
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Moon Il Kim | + 2321 word(s) | 2321 | 2021-08-23 08:56:52 | | | |
| 2 | Beatrix Zheng | + 308 word(s) | 2629 | 2021-08-23 12:07:37 | | |
Video Upload Options
The appearance and evolution of biofuel cells can be categorized into three groups: microbial biofuel cells (MBFCs), enzymatic biofuel cells (EBFCs), and enzyme-like nanomaterial (nanozyme)-based biofuel cells (NBFCs). MBFCs can produce electricity from waste; however, they have significantly low power output as well as difficulty in controlling electron transfer and microbial growth. EBFCs are more productive in generating electricity with the assistance of natural enzymes, but their vulnerability under diverse environmental conditions has critically hindered practical applications.
1. Introduction
Currently, humans are facing a shortage of energy sources and a wide range of environmental challenges caused by over-exploitation and over-consumption of fossil fuels. To overcome this crisis, new energy sources with sustainable and environmentally friendly characteristics are urgently required [1][2][3][4][5]. In the field of bio-electrochemical research, biofuel cells have emerged as an alternative energy conversion device to generate electricity from biomass while simultaneously preventing environmental pollution. Moreover, the biofuel cell is recognized as a key factor for realizing self-powered sensors, wearable devices, and implantable devices [6]. Biofuel cells can be categorized into three groups: microbial biofuel cells (MBFCs), enzymatic biofuel cells (EBFCs), and enzyme-like nanomaterial (nanozyme)-based (nanozymatic) biofuel cells (NBFCs) ( Figure 1 ) [4][6][7].
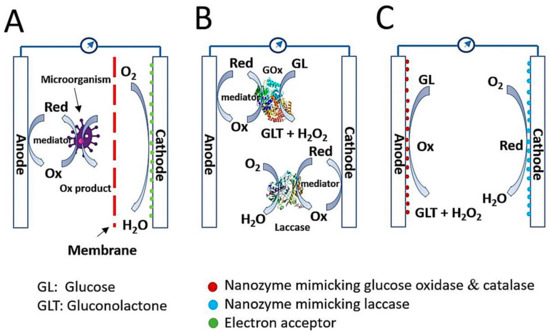
The MBFC is a system that utilizes a microorganism as a reactor to catalyze the oxidation of biomass or waste and thus convert biochemical energy to electrical energy [1]. Fundamentally, the prototype architecture of MBFCs includes the anode, cathode, and membrane between the two ( Figure 1 A). The anodic compartment consists of electrochemically active microorganisms on a supportive electrode, and exoelectrogens, as defined by Logan et al. [8], play a role in donating electrons to the electrode by oxidizing substrates, whereas the large amount of oxygen in the cathodic compartment can accept electrons and protons to form harmless water. Supportive electrodes can be fabricated from carbon-based materials such as carbon fibers, carbon sheets, carbon cloth, carbon graphite, and carbon nanotubes [9][10][11]. Membrane is also an important component, That inhibits the diffusion of mediators and substrates, thereby reducing unwanted flux between the two electrodes but keeping them ionically and chemically conjugated [1]. Without membrane, severe biofouling can happen on the electrode surface, yielding instability and low efficiency in power generation during long-term operation. MBFCs have diverse biofuels, including wastewater, marine sediment soil, freshwater soil, and active sludge [1][12][13]. Electron transfer in MBFCs can be performed through direct contact between bacterial pili and electrode or short-range electron transfer [14], and indirect processes are possible via electron shuttles [15]. Although the MBFC possesses many advantages in waste processing and environmental protection, the critical drawbacks that limit its wide application and commercialization are significantly low power output and extreme difficulty of electron transfer control inside the microorganisms.
