
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hugues Portier | + 1644 word(s) | 1644 | 2021-08-15 12:52:21 | | | |
| 2 | Dean Liu | Meta information modification | 1644 | 2021-09-28 06:03:04 | | |
Video Upload Options
As vascularization is an integral part of bone repair process, the analysis of the preventive and/or curative effects of physical exercise is currently very undeveloped. Angiogenesis–osteogenesis coupling may constitute a new way for understanding the role of physical activity, especially in fracturing or in the integration of bone biomaterials.
1. Introduction
Physical activity is widely recognized as a biotherapy by WHO (World Health Organization) in the fight and prevention of bone diseases such as osteoporosis in older subjects [1]. Physical exercise-induced improvement in bone mineral density (BMD) has also been observed in young patients treated with glucocorticoids [2] or suffering from excessive alcohol consumption [3], both of these substances affecting bone viability. Exercise reduces the risk of disabling fractures associated with many comorbidities, and whose repair is a major public health and economic issue. It is classically accepted that physical activity in this context helps maintaining or increasing bone mass through mechanical stimulation [4][5] and systemic factors’ (hormones, growth factors, etc.) production [6]. Relevant mechanisms may also include an increase in vascularization within bone tissue, such as reported in previous studies that tend to show a link between the processes of angiogenesis, vascularization foundation, and osteogenesis involved in bone tissue formation [7].
As physical activity improves vascularization, it also improves bone healing.
Thus, physical activity could promote the link between angiogenesis and osteogenesis through both the compressions generated and the growth factors produced. The effects of exercise on these parameters require, however, further investigations [7]. Finally, in the treatment of osteoporosis, particular attention should be paid to the effects of physical activity on bone defect or fracture repair processes that take place in the osteoporotic bone. Although vascularization is highly involved in the bone repair process, studies demonstrating the preventive/curative effects of physical activity on bone repair have not investigated the contribution of vascularization to these effects specifically. Such studies are probably hindered, at least in humans, by the complexity of the approach and visualization of the results. That being said, the role of physical activity on the vascularization and repair of skeletal muscles or cardiovascular tissues is no longer to be demonstrated, which provides sound grounds for hypotheses to explore in a bone repair context.
2. Physical Activities and Their Effects on Angiogenesis and Osteogenesis
It has been proven that moderate to vigorous physical activity improves health [5][8][9]. Increased attention is being paid to the effects of physical exercise on the skeleton and the whole body. Moderate to intense training or running exercise is known to improve body composition in humans [10]. In addition, intermittent (interval or fractionated) high-intensity training improves aerobic capacity and oxygen consumption in individuals [11]. Physical exercise is also characterized by its anti-inflammatory effect [12]. For bone, numerous studies have focused on the effects of different physical exercise modalities on bone quality [13] and on the bone parameters determining this quality: geometric parameters (cortical bone thickness, bone size, femoral neck geometry), architectural parameters (cortical porosity, trabeculae shape), and tissue properties (mineralization, cell density, osteocyte networks) [14]. Bone tissue is known for its sensitivity to mechanical stresses and its ability to support these [15]. Consequently, it is capable of adapting to these constraints in order to maintain its metabolic and phosphocalcic balance and constant remodeling [16]. The theories about bone response to stress include the production of multiple mechanical loads, along with the circulation of interstitial fluid in the lacuna-canalicular system [16]. To respond to mechanical strains and strengthen bone tissue, as a dynamic tissue, requires a combination of elements, including angiogenesis, which, besides, proved to be essential in bone repair and regeneration [17]. To date, literature data on the effects of exercise on angiogenic–osteogenic responses are scarce. Moreover, not to even mention the effects of physical activity, very little is known about the cellular and molecular mechanisms related to angiogenesis–osteogenesis coupling [18]. Yet, understanding these mechanisms should enable identifying new therapeutic approaches against bone diseases [19]. Indeed, the increased vascularization of bone tissue and the great impact induced by physical exercise on this tissue makes bone angiogenesis a target of choice for studies aiming at the prevention of bone loss diseases via non-pharmaceutical pathways. The works investigating the effect of physical exercise on bone vascular status have focused on different sets of angiogenic and vascular parameters including blood flow, endothelial function, arterial properties, etc.
