
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Damien Gaboury | + 1887 word(s) | 1887 | 2021-08-25 11:18:47 |
Video Upload Options
Carbonaceous organic matter occurs under various phases and forms, where its fine characterization is mostly restricted to petroleum and coal geology. As a consequence, few studies have integrated the complete link between various forms of organic matter and metals to decipher hydrothermal ore concentrating processes. The study of Dill et al., integrating the concentration of sulfides and oxides with the interaction of silicates and organic matters, is an example of the next step to reach for defining the complex role of organic matter for the formation of orogenic gold deposits.
1. Introduction
For at least 6000 years, the use of gold marked a change for humanity: the use of metals. Gold was the first metal used because it occurs in a native form. Gold is insoluble under surface conditions and non-oxidable, so its physical properties are conserved. Presenting a shining yellowish color like that of the sun and exhibiting extreme malleability, gold was first used for ornamental purposes and later as a medium of exchange and coinage. The first exploitations were from rivers or dried riverbeds, where gold was physically concentrated as nuggets. Later, gold was extracted from quartz vein outcroppings at surfaces. Both types of gold extraction are still in use today.
Humans have always had a fascination and irrational relationship with gold. Gold was and is still a physical means for conserving values (e.g., [1]). Consequently, wars, invasions, colonizations, and territorial conquests (gold rushes) were established and driven. Gold, as has any other substance, positively and negatively impacted human development. Artisanal gold extractions are still providing revenue for 15–20 million persons worldwide [2], whereas hundreds of mines are producing gold commercially in more than 42 countries ( www.gold.org (accessed on 21 March 2021)).
In the inorganic world of metals, minerals, and rocks, consideration of the roles of organic matter in accumulating, solubilizing, and precipitating gold in lodes was not a natural way of thinking for geologists. At first, it may appear paradoxical that the ultimate noble metal requires organic matter for concentration. In this contribution, I address recent advances regarding the role of carbon-rich organic matter in forming rich and large gold deposits in three stages: (1) the source stage, when gold in seawater accumulates in organic-rich sediments; (2) the mobilization stage, when gold is solubilized by hydrocarbon-metal complexes and colloidal nanoparticles for hydrothermal transport along faults; and (3) the precipitation stage. It is demonstrated that unusual CO2-rich, H2O-poor fluids, documented for some of the largest and richest orogenic gold deposits, are the result of chemical reactions involving hydrocarbon degradation, hence demonstrating the fundamental role of carbonaceous organic matter.
2. Fluid Composition and Generation
The fluid composition of orogenic gold deposits was estimated from the study of fluid inclusions for more than 70 years (e.g., [3][4]). Hundreds of studies have detailed the mineralizing fluids from worldwide examples covering all ages (e.g., [5]). The fluids are aqueous with low salinity (<5 wt% NaCl equiv.), ubiquitous CO2, and variable contents of N2, CH4, and, in some cases, H2, C2H6, H2S, He, and Ar. Thermodynamic calculations have demonstrated that metamorphic dehydration of seafloor rocks is a viable mechanism for producing abundant aqueous-carbonic and low-salinity fluids. Elmer et al. [6] and Phillip and Powell [7] demonstrated that seafloor rocks, hydrated initially by hydrothermal seawater convection cells at oceanic ridges [8], release fluids at the metamorphic transition of greenschist to amphibolite, mostly when chlorite is converted to amphibole. Metamorphic fluids have a more diverse volatile composition than other fluids, such as seawater, magmatic fluids, or meteoric fluids, because they are generated by devolatilization of lithologies, where organic compounds in sedimentary rocks contribute to C-O-H-S-N contents [9].
Of particular interest, CO2-rich and H2O-poor fluid inclusions have been documented from some world-class gold districts and deposits, such as those at the Red Lake [10], Ashanti [11] and Tarkwa goldfields [12], and the Detour Gold and Wona deposits [9]. Fluids for these deposits also contain CH4, N2, and C2H6. The origins of these fluids are still debated (e.g., [3][9]).