Unlike MBFCs, EBFCs catalyze the oxidation of biofuels with the assistance of natural enzymes to produce electricity. Biofuels for EBFCs are generally sugar relatives, such as glucose, sucrose, fructose, alcohols, including ethanol and methanol, organic acids, and organic salts such as sulfite salts [4][16][17]. Among them, the glucose-based EBFC is a well-known object because of the ubiquitous nature of glucose in the physiological fluids of plants, animals, and humans with high potential energy, facile mass production, low financial expenditure, and biocompatibility [18]. The estimated availability of glucose concentration in the human blood is approximately 2–10 mM [19], which is sufficient for developing enzymatic glucose biofuel cells. Like MBFCs, the EBFC comprises an anode and cathode, but the membrane may not be involved because of the high substrate specificity of natural enzymes on each electrode ( Figure 1 B) [20]. In the glucose biofuel cell, the anode generally consists of two kinds of enzymes, glucose oxidase (GOx) and catalase, to convert glucose to gluconolactone with hydrogen peroxide (H 2O 2) and H 2O 2 to water with oxygen, respectively. The cathode comprises laccase, which catalyzes the conversion of oxygen into harmless water. During the catalysis of one glucose molecule, two electrons are transferred through the electrode either directly or indirectly, which is also important for cell performance [4][5]. Glucose-based EBFCs have attracted much attention because of their capability to utilize glucose in physiological fluids to generate electricity, as well as improved power output in comparison with MBFCs. Nevertheless, glucose-based EBFCs have many limitations derived from natural enzymes, such as easy denaturation and instability, high production cost, and difficult electron transfer. Thus, exploring an alternative to natural enzymes that resolve these limitations is urgently needed. [7].
In this scenario, nanozymes take the spotlight with their preferable intrinsic peculiarities in comparison to those of natural enzymes, such as long-term stability, ease of synthesis with low cost, and tunable enzyme-mimicking activities, that present them as potential catalytic material for developing a glucose biofuel cell, namely the nanozyme-based glucose biofuel cell ( Figure 1 C). Nanozymes are functional nanomaterials having an ability to mimic the actions of natural enzymes, and until now, nanozymes typically include metals, metal oxides, metal chalcogenides, nanocarbons, and their composites, that induce distinct catalytic functions [21]. Nanozymes have been exploited in a wide range of applications for biosensors, environmental treatments, therapeutics, and particularly for glucose biofuel cell. To the best of our knowledge, no review paper has specifically discussed nanozyme-based glucose biofuel cell. In this review, we describe recent research progress on the representative synthetic strategies and catalytic characteristics of nanozymes, mechanisms of electricity generation from glucose, and application studies. We also describe the current challenges and prospects of advanced glucose-based NBFCs, based on the unique properties of nanozymes.
2. Synthetic Strategies and Catalytic Characteristics of Nanozymes to Replace Natural Enzymes in Glucose Biofuel Cells
To develop glucose-based NBFCs, nanozymes that mimic GOx and catalase are required to construct an anode for catalyzing glucose oxidation without accumulating H 2O 2, as well as laccase-mimicking nanozymes to construct a cathode for accepting electrons, which are produced and transferred to the cathode during glucose oxidation. Glucose-based NBFCs can be constructed by placing appropriate nanozymes in either both electrodes or a single electrode. Diverse types of nanozymes have been reported to mimic GOx, catalase, and laccase, and they can be categorized into noble metal and metal oxide-based nanozymes based on their composition. In this section, we describe the representative synthetic strategies and catalytic activities that are essentially utilized to develop glucose biofuel cells.
Nanozymes are generally synthesized via physicochemical routes similar to conventional nanomaterials. Different methods are available, and depending on the experimental orientation and application purpose, researchers can select between the appropriate top-down or bottom-up approach. The top-down approach includes a solid-state reaction route, in which the starting materials are scaled down to the synthesized product via ball milling, nanolithography, sputtering, and thermal decomposition processes; the bottom-up approach is a wet chemical route comprising sol-gel, reverse micelle, chemical vapor decomposition, pyrolysis, biosynthesis, microwave-assisted, and flow synthesis processes [22]. Between the two, the bottom-up approach is considered more efficient, and thus, it has been generally employed to synthesize diverse kinds of nanozymes. The bottom-up approach is capable of precisely controlling the size, morphology, crystalline structure, and surface properties of nanostructures. These features not only affect the physicochemical properties, but also significantly affect enzyme-mimicking activities. For example, smaller nanozymes around 10 nm in their diameter generally yielded higher activity than larger nanozymes, possibly due to their higher surface-to-volume ratio to combine with substrate [23]. Nanozymes preserving more active crystallographic facets showed higher activity due to higher surface reactivity [24]. Moreover, recent studies have been conducted to chemically mimic the structures of natural enzymes, including the active center or substrate-binding pocket, that yielded further enhancement of activity as well as selectivity toward the target substrate [25]. Therefore, material processing is a key factor for tailoring an arbitrary nanomaterial to obtain the required properties and realize the desired applications.