Additionally, in rats, Dominguez et al. (2010) showed that the treadmill exercise-induced increase in bone blood flow and the NOS signaling pathway were associated with increased trabecular bone volume in the distal femur from young and old animals by 34% and 26%, respectively, compared to respective results obtained in sedentary animals [20]. In the same vein, a recent study conducted in ovariectomized rats demonstrated that, in addition to trabecular bone integrity, femoral angiogenesis was significantly improved in running rats. This was associated with improved femoral artery vasodilatation in running rats [21]. Taken together, these results confirm that bone angiogenesis is closely linked to exercise-induced osteogenesis and is essential for the bone gain sought by exercise, both in healthy [22], aging, or pathological contexts (osteoporosis) [20][21]. This coupling is generally explained by the increased NO-dependent vasodilation induced by physical training, associated with increased production and number of circulating endothelial progenitor cells (EPCs) in the bony vascular system in animals [23]. This vasodilatation promotes blood supply and angiogenesis, which, in turn, facilitates osteogenesis at different bone sites.
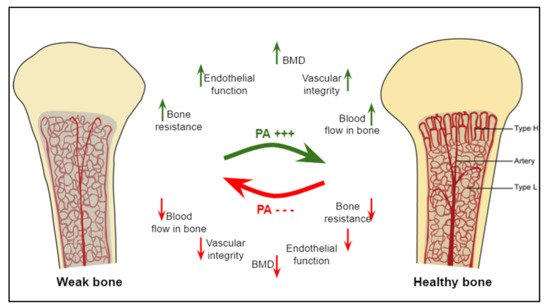
Overall, physical exercise is classically known as a bone anabolic agent [24]. Additionally, exercise is also known to improve bone angiogenic adaptations through the regulation of key angiogenic mediators (VEGF, FGF, etc.) [7]. These adaptations in the vascular system precede those occurring in bone in response to mechanical stimulations. Therefore, a better understanding of the angiogenic and osteogenic mechanisms induced by different types of physical exercise should allow the identification of new non-pharmacological strategies to prevent bone fragility ( Figure 1 ).
3. Vascularization and Bone Repair
Few studies have focused on the impact and evolution of vascularization in bone repair. Fractures are the most common large-organ, traumatic injuries to humans with, however, a very high regeneration capacity [26][27]. As for the development of bone, bone fracture healing is a complex multistep process. It starts with the formation of a hematoma around the fractured region of the bone and involves cytokine-secreting inflammatory cells for forming a fibrinous clot [17][28]. In the early callus, mesenchymal stem cells differentiate into chondrocytes, which further promote vascularization and bone formation through the secretion of several proteins (BMPs, MMP-13, alkaline phosphatase, VEGF, and placental growth factor). Chondrocytes in the callus also release anti-angiogenic factors, which limit blood vessel growth [29]. Additionally, the vascular network is essential for proper bone formation and functional bone restoration [17][18][30][31][32][33][34]. If blood flow is impaired, bone healing and repair may be delayed [35]. Indeed, it is admitted that the presence of blood vessels is critical for a successful bone regeneration [36][37]. The lack of blood supply induces local hypoxia, which may be maintained by subsequent inflammation [38]. The observed pO2 (partial pressure of oxygen) post-fracture is 1–3% [33]. This trauma-induced hypoxia causes endothelial cells (ECs) to upregulate bone morphogenic proteins (BMPs; i.e., BMP-2) to promote osteogenesis [17][34]. The hypoxia-induced transcription factor (HIF)-α pathway, activated in hypoxia, is considered a key mechanism for coupling bone growth to angiogenesis via increased expression of VEGF, the major vascular growth factor expressed by hypoxic osteoblasts [33]. In mice, where HIFα is overexpressed, the bones are highly vascularized [39]. In addition, the placental growth factor (PlGF, a VEGF homologue), which acts through the VEGF receptor, also appears to play a significant role in fracture healing [38]. VEGF stimulates the regrowth of blood vessels into the injury site, so that oxygen and nutrient levels return to normal values [33]. The general pattern for bone healing consists in a recruitment of osteoclasts in the early hypoxic phase, while revascularization progressively favors osteoblast function (proliferation, differentiation, and bone formation). Osteoblast precursors could also move into fractured bones concomitant with the invasion of the blood vessels [28]. Additionally, osteoblasts accumulate HIF-1a, leading to VEGF-A production, which further enhances angiocrine BMP production [29][34][39][40][41]. As for embryonic bone development, fibroblast growth factors (FGFs) are also required for bone repair. In Behr et al. ’s study (2011), mice with heterozygous FGF9 loss, neovascularization, and cortical repair were reduced, in part due to reduced VEGF-A, and after a combined administration of FGF9 and VEGF-A the defect was significantly reduced [42]. Further, Kigami et al. (2013) showed that FGF-2 enhanced angiogenesis in rat calvarial critical-sized bone defects [43]. Even if knowledge has yet to be widely developed, other molecular processes are also involved. For example, some works highlight the central roles of Notch signaling in bone endothelium and its regulation by blood flow, which is relevant for age-related bone loss and, potentially, for therapeutic approaches aiming at the maintenance or restoration of bone mass. Notch pathway genes are highly expressed in arterial ECs, which are exposed to high flow rates and shear stress [17][44]. Based on the principle that active blood supply is essential for callus formation during fracture healing and repair [31], methods to artificially increase blood flow during early steps of repair are used as treatment [29]. However, the relationship between angiogenesis and bone repair may be more nuanced. This suggests a more complex balance or interplay between pro- and anti-angiogenic factors in the healing process.