For the Paleozoic Ashanti gold belt, Western Africa, Goldfarb et al. [13] suggested that devolatilization of abundant carbonaceous schists and cherts could lead to a variety of carbon-bearing molecular components within metamorphic C-O-H-S fluids bearing gold. Such a sedimentary source is confirmed by the compositions of stable carbon isotopic mixture in quartz-hosted, CO2-rich fluid inclusions [14]. These unusual fluids are thus likely derived from the metamorphism of carbonaceous-rich sedimentary rocks. Nonetheless, these fluids are associated with either very high-grade or very large gold deposits, suggesting that CO2-rich and H2O-poor fluids have unrecognized potential for forming exceptional orogenic gold deposits.
3. Sources of Gold
The sources of gold for orogenic deposits have been reviewed by numerous authors (e.g., [15][16][17]). Gold can be sourced from intrusion degassing (e.g., [18]) and oceanic basalt devolatilization (e.g., [19][20][21]). However, carbonaceous- and pyrite-rich sedimentary rocks, commonly referred to as black shales, are considered one of the most important sources (e.g., [15][22][23][24][25][26][27][28]).
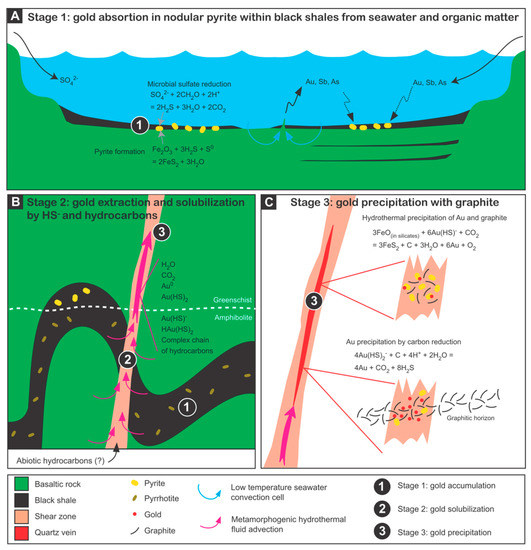
Gold and other trace metals occurring in sedimentary pyrite can be liberated by recrystallization and hydrothermal replacement processes occurring under metamorphic conditions, corresponding to the pyrite–pyrrhotite transition [23][24][29][30][31]. Gold concentrations in nodular pyrite average 0.09 ppm [32] but can reach 10 ppm Au in orogenic gold districts (e.g., [33][34][35]). Considering that nodular pyrite can constitute up to 20% of black shales and that black shales are very extensive marine sediments, these rocks may constitute a significant volume for providing gold. In addition, because pyrrhotite (Fe1-xS) has a lower S content (37.67% vs. 53.45%) than pyrite (FeS2), the conversion can also provide S in solution for solubilizing gold [36]. Gaboury [15] stated that, in addition to gold, fluids and ligands (HS-) can all be sourced from the metamorphism of black shales and associated rocks under amphibolite conditions.
Using a solid-probe mass spectrometer system [37], Gaboury [9] documented that ethane (C2H6) is present in fluid inclusions from orogenic gold deposits, ranging in age from ~2800 Ma to ~100 Ma. Ethane is sourced from thermally degraded organic matter because the values of CH4/(C2H6 + C3H8), expressed as C1/C2+ in hydrothermal fluids, are lower than 100 [38]. Consequently, ethane provides a reliable tracer for the involvement of carbonaceous and pyritic shales at depth in the formation of gold deposits [9].
The ultimate support for a sedimentary gold source model is provided by the link between gold dissolved in oceans and the temporal distribution of orogenic gold deposits. This was first proposed by Tomkins [39] and later documented by Large et al. [32] by using gold concentrations in primary pyrites from black shales. Oxidizing seawater conditions are favorable for gathering gold in nodular sedimentary pyrite in black shales [32]. The lack of major orogenic gold deposits from the middle to late Proterozoic (~1800 to 800 Ma—the boring period) is interpreted as being related to low levels of Au in the oceans [32]. During this period, the deep oceans were anoxic and sulfidic [40], hence limiting the bacterial reduction of sulfate and the incorporation of gold in primary pyrite [15]. The occurrence of orogenic gold deposits in Neoproterozoic time, such as those in Sudan, which also contain ethane [25], coincides with the reappearance of oxygenic conditions in the oceans [32][41].
