Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Federica Grillo | + 3218 word(s) | 3218 | 2021-08-25 08:49:18 | | | |
| 2 | Vivi Li | Meta information modification | 3218 | 2021-08-26 04:20:12 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Grillo, F. Rare Colorectal Carcinoma Histotypes. Encyclopedia. Available online: https://encyclopedia.pub/entry/13528 (accessed on 07 February 2026).
Grillo F. Rare Colorectal Carcinoma Histotypes. Encyclopedia. Available at: https://encyclopedia.pub/entry/13528. Accessed February 07, 2026.
Grillo, Federica. "Rare Colorectal Carcinoma Histotypes" Encyclopedia, https://encyclopedia.pub/entry/13528 (accessed February 07, 2026).
Grillo, F. (2021, August 25). Rare Colorectal Carcinoma Histotypes. In Encyclopedia. https://encyclopedia.pub/entry/13528
Grillo, Federica. "Rare Colorectal Carcinoma Histotypes." Encyclopedia. Web. 25 August, 2021.
Copy Citation
Several histopathological variants of colorectal carcinoma can be distinguished, some associated with specific molecular profiles. However, in routine practice, ninety/ninety-five percent of all large bowel tumors are diagnosed as conventional adenocarcinoma, even though they are a heterogeneous group including rare histotypes, which are often under-recognized. Indeed, colorectal cancer exhibits differences in incidence, location of tumor, pathogenesis, molecular pathways and outcome depending on histotype.
colorectal cancer histotypes
signet ring cell carcinoma
medullary carcinoma
lymphoepitelioma-like carcinoma
cribriform/comedo-type carcinoma
micropapillary carcinoma
clear cell carcinoma
hepatoid carcinoma
adenocarcinoma with osseous metaplasia
rhabdoid c
1. Introduction
Colorectal cancer (CRC) is the third most frequent malignant neoplasm worldwide. The CRC risk increases with age, as the majority of cases are diagnosed in patients with more than 50 years of age. CRCs exhibit biological differences in both pathogenesis and molecular pathways reflecting different incidences, sidedness and outcome [1][2]. Most CRC are located in the sigmoid colon/rectum, but the proportion of carcinomas in the right colon increases with age [3].
Three kinds of alterations are involved in CRC development: (1) chromosomal instability (CIN); (2) microsatellite instability (MSI); (3) CpG island methylator phenotype (CIMP). A different association between these pathogenetic alterations determines distinct molecular pathways: (i) traditional, (ii) alternative and (iii) serrated [1]:
- (i)
-
The traditional pathway is based on APC and KRAS mutations (by CIN alterations). These neoplasms usually involve the left colon.
- (ii)
-
The alternative pathway is characterized by a CIMP-low phenotype, predominant KRAS and occasional BRAF mutations, with no CIN. The prognosis of these CRC is aggressive.
- (iii)
-
The serrated pathway is characterized by BRAF mutations and epigenomic instability (CIMP-high). These lesions are located mainly in the right colon with MSI morphology (mucinous, medullary and tumours with intraepithelial lymphocytes) or MSS with a serrated morphology (eosinophilic cytoplasm, epithelial serration and tufts and vescicular basal nuclei) [1][3].
Tumor development through the traditional pathway is relatively slow (5–20 years), probably due to the fact that the initial events occur in the fully differentiated cells of the colonic crypt. APC mutations, generally, are detected in the cells of the upper crypt compartment according to the top-down morphogenetic model [4]. The causal events underlying the serrated pathway, however, may take place in the cells of the lower crypt compartment which are less differentiated and rapidly progressive [5]. This could explain the morphologic heterogeneity of tumors arising in the right colon, in older patients which are usually BRAF mutated. These CRCs are thought to develop rapidly and may in part explain interval cancers [6]. These new insights into the molecular pathogenesis of CRC have contributed to the distinction of right- and left-sided CRCs, identifying them as two distinct clinical, pathological and molecular entities [7]. This distinction is especially useful considering the impact which sidedness may have on treatment choice.
Several epithelial histopathological variants of CRC can be distinguished, some associated with specific molecular profiles. In routine practice, 90–95% of all large bowel tumors are diagnosed as classic adenocarcinoma, however this group is actually a heterogeneous population including rare histotypes which are often underdiagnosed but which may collectively reach up to 50% of CRCs in histologically classified series (Figure 1). Indeed, a frequent downfall in large studies is that CRCs are collected regardless of histotype with no importance being given to rare histotypes. The aim is to review the morphologic and molecular features of these rare histotypes which may be seen as pure/prevalent forms (Table 1) or as composite/mixed morphologically heterogeneous neoplasms (in which each separate morphological entity should be reported and quantified). The understanding of the morphological complexity of a group of tumors which are often all placed together in the CRC basket is especially important for non-pathology clinical colleagues or researchers who may not be aware or appreciate the subtleties of morphology (phenotype) with its possible molecular implications (genotype).