Natural enzymes are categorized into six groups based on the type of catalytic reaction: oxidoreductase, transferase, hydrolase, lyase, ligase, and isomerase [26]. Four groups have already been mimicked by nanozymes, including oxidoreductase [27][28], hydrolase [29][30], isomerase [31][32][33], and lyase [34]. Diverse material types, such as noble metals, bi- or tri-metallic alloys, metal oxides, carbon, and hybrid-like metal-organic frameworks, have been reported as nanozymes. As nanozymes mimicking GOx, catalase, and laccase are utilized to develop glucose biofuel cells; the catalytic features of these nanozymes are discussed with recent examples.
Catalase-like nanozymes cannot be utilized solely for glucose biofuel cell development; however, bi-enzymatic nanozymes showing GOx and catalase have been reported to serve as an anode in glucose biofuel cells [35][36]. In this regard, finding efficient catalase-like nanozymes or bi-enzymatic nanozymes showing both GOx and catalase-like activity, for preparing an efficient anode in glucose biofuel cells is crucial for decomposing the byproduct H 2O 2 and enhancing the power output and extending the lifetime of glucose-based NBFCs.
3. Recent Research Examples of Glucose-Based NBFCs
The glucose-based NBFC was composed of an anode, a cathode, and an electrolyte containing glucose. Depending on the working environment, components in the NBFC can be modified to improve power output, conductivity, OCV, stability, and lifetime. In this section, we briefly summarize recently reported and representative examples of glucose-based NBFCs. We categorize our discussion into two main groups based on the types of nanozymes employed, primarily noble metals and several metal oxide-based ones. In most studies, these nanozymes were anchored on carbon-based materials, and then utilized to construct electrodes of the biofuel cell system, which could provide much enhancement in electrical conductivity as well as other catalytic advantages including facilitated substrate or mediator transfer.
Noble metals such as Au, Pt, and Pd, which show outstanding GOx-mimicking activity, have been widely applied in glucose biofuel cells [37][38][39]. Xie et al. [37] introduced an Au nanozyme-based glucose biofuel cell, where Au salt was dispersed into a glucose electrolyte and deposited on the electrode during glucose oxidation to obtain a high-performance NBFC. Another NBFC comprising GOx-like Au nanowires as the anode and Pt/carbon as the cathode was reported [38]. Thanks to the large surface area and small particle size of the Au nanowires, a high-power output of 126 µW/cm 2 and an OCV of 0.425 V were obtained. Another glucose-based NBFC incorporating an Au NP-based anode, graphene-based cathode, and Nafion membrane was developed to yield a high-power output of 10.7 mW/cm 2, which is among the best results for glucose-based NBFCs [39].
Binary [40][41][42][43][44][45][46] or ternary [42][46] noble metal alloys have also been employed for developing glucose-based NBFCs, based on their synergistically enhanced enzyme-like activity and ability to circumvent the poisonous intermediates generated during the catalytic oxidation at the anodic electrode [47][42][44][45]. Chu et al. [45] developed a Pt/Au alloy-based glucose biofuel cell consisting of an anode of a GOx-like Pt/Au nanozyme and a cathode of graphene with a Nafion membrane. In this study, the optimized Pt/Au ratio (1:4) and glucose (6 mM) with saturated oxygen environment yielded good biofuel cell performances with power output of 0.32 mW/cm 2 and OCV of 0.42 V. Guo et al. [40] designed and prepared another glucose-based NBFC consisting of nanoporous Au-PtBi as the anode, Pt/carbon as the cathode, and glucose electrolyte mixed with NaOH. At an optimized glucose concentration (0.5 M), a high-power output performance of 8 mW/cm 2 and an OCV of 0.95 V were achieved. A bimetallic nanozyme-based glucose biofuel cell comprising an anode constructed of GOx-like Au 80Pt 20/carbon and a cathode of Pt/carbon with a glucose solution containing KOH, has been reported [47]. In this study, a series of experimental parameters were tested, and the glucose and KOH concentration, reaction temperature, and flow rate of glucose solution were optimized to be 0.6 M, 4 M, 328 K, and 50 mL/min, respectively. By adopting the optimized conditions, a high-power output of 95.7 mW/cm 2 and an OCV of 0.34 V were achieved, but the initial power output decreased to 82.5 mW/cm 2 after only 20 min of operation because of the deterioration of the electrodes.