4. The Fundamental Involvement of Organic Matter
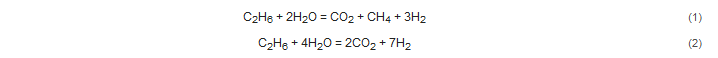
References
- Rickard, D.; Mussmann, M.; Steadman, J.A. Sedimentary sulfide. Elements 2017, 13, 117–122.
- Reuters Staff. Available online: https://www.reuters.com/article/us-gold-mining-artisanal-explainer-idUSKBN1ZE0YU (accessed on 20 March 2021).
- Ridley, J.R.; Diamond, L.W. Fluid chemistry of orogenic lode gold deposits and implications for genetic models. Rev. Econ. Geol. 2000, 13, 146–162.
- Yardley, B.W.D.; Bodnar, R.J. Fluids in the continental crust. Geochem. Perspect. 2014, 3, 1–127.
- Prokofiev, V.Y.; Naumov, V.B. Physicochemical parameters and geochemical features of ore-forming fluids for orogenic gold deposits throughout geological time. Minerals 2020, 10, 50.
- Elmer, F.L.; White, R.W.; Powell, R. Devolatilization of metabasic rocks during greenschist–amphibolite facies metamorphism. J. Metam. Geol. 2006, 24, 497–513.
- Phillips, G.N.; Powell, R. Formation of gold deposits: A metamorphic devolatilization model. J. Metamorph. Geol. 2010, 28, 689–718.
- Gretchen, L.; Früh-Green, G.L. Fluids in metamorphism: Alteration of the oceanic lithosphere and implications for seafloor processes. Elements 2010, 6, 173–178.
- Gaboury, D. Does gold in orogenic deposits come from pyrite in deeply buried carbon-rich sediments?: Insight from volatiles in fluid inclusions. Geology 2013, 41, 1207–1210.
- Chi, G.; Dubé, B.; Williamson, K.; Williams-Jones, A.E. Formation of the Campbell-Red Lake gold deposit by H2O-poor, CO2-dominated fluids. Miner. Depos. 2006, 40, 726–741.
- Mumm, A.S.; Oberthür, T.; Vetter, U.; Blenkinsop, T.G. High CO2 content of fluid inclusions in gold mineralisations in the Ashanti Belt, Ghana: A new category of ore forming fluids? Miner. Depos. 1997, 32, 107–118.
- Klemd, R.; Hirdes, W. Origin of an unusual fluid composition in Early Proterozoic Palaeoplacer and lode-gold deposits in Birimian greenstone terranes of West Africa. S. Afr. J. Geol. 1997, 100, 405–414.
- Goldfarb, R.J.; André-Mayer, A.-S.; Jowitt, S.M.; Mudd, G.M. West Africa: The World’s premier paleoproterozoic gold province. Econ. Geol. 2017, 112, 123–143.
- Lüders, V.; Klemd, R.; Oberthür, T.; Plessen, B. Different carbon reservoirs of auriferous fluids in African Archean and Proterozoic gold deposits? Constraints from stable carbon isotopic compositions of quartz-hosted CO2-rich fluid inclusions. Miner. Depos. 2015, 50, 449–454.
- Gaboury, D. Parameters for the formation of orogenic gold deposits. App. Earth Sci. 2019, 128, 124–133.
- Goldfarb, R.J.; Groves, D.I. Orogenic gold: Common vs evolving fluid and metal sources through time. Lithos 2015, 223, 2–26.
- Frimmel, H.E. Earth’s continental crustal gold endowment. Earth Planet. Sci. Lett. 2008, 267, 45–55.
- Sillitoe, R.H.; Thompson, J.F.H. Intrusion-related vein gold deposits: Types, tectono-magmatic settings and difficulties of distinction from orogenic gold deposits. Res. Geol. 1998, 48, 237–250.
- Pitcairn, I.K.; Craw, D.; Teagle, D.A.H. Metabasalts as sources of metals in orogenic gold deposits. Miner Depos. 2015, 50, 373–390.
