Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Niki Christou | + 2882 word(s) | 2882 | 2021-08-09 09:01:51 | | | |
| 2 | Camila Xu | + 218 word(s) | 3100 | 2021-08-19 05:06:20 | | | | |
| 3 | Camila Xu | Meta information modification | 3100 | 2021-08-19 05:07:23 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Christou, N. Colorectal Cancer (CRC). Encyclopedia. Available online: https://encyclopedia.pub/entry/13306 (accessed on 07 February 2026).
Christou N. Colorectal Cancer (CRC). Encyclopedia. Available at: https://encyclopedia.pub/entry/13306. Accessed February 07, 2026.
Christou, Niki. "Colorectal Cancer (CRC)" Encyclopedia, https://encyclopedia.pub/entry/13306 (accessed February 07, 2026).
Christou, N. (2021, August 18). Colorectal Cancer (CRC). In Encyclopedia. https://encyclopedia.pub/entry/13306
Christou, Niki. "Colorectal Cancer (CRC)." Encyclopedia. Web. 18 August, 2021.
Copy Citation
Colorectal cancer (CRC) is the third most common cancer type, only behind breast cancer and lung cancer in females and prostate cancer and lung cancer in males. CRC is also a leading cause of cancer-related deaths globally.
colorectal cancer
liver metastases
immunotherapy
chemotherapy and biological agents
1. Introduction
Colorectal cancer (CRC) is the third most common cancer type, only behind breast cancer and lung cancer in females and prostate cancer and lung cancer in males. CRC is also a leading cause of cancer-related deaths globally. The liver is of anatomical and physiological importance in regard to the natural course of CRC. The liver and lung are the commonest sites for CRC metastasis to occur. The liver’s predominant blood supply arises from the confluence of the GI tract supplying blood vessels via the hepatic portal vein; this circulation aids the transference of the colorectal cancer cells to the hepatic parenchyma, migrating and forming colorectal metastases in the liver. The overall prognosis and survival of patients with CRLM are poor, and most patients initially are unable to undergo surgery.
The management of colorectal metastases of the liver varies on CRC disease burden, patient suitability, clinical correlation and appropriateness of treatments decided by cancer multidisciplinary team (MDT). Conventional treatment methods of CRRLM include hepatic resection with or without chemo-radiotherapy (neoadjuvant or adjuvant) and other conservative management. The development of novel and effective biotherapies in conjunction with chemotherapies for colorectal metastases have changed the natural course of CRC by enhancing the host’s own immunological anti-tumour responses against CRC and already shown to have a good potential and improve the prognosis of the disease. Treatment strategies utilizing and evaluating the benefit of the use of biotherapies in CRLM of the liver remain unclear.
2. Biology of the Metastatic Colorectal Cancer
Colorectal adenocarcinoma forms the largest disease type for CRCs. These adenocarcinomas derive from sequential changes involving mutations and key oncogenes and the loss of suppressor genes driving changes of adenomas into adenocarcinomas [1]. These mutational changes include KRAS and inactivation of tumour suppressor gene p53, which drive the pathogenesis of adenocarcinoma from adenomas [2]. The adenoma–adenocarcinoma sequence highlights significant avenues for targeted biological therapeutics. KRAS primarily belongs to the family of GTPases. Upon activation, KRAS induces the mitogen-activated protein kinase (MAPK) cascades and facilitates the transmission of signals from the cell membrane to the nucleus; the RAS gene, through downstream signalling, activates RAF proteins (including ARAF, BRAF, and RAF-1) [3][4].
The signalling pathway of the RAS–RAF–MEK–MAPK cascade regulates gene transcription controlling cancer cell proliferation, survival, migration and angiogenesis, abetting the progression of colorectal cancer (CRC) and promotes metastasis. Therefore, direct inhibition of MEK binding and effector function, consequently, may uncover a promising targeted therapeutic strategy for CRCs. There are several targeted inhibitors that are currently under evaluation in clinical trials showing initial clinical activity in a variety of tumours, including mCRC [4][5][6]. Specific markers that targeted therapies are aimed against include: Epidermal Growth Factor Receptor (EGFR), BRAF and tyrosine kinases, amongst others.
Resistance to molecular biological targeted therapies can be detected in the early and advanced stages of the disease, and this can occur in an estimated prevalence of up to 45% in patients with CRLM. Studies have shown that such mutations are independently associated with worse survival and poor overall outcomes [7][8].