The high synthesis cost of noble metal-type nanozymes can be surpassed by metal oxide-type nanozymes, which can be easily synthesized at a low cost. An interesting metal oxide-based NBFC was presented by Ho et al. [48], where CoMn 2O 4/carbon was employed as a GOx-like anode catalyst in a biofuel cell system. The NBFC showed a power output of 2.372 mW/cm 2, which is comparable to that of a commercial Pt/carbon-based biofuel cell system. Another NBFC utilized Co 3O 4 grown on graphene oxide as a GOx-like nanozyme to prepare the anode, and N and Fe co-doped biowaste-derived activated carbon to prepare the cathode [49]. The biofuel cell showed a maximum power output of 12.81 μW/cm 2 and OCV of 0.442 V, with 10 mM glucose. Interestingly, as the glucose concentration increased to 10 mM, the power output increased gradually and dramatically dropped at approximately 30 mM. This was ascribed to the effect of high glucose concentration, which might prevent the hydroxyl radical from approaching the electrode anode. According to the study by Slaughter et al. [50], ZnO nanosol was deposited on an Al/Au substrate to construct an anode, and Pt was used to prepare the cathode. This NBFC showed a power output of 16.2 μW/cm 2 with an OCV of 0.84 V. The electricity-generating mechanism from glucose could be interpreted by the unique electronic structure of the anode, where Zn and O within ZnO served as the electron acceptor and donor, respectively, owing to the special valence band of ZnO.
Likewise, although the glucose oxidizing activity of metal oxide-based nanozymes is relatively lower than that of noble metal-based nanozymes, there have been many approaches for their unique utilization in the construction of glucose-based NBFCs.
4. Conclusions, Current Challenges, and Prospects
Recently, enzyme-mimicking nanozymes have attracted intense interest as promising alternatives to natural enzymes. In particular, diverse types of noble metal and metal oxide-based nanozymes can mimic natural GOx and catalase to efficiently oxidize glucose without accumulating harmful H 2O 2, as well as laccase to accept electrons, both of which are essential for constructing the anode and cathode, respectively, in glucose biofuel cells. Compared with EBFCs, NBFCs have distinct advantages such as high operational stability and robustness with extended lifetime, sufficient power output by high catalytic activity for glucose oxidation, low production cost by facile large-scale synthesis and manufacturing, and further tailored functionalities derived from the uniqueness of nanozymes. In this regard, the use of nanozymes in the glucose biofuel cell system significantly enhanced power generation performance ( Table 1 ).