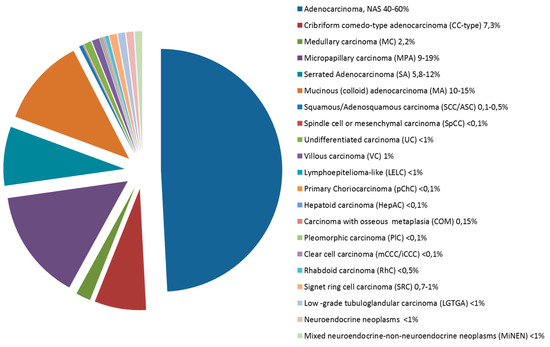
Figure 1. Pie chart showing the frequency of colorectal carcinomas by histologic type.
Table 1. Clinico-pathologic and immune-molecular characteristics of rare type colorectal carcinomas.
| Histotype | Mean Age | Site | Prognosis# | Main Diagnostic Criteria | Immunoprofile | Molecular Profile | |
|---|---|---|---|---|---|---|---|
| Serrated Adenocarcinoma (SA) | 67 | Right colon and rectum | Aggressive | Epithelial serrations +/− tufts; eosinophilic cytoplasm; vescicular nuclei | KRASmut BRAFmut MSI | ||
| Mucinous (colloid) adenocarcinoma (MA) | 60 | Right colon | Similar to conventional | Abundant extracellular mucin in more than 50% | MMRd, PDL1+ | KRASmut BRAFmut, MSI | |
| Signet ring cell carcinoma (SRC) | 65 | No site predilection | Aggressive | Signet ring cells in more than 50% | MMRd, PDL1+ | KRASmut BRAFmut, MSI | |
| Medullary carcinoma (MC) | 70 | Right colon | Favourable | Solid growth pattern with circumscribed tumor borders; tumor cells with a syncytial appearance; conspicuous intra and peri tumor lymphocytes. | MMRd, CDX-2−, CK20+, calretinin+ | MSI, BRAFmut | |
| Lymphoepitelioma-like (LELC) | 62 | No site predilection | Favourable | Poorly differentiated carcinoma with abundant intratumour infiltrating lymphocytes; presence of EBV | MMRd | EBV+ | |
| Cribriform comedo-type adenocarcinoma (CC-type) | 56 | No site predilection | Aggressive | Tightly packed neoplastic glands and cribriform architecture and large glands with central necrosis | CK20+, CDX-2+, MUC2+ | ||
| Micropapillary carcinoma (MPA) | 69 | Right colon and rectum | Aggressive | Clusters with lacunar space of more than 5 neoplastic cells; inverse polarity | Inverted MUC1, MUC2−, E-cadherin altered pattern | TP53mut, KRASmut, BRAFmut, CIN | |
| Low -grade tubuloglandular carcinoma (LGTGA) | 42 | No site predilection | Favourable | Tubular architecture composed of neoplastic glands with little atypia | MMRd (MLH1d) | MSI, KRASmut, IDH1mut | |
| Villous carcinoma (VC) | 66 | Left colon | Favourable | Villous architecture in >50% | KRASmut | ||
| Squamous/Adenosquamous carcinoma (SCC/ASC) | 60 | Right colon | Aggressive | Squamous differentiation either pure or composite with glandular component | p63+, CK5/6+ | ||
| Clear cell carcinoma | Mullerian-mCCC | 52 | Exclusively rectum | Favourable | Clear cells in more than 50%; endometriosis or pregnancy | CK20−, CK7+, CEA−, CA125+ | |
| Intestinal-iCCC | 61 | No site predilection | Aggressive | Clear cell in more than 50% | CK20+, CK7−, CEA+, CDX-2+) | KRASmut, MSI | |
| Hepatoid carcinoma (HepAC) | 50 | Rectum | Aggressive | Neoplastic cells with hepatoid appearence in solid, trabecular o pseudoacinar architectural patterns | AFP+ (also serum), Glypican-3+, CK18+, CK19+, CEA+, Hep Par1 + (40%) | ||
| Primary Choriocarcinoma (pChC) | 54 | Left colon | Aggressive | Syncytiotrophoblast-like cells | β-HCG (also serum) | ||
| Rhabdoid carcinoma (RhC) | 70 | Right colon | Aggressive | Rhabdoid cells >5% | CK20−, Vimentin+, CDX-2−, INI1−, CROCC reduction signals. | BRAFmut, MSI, CROCCmut | |
| Carcinoma with osseous metaplasia (COM) | 58 | Left colon | Similar to conventional | Presence of osseous metaplasia in a conventional adenocarcinoma | |||
| Spindle cell or mesenchymal carcinoma* (SpCC) | 70 | Left colon and Rectum | Aggressive | Biphasic carcinoma with a spindle-cell sarcomatoid component (cytokeratin +); may have giant cells | Vimentin+, CK+ (focal) | ||
| Undifferentiated carcinoma (UC) | 70 | No site predilection | Aggressive | Evidence of epithelial differentiation with minimal or without gland formation | CK+, absence of other differentiation markers | ||
* Pleomorphic carcinoma is considered within this type. # Prognosis is compared to conventional colorectal adenocarcinoma. MSI—microsatellite instability; MMRd—mismatch repair protein deficiency; CK—cytokeratins; EBV—Ebstein-Barr virus; CIN—Chromosomal instability.