The use of biological therapies in combination with chemotherapy is an essential option towards “conversional” strategies that aim to transform initially unresectable CRLM to resectable CRLM, and thereby has evidently increased the proportion of patients eligible for hepatic resection. Along with the advancements in the amalgamation of perioperative and surgical management, the use of effective chemotherapies, targeted biological therapies and novel methods of delivering these targeted therapies locally (e.g., hepatic intra-arterial chemotherapy, RFA, stereotactic radiotherapy) [9], as we strive to decrease perioperative morbidity and mortality, increase long-term survival by increasing the number of patients who are able to undergo complete hepatic resections (Figure 1).
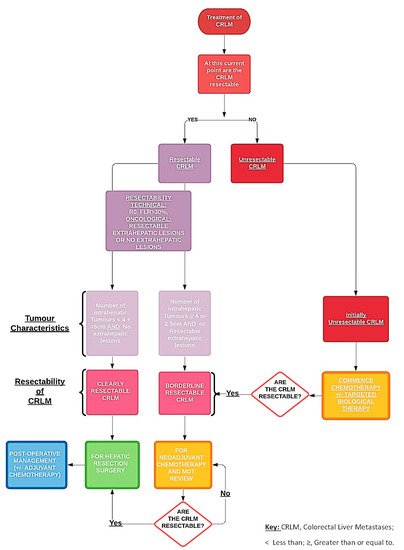
Figure 1. Pathway of managing resectable and unresectable colorectal liver metastases.
Chemotherapy is a chemically constituted anticancer therapy that acts by reducing the tumour burden and facilitates the destruction of rapidly growing cancer cells in the body. In colorectal cancer, the most used are shown in Table 1.
Table 1. Combined Chemotherapy Regimens.
| Names of Combined Chemotherapy Regimens | Components of Combined Chemotherapy |
|---|---|
| FOLFOX | Folinic Acid, Fluorouracil and Oxaliplatin. |
| FOLFORI | 5-Fluorouracil and High-Dose Leucovorin |
| FOLFIRI | Folinic Acid, Fluorouracil and Irinotecan |
| FOLFOXIRI | Folinic Acid, Fluorouracil and Oxaliplatin. |
| CAPOX or XELOX | Oxaliplatin and Capecitabine |
With regards to targeted therapies used in the treatment of colorectal cancer and their metastases, the main forms of this type of therapy are Tyrosine Kinase Inhibitors, BRAF (proto-oncogene B-Raf or v-Raf murine sarcoma viral oncogene homolog B) inhibitors and epidermal growth factor receptor (EGFR) inhibitors (Figure 2).
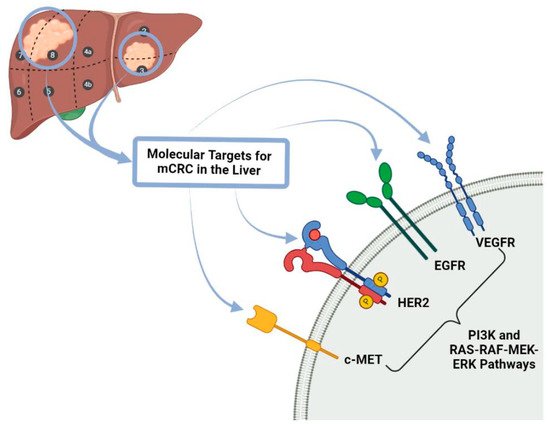
Figure 2. Molecular Targets for mCRC in the liver. c-MET: tyrosine-protein kinase Met receptor, HER 2: Human Epidermal Growth Factor Receptor-2, EGFR: Epidermal growth factor receptor, VEGFR: Vascular Endothelial Growth Factor Receptor PI3K: Phosphoinositide 3-kinase, RAS-RAF-MEK-ERK pathways also known MAPK/ERK pathway corresponds to the Ras/Raf/Mitogen-activated protein kinase/ERK kinase (MEK)/extracellular-signal-regulated kinase (ERK) cascade couples signals from cell surface receptors to transcription factors, regulating gene expression and mCRC: metastatic colorectal cancer.
Tyrosine Kinase Inhibitors, also known as kinase inhibitors, located on cellular surfaces and intracellularly, are proteins involved in cell to cell signalling and are key elements in cell function. Kinase inhibitors like Regorafenib block several kinase proteins that assist the tumour cell growth and tumour blood vessel development, similar to anti-angiogenic inhibitors (Table 2). Blocking these proteins can impair tumour growth and function. BRAF gene and its proteins play a fundamental role in colorectal cancer (CRC). Some CRC cells produce abnormal BRAF proteins that promote tumour growth, hence being an important therapeutic target. A limiting factor in using BRAF inhibitors is that BRAF inhibitors are unlikely to work on patients with colorectal cancers that have normal BRAF genes. Epidermal growth factor receptor (EGFR) is a protein-based receptor that also assists cancer cell growth. Primarily, EGFR inhibitors are used in patients with advanced colon or rectal cancers.
