliximab compared with and without non-biological systemic therapy. The rates of serious infections among patients treated with infliximab, adalimumab, etanercept, and ustekinumab were 2.49, 1.97, 1.47, and 0.83 per 100 patient-years, respectively
[19]. No similar infections were recorded in the follow-up.