2. Serrated Adenocarcinoma (SA)
2.1. Background
Following the first description of five CRCs histologically resembling serrated polyps by Jass and Smith [8], Mäkinen et al. reported twenty-seven CRCs associated with an adjacent serrated adenoma [9]. They noted that such cases exhibited distinctive clinical, histological and molecular features, suggesting that serrated adenocarcinomas (SAs) may be considered a distinct entity, probably representing an end-point of the serrated pathway. Several subsequent studies have confirmed the clinico-pathologic and molecular differences between SA and conventional CRC [10][11], recognizing SA as a distinct CRC subtype in the 2010 WHO classification. SA can be identified either by the presence of a residual serrated polyp or by its peculiar histologic characteristics, even when precursor lesions are no longer visible [10][12]. Considering this definition, SA accounts for 5.8–12% of all CRCs and up to 17% of proximal CRCs.
2.2. Clinical Presentation
2.3. Sidedness
Most SA are located in either the right colon (47–57%) or the rectum (15–29%).
2.4. Morphologic Diagnostic Criteria
The histologic criteria for SA diagnosis include: epithelial serrations and tufts, abundant eosinophilic or clear cytoplasm, vesicular basal nuclei with chromatin condensation around the nuclear envelope, easily distinguishable nuclei and preserved polarity, absence or less than 10% necrosis of the total surface area and, within mucinous areas, the presence of cell balls and papillary rods [12]. Importantly, serrations of SAs are composed of epithelium with or without basement membrane, but lack the fibro-vascular cores seen in non-serrated CRCs (Figure 2A). Three growth patterns have been described-serrated, mucinous and trabecular, the latter being characteristic of poorly differentiated SAs, which may be challenging to recognize.
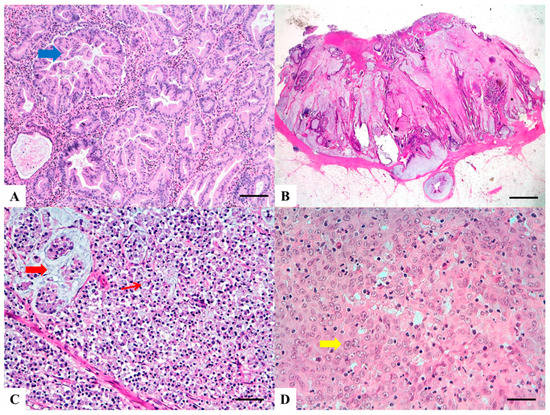
Figure 2. Haematoxylin and Eosin stained sections of rare type colorectal carcinomas. (A) Serrated Adenocarcinoma: epithelial serrations or tufts (thick blue arrow), abundant eosinophilic or clear cytoplasm, vesicular basal nuclei with preserved polarity. Scale bar 200 micron. (B) Mucinous Carcinoma: presence of extracellular mucin (>50%) associated with ribbons or tubular structures of neoplastic epithelium. Scale bar 2 mm. (C) Signet Ring Carcinoma: more than 50% of signet cells with infiltrative growth pattern (thin red arrow) or floating in large pools of mucin (thick red arrow). Scale bar 200 micron. (D) Medullary carcinoma: neoplastic cells with syncytial appearance (thick yellow arrow) and eosinophilic cytoplasm associated with abundant peritumoral and intratumoral lymphocytes. Scale bar 100 micron.
2.5. Molecular Alterations
Gene expression and methylome profiling analysis has highlighted a clear distinction between SAs and conventional CRCs [13][14][15], showing higher representation of morphogenesis-, hypoxia-, cytoskeleton- and vesicle transport-related functions in SA. Among the most relevant differentially expressed genes, hypoxia-inducible factor 1-alpha (HIF-1α), fascin 1, the anti-apoptotic gene hippocalcin and annexin A10, are specifically upregulated in SA and, therefore, have been proposed as immunohistochemical markers for SA diagnosis. KRAS and BRAF mutations are common in SA, being found in 33% and 45% of cases, respectively, and may contribute to stabilize HIF-1α [11]. BRAF mutation, in particular, is strongly associated with serrated morphology in CRCs. Up to 20% of SA harbor microsatellite instability (MSI).
2.6. Prognosis
Garcia-Solano et al. [10] demonstrated that SA patients more frequently presented lymph node metastases (52%) and they showed a less favorable prognosis compared to conventional CRCs, especially in the case of left-side SA. In addition, SAs are characterized by higher frequency of adverse histologic features at the invasive front, such as high-grade tumor budding and weak peritumoural lymphocytic infiltration [16]. Interestingly, Zhu et al. highlighted that high PD-L1 expression by SAs is frequent (25%) and associated with poor survival [17].