Table 2. Types of anticancer therapies.
| Types of Therapy | Definition | Examples of Type of Therapy |
|---|---|---|
| “Classical” Cytotoxic Chemotherapies | Therapies that can be delivered intravenously or orally. It can be given during the neoadjuvant, adjuvant or palliative setting and can be given either systemically or regionally. | FOLFOX (also known as Oxaliplatin de Gramont or OxMdG, which means modified Oxaliplatin de Gramont) (Folinic acid, fluorouracil and oxaliplatin) FOLFORI (5-Fluorouracil and High-Dose Leucovorin) FOLFIRI (Folinic acid, fluorouracil and irinotecan) FOLFOXIRI (folinic acid, 5-fluorouracil, oxaliplatin and irinotecan) CAPOX (capecitabine plus oxaliplatin) or XELOX (xeloda® = capecitabine plus oxaliplatine) |
| Targeted Therapies | Therapies that target specific molecules, including receptors, proteins, genes which impair the development and propagation of tumour growth. | Epidermal Growth Factor Receptor Inhibitor: Cetuximab and Panitumumab Tyrosine Kinase Inhibitor: Regorafenib BRAF Inhibitors: Encorafenib |
| Anti-angiogenic Therapies | Therapies against which targets the protein (VEGF) that promotes vessel development and growth in order to facilitate transportation of nutrients to the tumour in order for tumour growth. | Bevacizumab Ramucirumab Ziv-aflibercept |
| Biotherapies | Therapies that utilize and facilitate a patient’s own immune system in recognizing and killing present cancer cells, e.g., immunotherapies. | Monoclonal Antibodies CAR T-Cell Therapy Immune Checkpoint Inhibitors Cancer Vaccines Immunomodulators |
Biotherapies are a form of therapies encompassed under the umbrella term of immunotherapies in which these agents utilize and enhance a patient’s own immune system to treat cancer.
3. Current Management of Liver Metastases from Colorectal Cancers
3.1. Initial Assessment
Patients with suspected CRC will have undergone several pre-operative evaluations, including have CT Thorax, Abdomen and Pelvis with Contrast. Occasionally, these preliminary imaging modalities identify the presence of a potential primary CRC and/or lesions that are detected within the liver (metachronous or synchronous) [10][11]. We then opt for additional imaging in the format of a contrast magnetic resonance imaging (MRI) of the Pelvis (in case of rectal cancers) and an MRI of the Liver with contrast. MDT involving colorectal surgeons, HPB surgeons, radiologists and oncologists decide on the most favourable management plan given the patient’s pathology encompassing CRC resection, chemotherapy with or without biological agents and hepatic resection. Resectable hepatic disease is considered for pre-operative chemotherapy followed by resection or attempt for immediate curative resection (R0) if pathology meets resectability criteria [12]: (i) CRLM must be resectable with negative margins and allow for the preservation of at least two contiguous liver segments with intact inflow, venous outflow and biliary drainage; (ii) the volume of this future liver remnant (FLR) depends on the functionality (~30% of total liver volume for a normal liver, FLR > 30%, if liver fibrosis is present). The tumour number and location and position are determined with imaging pre-operatively and intra-operatively as previously discussed, and for deeper-seated lesions in the liver parenchyma and smaller than 2 cm in diameter, combined radiofrequency ablation (RFA) is frequently used to minimize liver tissue loss and limits post-resection hepatic dysfunction.
Hepatic resection is considered the primary operation for mCRC if patients have a high tumour burden or with primary tumours located in the rectum, which will require pre-operative chemo-radiotherapy [13][14]. Major hepatic resection is defined as the resection of 3 or more hepatic segments associated with an increased mortality rate of ~15%. The use of neoadjuvant chemotherapy in combination with targeted biotherapies has shown to increase resectability in complex CRLM and unresectable CRLM prior to hepatic resection. In spite of the neoadjuvant therapies, there a credible risk associated with chemotherapy-associated liver injury (CALI), which can impact patient suitability for hepatic resection [15][16]. Hepatic changes such as sinusoidal obstruction, peri-portal inflammation, and steatohepatitis are associated with neoadjuvant therapies, which can alter a patient’s overall outcome [17]. Specifically, the direct use of oxaliplatin is linked to the development of sinusoidal obstruction syndrome in up to 38% of patients, while steatosis and steatohepatitis complicated 9.3% of patients receiving irinotecan. Patients with the following complications were more likely to suffer post-operative complications (severe sinusoidal dilation (OR 1.73) or steatohepatitis (OR 2.08)) [12].