Recently, it has been shown that preventive moderate continuous running-exercise conditioning could improve the healing of non-critical-sized bone defects in male Wistar rats [45]. In another study, mice were put in cages that were supplied with running wheels after a cranial bone window. Spontaneous physical activity promoted angiogenesis during bone repair in this model over a time period of 21 days [46].
References
- Pinheiro, M.B.; Oliveira, J.; Bauman, A.; Fairhall, N.; Kwok, W.; Sherrington, C. Evidence on physical activity and osteoporosis prevention for people aged 65+ years: A systematic review to inform the WHO guidelines on physical activity and sedentary behaviour. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 1–53.
- Hofmann, C.; Girschick, H.; Lapa, C.; Semler, O.; Jakob, F. Frakturen und Knochendichte im Kindesalter. Z. Rheumatol. 2019, 78, 636–644.
- Sung, D.J.; Singh, H.; Oh, S.-B.; Kim, S. Bone-Loading Physical Activity and Alcohol Intake but not BMI Affect Areal Bone Mineral Density in Young College-Aged Korean Women: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2019, 16, 5063.
- Sañudo, B.; de Hoyo, M.; del Pozo-Cruz, J.; Carrasco, L.; Cruz, B.D.P.; Tejero, S.; Firth, E. A systematic review of the exercise effect on bone health: The importance of assessing mechanical loading in perimenopausal and postmenopausal women. Menopause 2017, 24, 1208–1216.
- Troy, K.L.; Mancuso, M.E.; Butler, T.A.; Johnson, J.E. Exercise Early and Often: Effects of Physical Activity and Exercise on Women’s Bone Health. Int. J. Environ. Res. Public Health 2018, 15, 878.
- Goolsby, M.A.; Boniquit, N. Bone Health in Athletes. Sports Health 2017, 9, 108–117.
- Tong, X.; Chen, X.; Zhang, S.; Huang, M.; Shen, X.; Xu, J.; Zou, J. The Effect of Exercise on the Prevention of Osteoporosis and Bone Angiogenesis. BioMed Res. Int. 2019, 2019, 1–8.
- Dibben, G.; Dalal, H.M.; Taylor, R.S.; Doherty, P.; Tang, L.; Hillsdon, M. Cardiac rehabilitation and physical activity: Systematic review and meta-analysis. Heart 2018, 104, 1394–1402.
- Hallal, P.C.; Victora, C.; Azevedo, M.R.; Wells, J. Adolescent physical activity and health. Sports Med. 2006, 36, 1019–1030.
- Wewege, M.; Berg, R.V.D.; Ward, R.E.; Keech, A. The effects of high-intensity interval training vs. moderate-intensity continuous training on body composition in overweight and obese adults: A systematic review and meta-analysis. Obes. Rev. 2017, 18, 635–646.
- Ojeda, A.H.; Maliqueo, S.G.; Guerra, M.C.; Barahona-Fuentes, G.D.F.; Villanueva, T.R.; Serrano, P.C. Efectos de un entrenamiento intervalado de alta intensidad en la capacidad aeróbica de adolescentes. Rev. Méd. Chile 2017, 145, 972–979.