3. Mucinous (Colloid) Adenocarcinoma (MA)
3.1. Background
Primary mucinous (colloid) colorectal adenocarcinoma (MA), is defined by the presence of more than 50% of extracellular mucin component containing malignant epithelium, according to the WHO 2010 classification criteria [18]. It constitutes approximately 10–15% of all CRCs and it is associated with peculiar clinico-pathological and prognostic features if compared to conventional CRCs [19].
3.2. Clinical Presentation
The mean age of presentation is 60 years (range from 10 to 93 years) with low female gender prevalence. Symptoms do not differ from those of conventional CRC however MA is often diagnosed in advanced stage, it is generally of larger size and it is associated with frequent loco-regional lymph node involvement and peritoneal implants [20].
3.3. Sidedness
MA is more often localized to the right colon [21], including caecum, ascending colon and proximal transverse colon.
3.4. Morphologic Diagnostic Criteria
Histology shows abundant extracellular mucin associated with ribbons or tubular structures of neoplastic epithelium (Figure 2B). Single cells, including signet ring cells, may be found floating within the mucin or attached to the adjacent stromal wall. Mucinous morphological changes in post neoadjuvant treatment cancers must not be interpreted as MA [22]. Presence of tumor infiltrating lymphocytes (TILs) is frequent, may show a Crohn-like appearance and is frequently associated with MSI [21].
3.5. Molecular Genotype
MA is one of the histotypes associated with MSI [20], both in sporadic and in Lynch syndrome associated CRCs. In sporadic CRCs, MSI is due to epigenetic silencing of the promoter region of mismatch repair (MMR) genes (predominantly MLH1) by CpG island hypermethylation. Diversely, Lynch syndrome associated patients show germline mutational inactivation of genes encoding the MMR proteins MLH1, MSH2, MSH6 or PMS2 and MA has a 22–40% prevalence in this setting. Furthermore the high mutational load of MA MSI-H, with its high production of tumor specific neoantigens, represents a strong immunogenic factor which explains the presence of TILs. MA has a greater incidence (65%) of KRAS mutations, compared to other CRC sub-types without mucin production, and often shows BRAF mutation.
3.6. Prognosis
There is no definitive evidence regarding prognostic differences between MA compared to conventional CRC. Indeed, the Literature is a well of conflicting data about prognosis and overall survival; in this context sidedness may have an impact (poorer prognosis in rectal MA versus colonic MA) [21]. A recent study has shown that there is no difference in prognosis, adjusted for stage, between non mucinous conventional adenocarcinoma and adenocarcinomas with mucin production of any percentage [20] making the 50% cut off debatable.
4. Signet Ring Cell Carcinoma (SRC)
4.1. Background
4.2. Clinical Presentation
This subtype of CRC occurs in younger individuals compared to conventional CRC (range at diagnosis 48 to 70 years) and it seems to occur more frequently in females.
4.3. Sidedness
SRC has been reported to be more frequently localized in the right colon, including caecum, ascending colon and proximal transverse colon even though discrepancies regarding site of tumor are present in the literature [24].
4.4. Morphologic Diagnostic Criteria
SRC is characterized by more than 50% of cells with prominent intracytoplasmic mucin and displacement of the nucleus (Figure 2C) [18]. These cells can be found associated with two main histological patterns of growth: (a) infiltrative, mucin poor (linitis plastica-like) pattern, often associated with adverse histological features such as vascular and perineural invasion; (b) mucin rich pattern with signet ring cells floating in large pools of mucin. There is no standardized method to separate SRC into the two morphological groups [25]. Adenocarcinomas with presence of less than 50 % of signet ring cells are defined as “adenocarcinomas with signet ring cell component” [18]. As for MA, high intra and peritumoral TILs are seen, especially in MSI associated SRC.
4.5. Molecular Genotype
SRC share molecular features with MA: they have a higher frequency of KRAS and BRAF mutations compared with conventional CRC, which are associated with a shorter median OS compared to KRAS and BRAF wild-type patients. SRC are often MSI-H tumors and have CpG island methylator phenotype-high (CIMP-H). Though SRC are a clinically aggressive tumours, MSI-H status correlates with a better prognosis and should be considered low grade tumors [26]. Two different molecular genotypes in SRC have been recognized: (a) the hypermethylated genotype with MSI-H, CIMP-H, BRAF-V600E, PDL1+, predominantly located in the right colon, which may be treated with immune checkpoint inhibitor therapy; (b) the hypomethylated genotype, predominantly located in left colon [27].
4.6. Prognosis
SRC is diagnosed at a more advanced stage with transmural extension, loco-regional lymph node metastases and peritoneal dissemination [28]. SRC has been considered an extremely aggressive tumor and is considered as an independent histologic prognostic factor of less favorable outcome and high risk of death [28]. However, as stated above, due to its heterogeneity and different molecular genotypes, SRC should not always be considered predictor of poor prognosis: SRC MMR/MSI-H should be considered as low grade tumors whereas SRC MSS/MSI low show aggressive behavior even though these data are still being debated. Another feature of promising prognostic value is represented by mucin phenotype. Some literature data have demonstrated that mucin-poor SRC have a worse prognosis, with an aggressive clinical outcome if compared with mucin-rich SRC [25].