| Nanozymes | Anode | Cathode | Lifetime | OCV (V) | Pmax (mW/cm2) | Ref |
|---|---|---|---|---|---|---|
| Au film | Au film | Pt | 90% retention after 60-day storage | 0.916 | 0.307 | [37] |
| Au nanowires | Au nanowires | Pt/carbon | 93% retention after 30-day storage | 0.425 | 0.126 | [38] |
| Nano/micro hybrid structured Au, graphene | Nano/micro hybrid structured Au | Graphene | 85% retention after 90-day storage | 8.2 | 10.7 | [39] |
| Pt/Au nano-alloy, graphene | Pt/Au | Graphene | NA i | 0.42 | 0.32 | [45] |
| PtBi decorated nanoporous gold and Pt/carbon | PtBi decorated nanoporous gold | Pt/carbon | NA | 0.95 | 8 | [40] |
| Au80Pt20 nanoparticles/carbon black and Pt/carbon | Au80Pt20 nanoparticles/carbon | Pt/carbon | NA | 0.34 | 95.7 | [47] |
| PdPtAu/carbon, PdPt/carbon and Pt | PdPtAu/carbon, PdPt/carbon | Pt | NA | 0.92 | 0.52 | [42] |
| PtNi alloy and Pd | PtNi alloy | Pd | NA | ~0.35 | ~0.00283 | [51] |
| Ni foam and CoMn2O4/NC nanocomposites ii | Ni foam | CoMn2O4/NC air cathode | ~80% retention after ~7 h running | 0.77 | 2.372 | [48] |
| Graphene-cobalt oxide nanocomposite on Ni foam substrate and N, Fe-codoped biomass carbon | Graphene-cobalt oxide nanocomposite on Ni foam substrate | N, Fe-codoped biomass carbon | ~80% retention after 10 h running | 0.442 | 0.01281 | [49] |
| Bimetallic Ni-Co composite anchored on reduced graphene oxide and Cu2O | Bimetallic Ni-Co composite anchored on reduced graphene oxide | Cu2O-Cu | NA | NA | 2.8807 | [52] |
| Pt/rGO and FeCo/Ketjen Black | Pt/rGO | Fe-Co/Ketjen Black | ~77% retention after 15 h running | 0.388 | NA | [53] |
| Pt and Pd graphene and nitrogen doped graphene oxide nanoribbons | Pt and Pd graphene | N-doped GO nanoribbons | ~92% retention after 7-day storage | 0.216 | 0.0249 | [54] |
| ZnO seed deposited on the Al/Au and single rod Pt | ZnO seed deposited on the Al/Au | Single rod Pt | 100% retention for 9 h running | 0.840 | 0.0162 | [50] |
References
- Slate, A.J.; Whitehead, K.A.; Brownson, D.A.C.; Banks, C.E. Microbial fuel cells: An overview of current technology. Renew. Sustain. Energy Rev. 2019, 101, 60–81.
- Xu, Z.; Chen, S.; Guo, S.; Wan, D.; Xu, H.; Yan, W.; Jin, X.; Feng, J. New insights in light-assisted microbial fuel cells for wastewater treatment and power generation: A win-win cooperation. J. Power Sources 2021, 501, 230000.
- Jung, S.-W.; Yeom, J.; Park, J.S.; Yoo, S.M. Recent advances in tuning the expression and regulation of genes for constructing microbial cell factories. Biotechnol. Adv. 2021, 50, 107767.
- Nasar, A.; Perveen, R. Applications of enzymatic biofuel cells in bioelectronic devices—A review. Int. J. Hydrog. Energy 2019, 44, 15287–15312.
- Xiao, X.; Xia, H.-Q.; Wu, R.; Bai, L.; Yan, L.; Magner, E.; Cosnier, S.; Lojou, E.; Zhu, Z.; Liu, A. Tackling the challenges of enzymatic (bio)fuel cells. Chem. Rev. 2019, 119, 9509–9558.
- Hao, S.; Sun, X.; Zhang, H.; Zhai, J.; Dong, S. Recent development of biofuel cell based self-powered biosensors. J. Mater. Chem. B 2020, 8, 3393–3407.
- Katz, E.; Bollella, P. Fuel cells and biofuel cells: From past to perspectives. Isr. J. Chem. 2021, 61, 68–84.
- Logan, B.E.; Regan, J.M. Electricity-producing bacterial communities in microbial fuel cells. Trends Microbiol. 2006, 14, 512–518.
- Qiao, Y.; Bao, S.-J.; Li, C.M. Electrocatalysis in microbial fuel cells—From electrode material to direct electrochemistry. Energy Environ. Sci. 2010, 3, 544–553.
- Zou, Y.; Xiang, C.; Yang, L.; Sun, L.-X.; Xu, F.; Cao, Z. A mediatorless microbial fuel cell using polypyrrole coated carbon nanotubes composite as anode material. Int. J. Hydrog. Energy 2008, 33, 4856–4862.
- Santoro, C.; Arbizzani, C.; Erable, B.; Ieropoulos, I. Microbial fuel cells: From fundamentals to applications. A review. J. Power Sources 2017, 356, 225–244.