- Neto, J.R.; Lira, F.S.; Oyama, L.M.; Zanchi, N.; Yamashita, A.S.; Batista, M.L.; Nascimento, C.M.O.D.; Seelaender, M. Exhaustive exercise causes an anti-inflammatory effect in skeletal muscle and a pro-inflammatory effect in adipose tissue in rats. Graefe Arch. Clin. Exp. Ophthalmol. 2009, 106, 697–704.
- Portier, H.; Benaitreau, D.; Pallu, S. Does Physical Exercise Always Improve Bone Quality in Rats? Life 2020, 10, 217.
- Fonseca, H.; Gonçalves, D.; Coriolano, H.-J.A.; Duarte, J.A. Bone Quality: The Determinants of Bone Strength and Fragility. Sports Med. 2014, 44, 37–53.
- Bonewald, L.F. Osteocytes as Dynamic Multifunctional Cells. Ann. N. Y. Acad. Sci. 2007, 1116, 281–290.
- Bonewald, L.F.; Johnson, M.L. Osteocytes, mechanosensing and Wnt signaling. Bone 2008, 42, 606–615.
- Sivan, U.; De Angelis, J.; Kusumbe, A.P. Role of angiocrine signals in bone development, homeostasis and disease. Open Biol. 2019, 9, 190144.
- Kusumbe, A.P.; Ramasamy, S.; Adams, R.H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nat. Cell Biol. 2014, 507, 323–328.
- Ramasamy, S.; Kusumbe, A.P.; Wang, L.; Adams, R.H. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nat. Cell Biol. 2014, 507, 376–380.
- Dominguez, J.M.; Prisby, R.D.; Muller-Delp, J.; Allen, M.R.; Delp, M.D. Increased nitric oxide-mediated vasodilation of bone resistance arteries is associated with increased trabecular bone volume after endurance training in rats. Bone 2010, 46, 813–819.
- Li, W.; Zhang, Y.; Xu, X.; Wang, K.; Ding, W. Relationship between osteogenesis and angiogenesis in ovariectomized osteoporotic rats after exercise training. Int. J. Clin. Exp. Pathol. 2017, 10, 11438–11449.
- Yao, Z.; Lafage-Proust, M.-H.; Plouët, J.; Bloomfield, S.; Alexandre, C.; Vico, L. Increase of Both Angiogenesis and Bone Mass in Response to Exercise Depends on VEGF. J. Bone Miner. Res. 2004, 19, 1471–1480.
- Laufs, U.; Werner, N.; Link, A.; Endres, M.; Wassmann, S.; Jürgens, K.; Miche, E.; Böhm, M.; Miche, E.; Nickenig, G. Physical Training Increases Endothelial Progenitor Cells, Inhibits Neointima Formation, and Enhances Angiogenesis. Circulation 2004, 109, 220–226.
- Prisby, R.D. Mechanical, hormonal and metabolic influences on blood vessels, blood flow and bone. J. Endocrinol. 2017, 235, R77–R100.
- Hendriks, M.; Ramasamy, S.K. Blood Vessels and Vascular Niches in Bone Development and Physiological Remodeling. Front. Cell Dev. Biol. 2020, 8, 602278.
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture healing: Mechanisms and interventions. Nat. Rev. Rheumatol. 2015, 11, 45–54.
- Watson, E.; Adams, R.H. Biology of Bone: The Vasculature of the Skeletal System. Cold Spring Harb. Perspect. Med. 2017, 8, a031559.
- Gerstenfeld, L.C.; Cullinane, D.M.; Barnes, G.L.; Graves, D.T.; Einhorn, T. Fracture healing as a post-natal developmental process: Molecular, spatial, and temporal aspects of its regulation. J. Cell. Biochem. 2003, 88, 873–884.
- Bahney, C.S.; Hu, D.P.; Emiclau, T.; Marcucio, R. The Multifaceted Role of the Vasculature in Endochondral Fracture Repair. Front. Endocrinol. 2015, 6, 4.
- Filipowska, J.; Tomaszewski, K.; Niedźwiedzki, Ł.; Walocha, J.A.; Niedźwiedzki, T. The role of vasculature in bone development, regeneration and proper systemic functioning. Angiogenesis 2017, 20, 291–302.
- Trueta, J. Blood supply and the rate of healing of tibial fractures. Clin. Orthop. Relat. Res. 1974, 105, 11–26.