5. Medullary Carcinoma (MC)
5.1. Background
The term “medullary (adeno)carcinoma” (MC) of the colon was first employed by Jessurun et al. in 1999 [29]. Subsequently, small series of MCs were reported and described as a distinct subgroup of CRCs showing minimal glandular differentiation and intense intratumoral and peritumoral lymphocytic infiltration [29][30][31]. These tumors were also characterized by proximal location, diploid flow cytometric nuclear DNA content and low levels of p53 protein expression. In addition, they demonstrated near always MSI by molecular analysis and better survival rate than other poorly differentiated CRCs [29][30][31]. Further studies confirmed and expanded these initial observations [32].
5.2. Clinical Presentation
The mean age of patients with MC is similar to that of patients with conventional CRC, with a prevalence in the female gender. MCs account for a small percentage (2.2%) of all CRCs, but represents about 20% of large bowel poorly differentiated adenocarcinomas [33]. MCs may be sporadic or develop in patients with Lynch syndrome.
5.3. Sidedness
5.4. Morphologic Diagnostic Criteria
MC is characterized by neoplastic cells with vesicular nuclei, prominent nucleoli and abundant eosinophilic cytoplasm, arranged in solid sheets and exhibiting prominent infiltration by intraepithelial lymphocytes (Figure 2D). The percentage of the tumor area which should exhibit medullary features in order to classify a tumor as medullary is not specified. In a recent meta-analysis, Pyo and coworkers [33] reported a great variation in the histologic criteria utilized for the definition of MC among different studies. In addition some Authors considered presence of MMR deficiency necessary for the diagnosis of MC [31][32]. For these reasons, the histological diagnosis of MC results poorly reproducible and the diagnostic criteria for differentiating MCs from non-medullary poorly differentiated carcinomas still need to be clarified [34].
5.5. Molecular Genotype
Most MCs are MSI-H at molecular analysis and show loss of expression of MMR proteins (generally MLH1 and PMS2) by immunohistochemistry. Moreover, MCs often demonstrate MLH1 promoter methylation and BRAF-V600E mutation. On the contrary, TP53 and KRAS mutations occur much less frequently in MC than in conventional CRC [33]. Immunophenotypically, MCs often show loss of CDX2 and cytokeratin (CK) 20 expression and positivity for calretinin [35][36]. The clinicopathologic and molecular features of MC are mainly related to their MMR deficient phenotype. However, recent studies indicate that MCs differ from the other types of MSI-H CRCs especially regarding the tumor immunoregulatory microenvironment [37][38].
5.6. Prognosis
6. Lymphoepitelioma-Like Carcinoma (LELC)
6.1. Background
Lymphoepitelioma-like carcinoma (LELC) is an undifferentiated carcinoma with prominent lymphoid stroma, found most frequently in the nasopharynx and associated with Epstein Bar Virus (EBV)-infection, although various other sites have been described [40]. In the gastrointestinal tract, the stomach is the most frequent site, while only 9 case have been described in the colon-rectum [41].
6.2. Clinical Presentation
Patient’s age is variable (range 25–86 years) with no gender predilection. Clinical presentation is unremarkable [40].
6.3. Sidedness
LELCs are present in the whole colon-rectum with the most frequent site being the sigmoid colon.
6.4. Morphologic Diagnostic Criteria
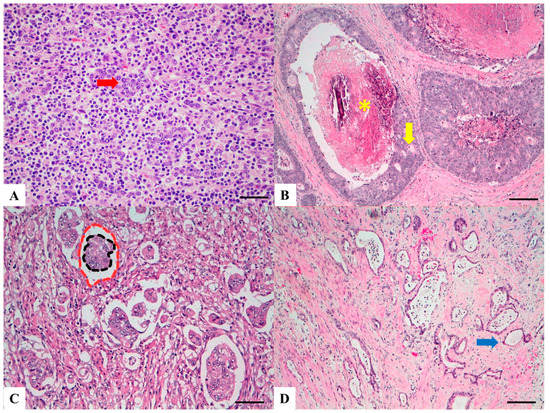
LELCs are composed of poorly differentiated cells arranged in solid nests, tubules and trabeculae with poorly demarcated, infiltrative margins. Inflammatory lymphoid infiltrate is extremely abundant and, differently from medullary carcinoma, is intratumoral rather than peritumoral, permeating between neoplastic cells (Figure 3A) [41]. Lymphoid follicles with germinal centers are usually present.