- Augustin, J.; Gaboury, D. Paleoproterozoic plume-related basaltic rocks in the Mana gold district in western Burkina Faso, West Africa: Implications for exploration and the source of gold in orogenic deposits. Afr. J. Earth Sci. 2017, 129, 17–30.
- Patten, C.G.C.; Pitcairn, I.K.; Molnár, F.; Kolb, J.; Beaudoin, G.; Guillemette, C.; Peillod, A. Gold mobilization during metamorphic devolatilization of Archean and Paleoproterozoic metavolcanic rocks. Geology 2020.
- Tomkins, A.G. On the source of orogenic gold. Geology 2013, 41, 1255–1256.
- Pitcairn, I.K.; Olivo, G.R.; Teagle, D.A.H.; Craw, D. Sulfide evolution during prograde metamorphism of the Otago and Alpine Schists, New Zealand. Can. Miner. 2010, 48, 1267–1295.
- Large, R.R.; Bull, S.W.; Maslennikov, V.V. A carbonaceous sedimentary source-rock model for Carlin-type and orogenic gold deposits. Econ. Geol. 2011, 106, 331–358.
- Gaboury, D.; Nabil, H.; Ennaciri, A.; Maacha, L. Structural setting and fluid composition of gold mineralization along the central segment of the Keraf suture, Neoproterozoic Nubian Shield, Sudan: Implications for the source of gold. Int. Geol. Rev. 2020.
- Gaboury, D.; Mackezie, D.; Craw, D. Fluid volatile composition associated with orogenic gold mineralization, Otago Schist, New Zealand: Implications of H2 and C2H6 for fluid evolution and gold source. Ore Geol. Rev. 2021, 133, 104086.
- Large, R.; Thomas, H.; Craw, D.; Henne, A.; Henderson, S. Diagenetic pyrite as a source for metals in orogenic gold deposits, Otago Schist, New Zealand. N. Z. J. Geol. Geophys. 2012, 55, 137–149.
- Pitcairn, I.K.; Leventis, N.; Beaudoin, G.; Faure, S.; Guilmette, C.; Dubé, B. A metasedimentary source of gold in Archean orogenic gold deposits. Geology 2021.
- Finch, E.G.; Tomkins, A.G. Pyrite-pyrrhotite stability in a metamorphic aureole: Implications for orogenic gold genesis. Econ. Geol. 2017, 112, 661–674.
- Zhong, R.; Brugger, J.; Tomkins, A.G.; Chen, Y.; Li, W. Fate of gold and base metals during metamorphic devolatilization of a pelite. Geochim. Cosmochim. Acta 2015, 171, 338–352.
- Wu, Y.-F.; Evans, K.; Fisher, L.A.; Zhou, M.-F.; Hu, S.-Y.; Fougerouse, D.; Large, R.R.; Li, J.W. Distribution of trace elements between carbonaceous matter and sulfides in a sediment-hosted orogenic gold system. Geochim. Cosmochim. Acta 2020, 276, 345–362.
- Large, R.R.; Gregory, D.D.; Steadman, J.A.; Tomkins, A.G.; Lounejeva, E.; Danyushevsky, L.V.; Halpin, J.A.; Maslennikov, V.; Sack, P.J.; Mukkerjee, I.; et al. Gold in the oceans through time. Earth Planet. Sci. Lett. 2015, 428, 139–150.
- Augustin, J.; Gaboury, D. Multi-stage and multi-sourced fluid and gold in the formation of orogenic gold deposits in the world-class Mana district of Burkina Faso—Revealed by LA-ICP-MS analysis of pyrites and arsenopyrites. Ore Geol. Rev. 2019, 104, 495–521.
- Augustin, J.; Gaboury, D.; Crevier, M. The world-class Wona-Kona gold deposit, Burkina Faso. Ore Geol. Rev. 2016, 78, 667–672.
- Dubé, B.; Mercier-Langevin, P.; Ayer, J.; Pilote, J.-L.; Monecke, T. Gold Deposits of the World-Class Timmins-Porcupine Camp, Abitibi Greenstone Belt, Canada; Society of Economic Geologists Special Publication: Littleton, CO, USA, 2020; Volume 23, pp. 53–80.