- Zhen, G.; Lu, X.; Kato, H.; Zhao, Y.; Li, Y.-Y. Overview of pretreatment strategies for enhancing sewage sludge disintegration and subsequent anaerobic digestion: Current advances, full-scale application and future perspectives. Renew. Sustain. Energy Rev. 2017, 69, 559–577.
- Grandclément, C.; Seyssiecq, I.; Piram, A.; Wong-Wah-Chung, P.; Vanot, G.; Tiliacos, N.; Roche, N.; Doumenq, P. From the conventional biological wastewater treatment to hybrid processes, the evaluation of organic micropollutant removal: A review. Water Res. 2017, 111, 297–317.
- Yue, H.; Khoshtariya, D.; Waldeck, D.H.; Grochol, J.; Hildebrandt, P.; Murgida, D.H. On the electron transfer mechanism between cytochrome c and metal electrodes. Evidence for dynamic control at short distances. J. Phys. Chem. B 2006, 110, 19906–19913.
- Velasquez-Orta, S.B.; Head, I.M.; Curtis, T.P.; Scott, K.; Lloyd, J.R.; von Canstein, H. The effect of flavin electron shuttles in microbial fuel cells current production. Appl. Microbiol. Biotechnol. 2010, 85, 1373–1381.
- Tang, J.; Werchmeister, R.M.L.; Preda, L.; Huang, W.; Zheng, Z.; Leimkühler, S.; Wollenberger, U.; Xiao, X.; Engelbrekt, C.; Ulstrup, J.; et al. Three-dimensional sulfite oxidase bioanodes based on graphene functionalized carbon paper for sulfite/O2 biofuel cells. ACS Catal. 2019, 9, 6543–6554.
- Gai, P.; Ji, Y.; Chen, Y.; Zhu, C.; Zhang, J.; Zhu, J.-J. A nitrogen-doped graphene/gold nanoparticle/formate dehydrogenase bioanode for high power output membrane-less formic acid/O2 biofuel cells. Analyst 2015, 140, 1822–1826.
- Zhu, Z.; Kin Tam, T.; Sun, F.; You, C.; Percival Zhang, Y.H. A high-energy-density sugar biobattery based on a synthetic enzymatic pathway. Nat. Commun. 2014, 5, 3026.
- Xiao, X.; Wang, M.E.; Li, H.; Pan, Y.; Si, P. Non-enzymatic glucose sensors based on controllable nanoporous gold/copper oxide nanohybrids. Talanta 2014, 125, 366–371.
- Holzinger, M.; Le Goff, A.; Cosnier, S. Carbon nanotube/enzyme biofuel cells. Electrochim. Acta 2012, 82, 179–190.
- Jiang, D.; Ni, D.; Rosenkrans, Z.T.; Huang, P.; Yan, X.; Cai, W. Nanozyme: New horizons for responsive biomedical applications. Chem. Soc. Rev. 2019, 48, 3683–3704.
- Mahmudunnabi, R.G.; Farhana, F.Z.; Kashaninejad, N.; Firoz, S.H.; Shim, Y.-B.; Shiddiky, M.J.A. Nanozyme-based electrochemical biosensors for disease biomarker detection. Analyst 2020, 145, 4398–4420.
- Huang, Y.; Ren, J.; Qu, X. Nanozymes: Classification, catalytic mechanisms, activity regulation, and applications. Chem. Rev. 2019, 119, 4357–4412.
- Yang, Y.; Mao, Z.; Huang, W.; Liu, L.; Li, J.; Li, J.; Wu, Q. Redox enzyme-mimicking activities of CeO2 nanostructures: Intrinsic influence of exposed facets. Sci. Rep. 2016, 6, 35344.
- Kim, M.S.; Lee, J.; Kim, H.S.; Cho, A.; Shim, K.H.; Le, T.N.; An, S.S.A.; Han, J.W.; Kim, M.I.; Lee, J. Heme cofactor-resembling Fe–N single site embedded graphene as nanozymes to selectively detect H2O2 with high sensitivity. Adv. Funct. Mater. 2020, 30, 1905410.