Figure 3. Haematoxylin and Eosin stained sections of rare type colorectal carcinomas. (A) Lymphoepitelioma-like carcinoma: poorly differentiated cells (red arrow) arranged in solid nests, tubules and trabeculae with poorly demarcated, infiltrative margins; intratumoral lymphoid infiltrate is extremely abundant. Scale bar 200 micron. (B) Cribiform comedo-type carcinoma: cribriform gland (yellow arrow) with central necrosis comedo-like (yellow asterisk). Scale bar 400 micron. (C) Micropapillary Carcinoma: small, tight round to oval cohesive clusters of neoplastic cells (>5 cells) floating in clear spaces (double circle red-black), without endothelial lining and with no evidence of inflammatory cells. Scale bar 200 micron. (D) Low grade tubulo-glandular carcinoma: very well-differentiated invasive glands with uniform circular or tubular profiles (blue arrow) with bland cytologic atypia. Scale bar 400 micron.
6.5. Molecular Genotype
While nasopharyngeal and gastric LELCs are frequently associated with EBV, only in 3/9 colonic cases has this association been reported [42]. Of interest, 2 cases were associated with ulcerative colitis [43] and a further 2 colonic LELCs showed microsatellite instability (1 due to epigenetic methylation of MLH1 promoter and 1 Lynch Syndrome associated) [41][44].
6.6. Prognosis
Prognosis of LELCs has been suggested to be more favorable than conventional CRC, but data are few; the role of inflammation, EBV and MSI status requires further investigation [40].
References
- Remo, A.; Pancione, M.; Zanella, C.; Vendraminelli, R. Molecular pathology of colorectal carcinoma. A systematic review centred on the new role of the pathologist. Pathologica 2012, 104, 432–441.
- Pancione, M.; Giordano, G.; Remo, A.; Febbraro, A.; Sabatino, L.; Manfrin, E.; Ceccarelli, M.; Colantuoni, V. Immune escape mechanisms in colorectal cancer pathogenesis and liver metastasis. J. Immunol. Res. 2014, 2014, 686879.
- Pancione, M.; Remo, A.; Colantuoni, V. Genetic and epigenetic events generate multiple pathways in colorectal cancer progression. Pathol. Res. Int. 2012, 2012, 509348.
- Shih, I.M.; Wang, T.L.; Traverso, G.; Romans, K.; Hamilton, S.R.; Ben-Sasson, S.; Kinzler, K.W.; Vogelstein, B. Top-down morphogenesis of colorectal tumors. Proc. Natl. Acad. Sci. USA 2001, 98, 2640–2645.
- Young, J.; Jenkins, M.; Parry, S.; Young, B.; Nancarrow, D.; English, D.; Giles, G.; Jass, J. Serrated pathway colorectal cancer in the population: Genetic consideration. Gut 2007, 56, 1453–1459.
- Hurt, C.; Ramaraj, R.; Farr, A.; Morgan, M.; Williams, N.; Philips, C.J.; Williams, G.T.; Gardner, G.; Porter, C.; Sampson, J.; et al. CONSCOP Clinical Research Consortium. Feasibility and economic assessment of chromocolonoscopy for detection of proximal serrated neoplasia within a population-based colorectal cancer screening programme (CONSCOP): An open-label, randomised controlled non-inferiority trial. Lancet Gastroenterol. Hepatol. 2019, 4, 364–375.
- Missiaglia, E.; Jacobs, B.; D’Ario, G.; Di Narzo, A.F.; Soneson, C.; Budinska, E.; Popovici, V.; Vecchione, L.; Gerster, S.; Yan, P.; et al. Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann. Oncol. 2014, 25, 1995–2001.
- Jass, J.R.; Smith, M. Sialic acid and epithelial differentiation in colorectal polyps and cancer—A morphological, mucin and lectinhistochemical study. Pathology 1992, 24, 233–242.
- Mäkinen, M.J.; George, S.M.; Jernvall, P.; Mäkelä, J.; Vihko, P.; Karttunen, T.J. Colorectal carcinoma associated with serrated adenoma—Prevalence, histological features, and prognosis. J. Pathol. 2001, 193, 286–294.
- García-Solano, J.; Pérez-Guillermo, M.; Conesa-Zamora, P.; Acosta-Ortega, J.; Trujillo-Santos, J.; Cerezuela-Fuentes, P.; Mäkinen, M.J. Clinicopathologic study of 85 colorectal serrated adenocarcinomas: Further insights into the full recognition of a new subset of colorectal carcinoma. Hum. Pathol. 2010, 41, 1359–1368.
- Stefanius, K.; Ylitalo, L.; Tuomisto, A.; Kuivila, R.; Kantola, T.; Sirniö, P.; Karttunen, T.J.; Mäkinen, M.J. Frequent mutations of KRAS in addition to BRAF in colorectal serrated adenocarcinoma. Histopathology 2011, 58, 679–692.
- Tuppurainen, K.; Mäkinen, J.M.; Junttila, O.; Liakka, A.; Kyllönen, A.P.; Tuominen, H.; Karttunen, T.J.; Mäkinen, M.J. Morphology and microsatellite instability in sporadic serrated and non-serrated colorectal cancer. J. Pathol. 2005, 207, 285–294.