3.2. Perioperative Chemotherapy
The use of chemotherapy in patients with CRLM is usually reserved for patients with borderline resectable disease and patients with unresectable disease (Figure 1). Chemotherapy aids in improving overall patient survival outcomes by facilitating hepatic resections. Recommended guidelines suggest that using FOLFOX or CAPOX, essentially oxaliplatin-based chemotherapy during the neoadjuvant period, is the ideal choice for patients with borderline resectable CRLMs, while FOLFIRI or FOLFOXIRI are alternative options (Table 1) [18]. During the period of delivery of chemotherapy, rigorous interval imaging is required to elicit the timepoint at which the CRLM become clearly resectable from borderline resectable and prepare for hepatic resection [19]. Monitoring CRLM during the active treatment with chemotherapy with or without other targeted therapies is extremely important as there is a genuine risk of in this group of patients developing “disappearing CRLM”, which can complicate the treatment pathway of affected patients [20].
For unresectable disease, conversional chemotherapy with and without biological therapies is used to convert unresectable disease into resectable disease [21][22]. In standard systemic chemotherapy regimens with oxaliplatin +/− irinotecan-based regimens in combination with 5-FU (FOLFOX, FOLFIRI and FOLFOXIRI), studies have shown that chemotherapy has been able to facilitate resections in ~40% of initially unresectable patients [23][24][25]. Neoadjuvant chemotherapy in unresectable disease has demonstrated a reduction in the size of the liver metastases by ~50% in tumour mass: this was seen in upwards of 60% of patients with unresectable CLM with complete resection in 40% of these patients [19][26][27].
A recent systematic review and meta-analysis reviewed the effectiveness of using neoadjuvant chemotherapy plus molecular targeted therapy in unresectable CRLM [28]. The study identified that using chemotherapy plus targeted biological therapy for unresectable CRLM patients had an impact on the overall response rate (ORR). ORR is a measure of the proportion of patients whose disease reduced (partial response–PR) and/or disappears (complete response–CR) after treatment [29]. The study highlighted that patients who received chemotherapy plus molecular targeted therapy had a higher overall response rate when compared with patients using chemotherapy alone (68% vs. 43%), but evidence to suggest improved overall survival (OS) remains inconclusive [28][30].
The EPOC trial also evaluated the Progression-Free Survival (PFS) in patients with unresectable CRLM, which is an important measure in treated metastatic disease. PFS is used as a primary endpoint in trials evaluating the treatment of metastatic cancer. PFS is the length of time during and after the treatment (chemotherapy +/− targeted biological therapies) of unresectable CRLM that a patient lives with stable or improved metastatic disease. PFS for unresectable CRLM patients undertaking perioperative systemic chemotherapy plus EGFR-inhibitor (Cetuximab) vs. chemotherapy indicated that this cohort of patients, who received specifically chemotherapy plus cetuximab, essentially experienced worse PFS when compared with the chemotherapy control group (14.1 vs. 20.5 months in control) [12][22]. The trial concluded that cetuximab should not be given with perioperative chemotherapy regimens; however, the trial emphasized the use of other groups of targeted biological therapies that showed improved PFS and OS. Further combinations of systemic chemotherapeutics and targeted biotherapies were reviewed; Bevacizumab, a vascular endothelial growth factor (VEGF) inhibitor, was combined with FOLFIRI (Folinic acid, fluorouracil and irinotecan) as joint neoadjuvant therapy and yielded a response rate of 66.7% in resectable CRLMs; however, the survival outcomes and benefits are still to be determined.
The PERIMAX trial evaluated the benefits and limitations of a highly active chemotherapy +/− targeted biological therapy regimens in the perioperative and post-operative setting for resectable and unresectable CRC. Patients with resectable liver metastases were randomized into perioperative treatment with FOLFOXIRI and bevacizumab or post-operative FOLFOX, and patients with unresectable mCRC being randomized between FOLFOX and bevacizumab with or without irinotecan. Analysis suggests that the use of cetuximab plus chemotherapy had no impact on overall survival compared with chemotherapy alone for the unresectable CRLM group patients and in resectable CRLM patient cohort, cetuximab use adversely affected OS (HR = 0.95 and 2.35, respectively) [22]. Perioperative chemotherapy offers an opportunity to reduce cancer recurrence post-resection in approximately 70% of patients after resection and drives complete eradication of CRC, and thereby imparting a survival benefit [31][32].