- Tomkins, A.G. Windows of metamorphic sulfur liberation in the crust: Implications for gold deposit genesis. Geochim. Cosmochim. Acta 2010, 74, 3246–3259.
- Gaboury, D.; Keita, M.; Guha, J.; Lu, H.-Z. Mass spectrometric analysis of volatiles in fluid inclusions decrepitated by controlled heating under vacuum. Econ. Geol. 2008, 103, 439–443.
- Whiticar, M.J. Carbon and hydrogen isotope systematics of bacterial formation and oxidation of methane. Chem. Geol. 1999, 161, 291–314.
- Tomkins, A.G. A biogeochemical influence on the secular distribution of orogenic gold. Econ. Geol. 2013, 108, 193–197.
- Canfield, D. A new model for Proterozoic ocean chemistry. Nature 1998, 396, 450–453.
- Steadman, J.A.; Large, R.R.; Blamey, N.J.; Mukherjee, I.; Corkrey, R.; Danyushevsky, L.V.; Maslennikov, V.; Hollings, P.; Garven, G.; Brand, U.; et al. Evidence for elevated and variable atmospheric oxygen in the Precambrian. Precambr. Res. 2020, 343, 105722.
- Dill, H.G.; Kus, J.; Goldmann, S.; Suárez Ruiz, I.; Neumann, T.; Kaufhold, S. The physical-chemical regime of a sulfide-bearing semi-graphite mineral assemblage in metabasic rocks (SE Germany)—A multidisciplinary study of the missing link between impsonite and graphite. Inter. J. Coal Geol. 2019, 214, 103262.
- Groves, D.I.; Goldfarb, R.J.; Robert, F.; Hart, C.J. Gold deposits in metamorphic belts: Overview of current understanding, outstanding problems, future research, and exploration significance. Econ. Geol. 2003, 98, 1–29.
- Fuchs, S.; Schumann, D.; Martin, R.F.; Couillard, M. The extensive hydrocarbon-mediated fixation of hydrothermal gold in the Witwatersrand Basin, South Africa. Ore Geo. Rev. 2021, 138, 104313.
- Magoon, L.B.; Dow, W.G. The Petroleum System—From Source to Trap; American Association of Petroleum Geologists: Tulsa, OK, USA, 1994; Volume 60.
- Fuchs, S.; Williams-Jones, A.E.; Jackson, S.E.; Przybylowicz, W.J. Metal distribution in pyrobitumen of the Carbon Leader Reef, Witwatersrand Supergroup, South Africa: Evidence for liquid hydrocarbon ore fluids. Chem. Geol. 2016, 426, 45–59.
- Sherwood Lollar, B.; Westgate, T.; Ward, J.; Slater, G.F.; Lacrampe-Couloume, G. Abiogenic formation of alkanes in the Earth’s crust as a minor source for global hydrocarbon reservoirs. Nature 2002, 416, 522–524.
- Reeves, E.P.; Fiebig, J. Abiotic Synthesis of Methane and Organic Compounds in Earth’s Lithosphere. Elements 2020, 16, 25–31.
- Gaboury, D.; Genna, D.; Trottier, J.; Bouchard, M.; Augustin, J.; Malcolm, K. The Perron gold deposit, Archean Abitibi belt, Canada: Exceptionally high-grade mineralization related to higher gold-carrying capacity of hydrocarbon-rich fluids. Minerals 2021, in press.
- Phillips, G.N.; Powell, R. Origin of Witwatersrand gold: A metamorphic devolatilisation—Hydrothermal replacement model. Appl. Earth Sci. 2011, 120, 112–129.
- Goldfarb, R.J.; Taylor, R.D.; Collins, G.S.; Goryachev, N.A.; Orlandini, O.F. Phanerozoic continental growth and gold metallogeny of Asia. Gondwana Res. 2014, 25, 48–102.
- Savchuk, Y.S.; Asadulin, E.E.; Volkov, A.V.; Aristov, V.V. The Muruntau deposit: Geodynamic position and a variant of genetic model of the ore-forming system. Geol. Ore Depos. 2018, 60, 365–397.




