Biological treatment is an important and effective therapy for psoriasis. During the COVID-19 pandemic, it remains unclear whether this type of therapy affects the course of SARS-CoV-2 infection. The aim of the study was to observe patients with psoriasis undergoing biological or other systemic treatment in relation to the impact of SARS-CoV-2 infection on the course of psoriasis and the COVID-19 disease itself. Materials and methods: A one-year observational study included 57 patients with diagnosed psoriasis who qualified for biological treatment and a group of 68 similar patients who were administered a different systemic treatment. Patients were analyzed monthly for psoriasis (including Psoriasis Area Severity Index (PASI) assessment) and constantly for SARS-CoV-2 infection (telephone contact). Cases of COVID-19 were confirmed by Polymerase Chain Reaction (PCR) at the study center. Results: SARS-CoV-2 infection was confirmed by a positive Real Time Polymerase Chain Reaction (RT-PCR) test in eight patients (14.0%) with psoriasis on biological therapy. None of the cases in this group required hospitalization for COVID-19. Similar data were obtained in the control group. Specifically, 11 (16%) patients were confirmed to be infected with SARS-CoV-2. These results were statistically comparable (p > 0.05). In the group of patients undergoing biological treatment, six (75%) of eight patients developed an exacerbation of psoriasis during SARS-CoV-2 infection, and similar results were noted in the control group, with eight (72%) patients experiencing an exacerbation of psoriasis. Conclusions: Patients with psoriasis who were administered biological treatment or other systemic therapy may experience a mild course of SARS-CoV-2 infection but might also experience a temporary exacerbation of skin lesions.
- psoriasis
- psoriatic arthritis
- COVID-19
- biological drugs
1. Introduction
2. Analysis on Results
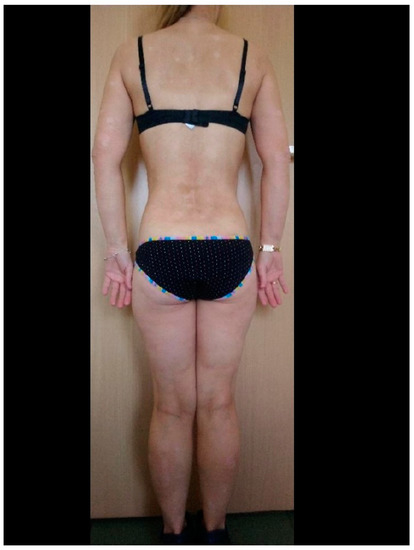
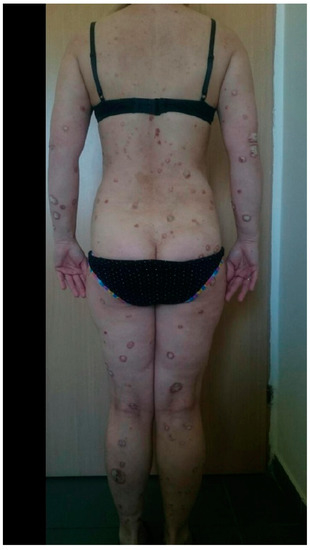
| N S/C |
Sex | Age | Type of Psoriasis According to ICD-10 | Drug Name | Exacerbation of Psoriasis PASI Score before vs. after |
Concomitant Diseases | Time of Exacerbation (days) |
|---|---|---|---|---|---|---|---|
| 1S | M | 72 | L40.0 | adalimumab | No 32–28 |
Arterial hypertension |
- |
| 2S | M | 60 | L40.0 | secukinumab | No 29–31 |
- | - |
| 3S | W | 50 | L40.0 | ustekinumab | Yes 4–64 |
Obesity | 12 |
| 4S | M | 44 | L40.0 | ustekinumab | Yes 23–51 |
- | 24 |
| 5S | W | 43 | L40.5 | adalimumab | Yes 18–33 |
- | 28 |
| 6S | M | 50 | L40.0 | risankizumab | Yes 4–31 |
Arterial hypertension |
28 |
| 7S | W | 52 | L40.0 | ustekinumab | Yes 8–44 |
- | 21 |
| 8S | W | 52 | L40.0 | adalimumab | Yes 15–25 |
- | 36 |
| 1C | M | 51 | L40.5 | cyclosporin | No 17–15 |
- | - |
| 2C | M | 42 | L40.5 | PUVA | Yes 32–46 |
Arterial hypertension |
24 |
| 3C | M | 45 | L40.0 | cyclosporin | Yes 28–39 |
- | 38 |
| 4C | W | 62 | L40.0 | PUVA | Yes 12–54 |
- | 42 |
| 5C | W | 55 | L40.0 | cyclosporin | No 19–42 |
- | - |
| 6C | M | 54 | L40.5 | cyclosporin | Yes 33–63 |
Arterial hypertension |
32 |
| 7C | M | 46 | L40.0 | methotrexate | No 8–7 |
- | - |
| 8C | W | 69 | L40.0 | methotrexate | Yes 5–26 |
Diabetes Arterial hypertension |
25 |
| 9C | M | 38 | L40.0 | cyclosporin | Yes 9–52 |
- | 60 |
| 10C | M | 44 | L40.0 | PUVA | Yes 12–33 |
- | 24 |
| 11C | W | 49 | L40.0 | methotrexate | Yes 25–38 |
- | 32 |