- McDonald, A.G.; Tipton, K.F. Fifty-five years of enzyme classification: Advances and difficulties. FEBS J. 2014, 281, 583–592.
- Wu, W.; Wang, Q.; Chen, J.; Huang, L.; Zhang, H.; Rong, K.; Dong, S. Biomimetic design for enhancing the peroxidase mimicking activity of hemin. Nanoscale 2019, 11, 12603–12609.
- Zhang, S.; Lin, F.; Yuan, Q.; Liu, J.; Li, Y.; Liang, H. Robust magnetic laccase-mimicking nanozyme for oxidizing o-phenylenediamine and removing phenolic pollutants. J. Environ. Sci. 2020, 88, 103–111.
- Garrido-González, J.J.; Iglesias Aparicio, M.M.; García, M.M.; Simón, L.; Sanz, F.; Morán, J.R.; Fuentes de Arriba, Á.L. An enzyme model which mimics chymotrypsin and N-terminal hydrolases. ACS Catal. 2020, 10, 11162–11170.
- Fu, T.; Xu, C.; Guo, R.; Lin, C.; Huang, Y.; Tang, Y.; Wang, H.; Zhou, Q.; Lin, Y. Zeolitic imidazolate framework-90 nanoparticles as nanozymes to mimic organophosphorus hydrolase. ACS Appl. Nano Mater. 2021, 4, 3345–3350.
- Vernekar, A.A.; Mugesh, G. Hemin-functionalized reduced graphene oxide nanosheets reveal peroxynitrite reduction and isomerization activity. Chem. Eur. J. 2012, 18, 15122–15132.
- Zhang, N.; Meng, X.-G.; Wu, Y.-Y.; Song, H.-J.; Huang, H.; Wang, F.; Lv, J. Highly selective isomerization of glucose into fructose catalyzed by a mimic glucose isomerase. ChemCatChem 2019, 11, 2355–2361.
- Krause, G.; Lundström, J.; Barea, J.L.; Pueyo de la Cuesta, C.; Holmgren, A. Mimicking the active site of protein disulfide-isomerase by substitution of proline 34 in Escherichia coli thioredoxin. J. Biol. Chem. 1991, 266, 9494–9500.
- Wang, M.; Qi, W. 16—Assembled Peptides for Biomimetic Catalysis. In Artificial Protein and Peptide Nanofibers; Wei, G., Kumbar, S.G., Eds.; Woodhead Publishing: Sawston, UK, 2020; pp. 383–413.
- Chung, M.; Nguyen, T.L.; Tran, T.Q.N.; Yoon, H.H.; Kim, I.T.; Kim, M.I. Ultrarapid sonochemical synthesis of enzyme-incorporated copper nanoflowers and their application to mediatorless glucose biofuel cell. Appl. Surf. 2018, 429, 203–209.
- Vo, T.N.; Tran, T.D.; Nguyen, H.K.; Kong, D.Y.; Kim, M.I.; Kim, I.T. In situ growth of hybrid nanoflowers on activated carbon fibers as electrodes for mediatorless enzymatic biofuel cells. Mater. Lett. 2020, 281, 128662.
- Xie, F.; Huang, Z.; Chen, C.; Xie, Q.; Huang, Y.; Qin, C.; Liu, Y.; Su, Z.; Yao, S. Preparation of Au-film electrodes in glucose-containing Au-electroplating aqueous bath for high-performance nonenzymatic glucose sensor and glucose/O2 fuel cell. Electrochem. Commun. 2012, 18, 108–111.
- Yang, L.; Zhang, Y.; Chu, M.; Deng, W.; Tan, Y.; Ma, M.; Su, X.; Xie, Q.; Yao, S. Facile fabrication of network film electrodes with ultrathin Au nanowires for nonenzymatic glucose sensing and glucose/O2 fuel cell. Biosens. Bioelectron. 2014, 52, 105–110.
- Chu, T.-F.; Rajendran, R.; Kuznetsova, I.; Wang, G.-J. High-power, non-enzymatic glucose biofuel cell based on a nano/micro hybrid-structured Au anode. J. Power Sources 2020, 453, 227844.