- Laiho, P.; Kokko, A.; Vanharanta, S.; Salovaara, R.; Sammalkorpi, H.; Järvinen, H.; Mecklin, J.P.; Karttunen, T.J.; Tuppurainen, K.; Davalos, V.; et al. Serrated carcinomas form a subclass of colorectal cancer with distinct molecular basis. Oncogene 2007, 26, 312–320.
- Conesa-Zamora, P.; García-Solano, J.; García-García, F.; Turpin Mdel, C.; Trujillo-Santos, J.; Torres-Moreno, D.; Oviedo-Ramírez, I.; Carbonell-Muñoz, R.; Muñoz-Delgado, E.; Rodriguez-Braun, E.; et al. Expression profiling shows differential molecular pathways and provides potential new diagnostic biomarkers for colorectal serrated adenocarcinoma. Int. J. Cancer 2013, 132, 297–307.
- Conesa-Zamora, P.; García-Solano, J.; TurpinMdel, C.; Sebastián-León, P.; Torres-Moreno, D.; Estrada, E.; Tuomisto, A.; Wilce, J.; Mäkinen, M.J.; Pérez-Guillermo, M.; et al. Methylome profiling reveals functions and genes which are differentially methylated in serrated compared to conventional colorectal carcinoma. Clin. Epigenetics 2015, 7, 101.
- García-Solano, J.; Conesa-Zamora, P.; Trujillo-Santos, J.; Mäkinen, M.J.; Pérez-Guillermo, M. Tumour budding and other prognostic pathological features at invasive margins in serrated colorectal adenocarcinoma: A comparative study with conventional carcinoma. Histopathology 2011, 59, 1046–1056.
- Zhu, H.; Qin, H.; Huang, Z.; Li, S.; Zhu, X.; He, J.; Yang, J.; Yu, X.; Yi, X. Clinical significance of programmed death ligand-1 (PD-L1) in colorectal serrated adenocarcinoma. Int. J. Clin. Exp. Pathol. 2015, 8, 9351–9359.
- Bosman, F.T.; Carneiro, F.; Hruban, R.H.; Theise, N.D. WHO Classification of Tumours of the Digestive System, 4th ed.; IARC: Lyon, France, 2010; pp. 137–138.
- Marzouk, O.; Schofield, J. Review of histopathological and molecular prognostic features in colorectal cancer. Cancers 2011, 3, 2767–2810.
- Green, J.B.; Timmcke, A.E.; Mitchell, W.T.; Hicks, T.C.; Gathright, J.B.J.; Ray, J.E. Mucinous carcinoma—Just another colon cancer? Dis. Colon Rectum 1993, 36, 49–54.
- Hugen, N.; van de Velde, C.J.; de Wilt, J.H.; Nagtegaal, I.D. Metastatic pattern in colorectal cancer is strongly influenced by histological subtype. Ann. Oncol. 2014, 25, 651–657.
- Gonzalez, R.S.; Cates, J.M.M.; Washington, K. Associations among histological characteristics and patient outcomes in colorectal carcinoma with a mucinous component. Histopathology 2019, 74, 406–414.
- Laufman, H.; Saphir, O. Primary linitis plastic type carcinoma of the colon. AMA Arch. Surg. 1951, 62, 79–91.
- Tajiri, K.; Sudou, T.; Fujita, F.; Hisaka, T.; Kinugasa, T.; Akagi, Y. Clinicopathological and corresponding genetic features of colorectal signet ring cell carcinoma. Anticancer Res. 2017, 37, 3817–3823.
- Hartman, D.J.; Nikiforova, M.N.; Chang, D.T.; Chu, E.; Bahary, N.; Brand, R.E.; Zureikat, A.H.; Zeh, H.J.; Choudry, H.; Pai, R.K. Signet ring cell colorectal carcinoma: A distinct subset of mucin-poor microsatellite-stable signet ring cell carcinoma associated with dismal prognosis. Am. J. Surg. Pathol. 2013, 37, 969–977.
- Wei, Q.; Wang, X.; Gao, J.; Li, J.; Li, J.; Qi, C.; Li, Y.; Li, Z.; Shen, L. Clinicopathologic and molecular features of colorectal adenocarcinoma with signet-ring cell component. PLoS ONE 2016, 11, e0156659.
- Alvi, M.A.; Loughrey, M.B.; Dunne, P.; McQuaid, S.; Turkington, R.; Fuchs, M.A.; McGready, C.; Bingham, V.; Pang, B.; Moore, W.; et al. Molecular profiling of signet ring cell colorectal cancer provides a strong rationale for genomic targeted and immune checkpoint inhibitor therapies. Br. J. Cancer 2017, 117, 203–209.
- Nitsche, U.; Zimmermann, A.; Späth, C.; Müller, T.; Maak, M.; Schuster, T.; Slotta-Huspenina, J.; Käser, S.A.; Michalski, C.W.; Janssen, K.P.; et al. Mucinous and signet-ring cell colorectal cancers differ from classical adenocarcinomas in tumor biology and prognosis. Ann. Surg. 2013, 258, 775–782.