- Trueta, J. The normal vascular anatomy of the femoral head in adult man. Clin. Orthop. Relat. Res. 1997, 334, 6–14.
- Marenzana, M.; Arnett, T.R. The Key Role of the Blood Supply to Bone. Bone Res. 2013, 1, 203–215.
- Hankenson, K.; Gagne, K.; Shaughnessy, M. Extracellular signaling molecules to promote fracture healing and bone regeneration. Adv. Drug Deliv. Rev. 2015, 94, 3–12.
- Wan, C.; Gilbert, S.R.; Wang, Y.; Cao, X.; Shen, X.; Ramaswamy, G.; Jacobsen, K.A.; Alaql, Z.S.; Eberhardt, A.W.; Gerstenfeld, L.C.; et al. Activation of the hypoxia-inducible factor-1 pathway accelerates bone regeneration. Proc. Natl. Acad. Sci. USA 2008, 105, 686–691.
- Sathy, B.N.; Mony, U.; Menon, D.; Baskaran, V.; Mikos, A.G.; Nair, S. Bone Tissue Engineering with Multilayered Scaffolds—Part I: An Approach for Vascularizing Engineered Constructs In Vivo. Tissue Eng. Part A 2015, 21, 2480–2494.
- Caliaperoumal, G.; Souyet, M.; Bensidhoum, M.; Petite, H.; Anagnostou, F. Type 2 diabetes impairs angiogenesis and osteogenesis in calvarial defects: MicroCT study in ZDF rats. Bone 2018, 112, 161–172.
- Maes, C.; Carmeliet, G.; Schipani, E. Hypoxia-driven pathways in bone development, regeneration and disease. Nat. Rev. Rheumatol. 2012, 8, 358–366.
- Wang, Y.; Wan, C.; Deng, L.; Liu, X.; Cao, X.; Gilbert, S.R.; Bouxsein, M.L.; Faugere, M.-C.; Guldberg, R.E.; Gerstenfeld, L.C.; et al. The hypoxia-inducible factor α pathway couples angiogenesis to osteogenesis during skeletal development. J. Clin. Investig. 2007, 117, 1616–1626.
- Brandi, M.L.; Collin-Osdoby, P. Vascular Biology and the Skeleton. J. Bone Miner. Res. 2006, 21, 183–192.
- Maes, C.; Kobayashi, T.; Selig, M.K.; Torrekens, S.; Roth, S.I.; Mackem, S.; Carmeliet, G.; Kronenberg, H.M. Osteoblast Precursors, but Not Mature Osteoblasts, Move into Developing and Fractured Bones along with Invading Blood Vessels. Dev. Cell 2010, 19, 329–344.
- Behr, B.; Sorkin, M.; Manu, A.; Lehnhardt, M.; Longaker, M.T.; Quarto, N. Fgf-18 Is Required for Osteogenesis But Not Angiogenesis During Long Bone Repair. Tissue Eng. Part A 2011, 17, 2061–2069.
- Kigami, R.; Sato, S.; Tsuchiya, N.; Yoshimakai, T.; Arai, Y.; Ito, K. FGF-2 Angiogenesis in Bone Regeneration Within Critical-Sized Bone Defects in Rat Calvaria. Implant. Dent. 2013, 22, 422–427.
- Laufs, U.; Urhausen, A.; Werner, N.; Scharhag, J.; Heitz, A.; Kissner, G.; Böhm, M.; Kindermann, W.; Nickenig, G. Running exercise of different duration and intensity: Effect on endothelial progenitor cells in healthy subjects. Eur. J. Cardiovasc. Prev. Rehabil. 2005, 12, 407–414.
- Bourzac, C.; Bensidhoum, M.; Manassero, M.; Chappard, C.; Michoux, N.; Pallu, S.; Portier, H. Preventive Moderate Continuous Running-Exercise Conditioning Improves the Healing of Non-Critical Size Bone Defects in Male Wistar Rats: A Pilot Study Using µCT. Life 2020, 10, 308.
- Holstein, J.H.; Becker, S.C.; Fiedler, M.; Scheuer, C.; Garcia, P.; Histing, T.; Klein, M.; Pohlemann, T.; Menger, M.D. Exercise Enhances Angiogenesis during Bone Defect Healing in Mice. J. Orthop. Res. 2011, 29, 1086–1092.