- Guo, H.; Yin, H.; Yan, X.; Shi, S.; Yu, Q.; Cao, Z.; Li, J. Pt-Bi decorated nanoporous gold for high performance direct glucose fuel cell. Sci. Rep. 2016, 6, 39162.
- Basu, D.; Basu, S. Synthesis and characterization of Pt–Au/C catalyst for glucose electro-oxidation for the application in direct glucose fuel cell. Int. J. Hydrog. Energy 2011, 36, 14923–14929.
- Basu, D.; Basu, S. Performance studies of Pd-Pt and Pt-Pd-Au catalyst for electro-oxidation of glucose in direct glucose fuel cell. Int. J. Hydrog. Energy 2012, 37, 4678–4684.
- Basu, D.; Sood, S.; Basu, S. Performance comparison of Pt-Au/C and Pt-Bi/C anode catalysts in batch and continuous direct glucose alkaline fuel cell. Chem. Eng. J. 2013, 228, 867–870.
- Chen, J.; Zheng, H.; Kang, J.; Yang, F.; Cao, Y.; Xiang, M. An alkaline direct oxidation glucose fuel cell using three-dimensional structural Au/Ni-foam as catalytic electrodes. RSC Adv. 2017, 7, 3035–3042.
- Chu, T.-F.; Lin, F.-Y.; Kuznetsova, I.; Wang, G.-J. A novel neutral non-enzymatic glucose biofuel cell based on a Pt/Au nano-alloy anode. J. Power Sources 2021, 486, 229374.
- Zhiani, M.; Abedini, A.; Majidi, S. Comparison of electro-catalytic activity of Fe-Ni-Co/C and Pd/C nanoparticles for glucose electro-oxidation in alkaline half-cell and direct glucose fuel cell. Electrocatalysis 2018, 9, 735–743.
- Torigoe, K.; Takahashi, M.; Tsuchiya, K.; Iwabata, K.; Ichihashi, T.; Sakaguchi, K.; Sugawara, F.; Abe, M. High-power abiotic direct glucose fuel cell using a gold–platinum bimetallic anode catalyst. ACS Omega 2018, 3, 18323–18333.
- Ho, J.; Li, Y.; Dai, Y.; Kim, T.; Wang, J.; Ren, J.; Yun, H.; Liu, X. Ionothermal synthesis of N-doped carbon supported CoMn2O4 nanoparticles as ORR catalyst in direct glucose alkaline fuel cell. Int. J. Hydrog. Energy 2021, 46, 20503–20515.
- Purkait, T.; Dey, R.S. Fabrication of a membrane-less non-enzymatic glucose-air fuel cell with graphene-cobalt oxide nanocomposite anode and Fe, N-doped biomass carbon cathode. J. Electroanal. Chem. 2020, 874, 114467.
- Slaughter, G.; Sunday, J. A membraneless single compartment abiotic glucose fuel cell. J. Power Sources 2014, 261, 332–336.
- Do, U.P.; Seland, F.; Johannessen, E.A. A micro fuel cell for abiotical catalysis of glucose. J. Power Sources 2020, 478, 229032.
- Irfan, M.; Liu, X.; Li, S.; Khan, I.U.; Li, Y.; Wang, J.; Wang, X.; Du, X.; Wang, G.; Zhang, P. High-performance glucose fuel cell with bimetallic Ni–Co composite anchored on reduced graphene oxide as anode catalyst. Renew. Energy 2020, 155, 1118–1126.
- Zhiani, M.; Barzi, S.; Gholamian, M.; Ahmadi, A. Synthesis and evaluation of Pt/rGO as the anode electrode in abiotic glucose fuel cell: Near to the human body physiological condition. Int. J. Hydrog. Energy 2020, 45, 13496–13507.
- Su, C.-H.; Sun, C.-L.; Peng, S.-Y.; Wu, J.-J.; Huang, Y.-H.; Liao, Y.-C. High performance non-enzymatic graphene-based glucose fuel cell operated under moderate temperatures and a neutral solution. J. Taiwan Inst. Chem. Eng. 2019, 95, 48–54.