- Jessurun, J.; Romero-Guadarrama, M.; Manivel, J.C. Medullary adenocarcinoma of the colon: Clinicopathologic study of 11 cases. Hum. Pathol. 1999, 30, 843–848.
- Kim, H.; Jen, J.; Vogelstein, B.; Hamilton, S.R. Clinical and pathological characteristics of sporadic colorectal carcinomas with DNA replication errors in microsatellite sequences. Am. J. Pathol. 1994, 145, 148–156.
- Rüschoff, J.; Dietmaier, W.; Lüttges, J.; Seitz, G.; Bocker, T.; Zirngibl, H.; Schlegel, J.; Schackert, H.K.; Jauch, K.W.; Hofstaedter, F. Poorly differentiated colonic adenocarcinoma, medullary type: Clinical, phenotypic, and molecular characteristics. Am. J. Pathol. 1997, 150, 1815–1825.
- Lanza, G.; Gafà, R.; Matteuzzi, M.; Santini, A. Medullary-type poorly differentiated adenocarcinoma of the large bowel: A distinct clinicopathologic entity characterized by microsatellite instability and improved survival. J. Clin. Oncol. 1999, 17, 2429–2438.
- Pyo, J.S.; Sohn, J.H.; Kang, G. Medullary carcinoma in the colorectum: A systematic review and meta-analysis. Hum. Pathol. 2016, 53, 91–96.
- Lee, L.H.; Yantiss, R.K.; Sadot, E.; Ren, B.; Calvacanti, M.S.; Hechtman, J.F.; Ivelja, S.; Huynh, B.; Xue, Y.; Shitilbans, T.; et al. Diagnosing colorectal medullary carcinoma: Interobserver variability and clinicopathological implications. Hum. Pathol. 2017, 62, 74–82.
- Hinoi, T.; Tani, M.; Lucas, P.C.; Caca, K.; Dunn, R.L.; Macri, E.; Loda, M.; Appelman, H.D.; Cho, K.R.; Fearon, E.R. Loss of CDX2 expression and microsatellite instability are prominent features of large cell minimally differentiated carcinomas of the colon. Am. J. Pathol. 2001, 159, 2239–2248.
- Winn, B.; Tavares, R.; Fanion, J.; Noble, L.; Gao, J.; Sabo, E.; Resnick, M.B. Differentiating the undifferentiated: Immunohistochemical profile of medullary carcinoma of the colon with an emphasis on intestinal differentiation. Hum. Pathol. 2009, 40, 398–404.
- Friedman, K.; Brodsky, A.S.; Lu, S.; Wood, S.; Gill, A.J.; Lombardo, K.; Yang, D.; Resnick, M.B. Medullary carcinoma of the colon: A distinct morphology reveals a distinctive immunoregulatory microenvironment. Mod. Pathol. 2016, 29, 528–541.
- Rosenbaum, M.W.; Bledsoe, J.R.; Morales-Oyarvide, V.; Huynh, T.G.; Mino-Kenudson, M. PD-L1 expression in colorectal cancer is associated with microsatellite instability, BRAF mutation, medullary morphology and cytotoxic tumor-infiltrating lymphocytes. Mod. Pathol. 2016, 29, 1104–1112.
- Johncilla, M.; Chen, Z.; Sweeney, J.; Yantiss, R.K. Tumor grade is prognostically relevant among mismatch repair deficient colorectal carcinomas. Am. J. Surg. Pathol. 2018, 42, 1686–1692.
- Del Arco, C.D.; Collazo, F.E.; Aceñero, M.J.F. Lymphoepithelioma-like carcinoma of the large intestine: A case report and literature review. Rev. Esp. Patol. 2018, 51, 18–22.
- De Petris, G.; Lev, R.; Quirk, D.M.; Ferbend, P.R.; Butmarc, J.R.; Elenitoba-Johnson, K. Lymphoepithelioma-like carcinoma of the colon in a patient with hereditary non polyposis colorectal cancer. Arch. Pathol. Lab. Med. 1999, 123, 720–724.
- Kon, S.; Kasai, K.; Tsuzuki, N.; Nishibe, M.; Kitagawa, T.; Nishibe, T.; Sato, N. Lymphoepithelioma-like carcinoma of rectum: Possible relation with EBV. Pathol. Res. Pract. 2001, 197, 577–582.
- Kojima, Y.; Mogaki, M.; Takagawa, R.; Ota, I.; Sugita, M.; Natori, S.; Hamaguchi, Y.; Kurosawa, H.; Fukushima, T.; Masui, H.; et al. A case of lymphoepithelioma-like carcinoma of the colon with ulcerative colitis. J. Gastroenterol. 2007, 42, 181–185.
- Delaney, D.; Chetty, R. Lymphoepithelioma-like carcinoma of the colon. Int. J. Clin. Exp. Pathol. 2012, 5, 105–109.
More
Information
Subjects:
Oncology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
781
Revisions:
2 times
(View History)
Update Date:
26 Aug 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