3.3. Synchronous Disease
The liver along with the lung are the most common sites of the metastases of colorectal cancer. Studies have shown about 40–50% of CRC patients will develop liver metastases at a point during the course of CRC disease [33][34]. About 20% of patients often have established synchronous liver metastases when the diagnosis of primary colorectal cancer is made. Synchronous liver metastases are associated with poor outcomes likely caused by poor tumour biology and complex treatment plans [13][35]. Hepatic resection is a definitive treatment option to achieve long-term survival and overall survival, as studies have shown that after primary and secondary resections. CRLM resection offered an overall median survival of 3.6 years; 5- and 10-year survival ranged from 16% to 74% (median 38%) and 9% to 69% (median 26%), respectively. Hepatic resections are divided into selective staged resection, delayed resection or simultaneous resections, with a higher proportion of teams opting for selective staged resection of CRCs, as this is associated with fewer risks accompanied with operating at two sites in simultaneous resection [36][37]. Risks associated with simultaneous resection include intraperitoneal infection, anastomotic fistula and hepatic insufficiency, as well as associated higher mortality. Hepatic resections are divided into three groups: (1) sequential resection (SeR), in which surgical teams are able to resect colorectal cancer and liver metastases without delivering interval chemotherapy; (2) delayed resection (DeR) in which we deliver interval chemotherapy between staged colonic cancer resection and hepatic resection of CRC metastases; and (3) simultaneous resection (SiR), single-stage resection of primary colorectal cancer and liver metastases simultaneously. SiR is performed in patients who fulfil certain criteria (1) the primary tumour was located in the right colon regardless of the tumour disease burden of liver metastases; (2) the tumour disease burden was not heavy, and the tumour number was less than two if the primary tumour was located in the left colon or rectum [38][39].
A staged resection is an important option in managing patients with high tumour burden (tumour number greater than three), rectal tumours requiring chemo-radiotherapy, and patients with significant co-morbidities who all are unlikely to tolerate simultaneous resections [40][41]. DeR selects patients post-primary surgery with resectable liver metastases who were treated with chemotherapy prior to the second resection operation and patients with unresectable liver metastases who were treated with chemotherapy +/− targeted biological therapies and then further evaluated and discussed in MDT after interval [12][42].
3.4. The Issue of Disappearing Liver Metastases
Pre-operative chemotherapy in patients with resectable CRLM can drive the phenomenon of “disappearing” CRLM (DLM) in a quarter of resectable CRLM lesions [20]. This phenomenon is identified using imaging. Despite the positive radiological response, 80% of these lesions remain viable metastases [43]. The risk of DLM is a valid concern when commencing resectable patients on chemotherapeutic agents. CRLMs, particularly in resectable patients, ideally will need to be identified on pre-operative contrast-enhanced imaging, and initially, resection or ablative therapies should be considered as initial treatment. However, the majority of these DLMs are still apparent and identifiable intraoperatively under direct vision and use of intraoperative ultrasonography (IOUS) imaging [44][45]. In patients with high-risk lesions (e.g., deep parenchymal lesions and smaller lesions), often, MDTs opt to limit the cycles of chemotherapeutics and proceed to surgery first. In the event where we are unable to excise all the DLMs or other sites of metastatic disease, these patients often rigorously followed up and managed upon macroscopic recurrence with a staged resection approach [46].
3.5. Patients with Initially Resectable Disease
The use of chemotherapy in patients with initially resectable disease varies from one centre to another; there is still no strict guidelines in the approach to integrating hepatic mCRC resection with systemic chemotherapeutics [47][48]. If patients have four or fewer mCRC resectable lesions with a primary colorectal tumour, then often centres opt for resection first over chemotherapy, unless there is a predicted strong response against chemotherapy. Initial chemotherapeutic treatment is reserved for patients with good exercise tolerance, few co-morbidities, multi-lobar tumour involvement and regional lymph node involvement. These patients are then re-evaluated with interval imaging 6–8 weeks to assess response to the chemotherapy and then re-discussed in MDT prior to surgical resection.
Beppu et al. demonstrated in the EPOC trial highlighted that the optimal neoadjuvant combination regime for patients with initially resectable CRLM with RAS mutations as such FOLFOX, FOLFIRI and/or CAPOX with or without bevacizumab; FOLFIRI with or without cetuximab or panitumumab; or FOLFOX with or without panitumumab or cetuximab (if RAS wild type) [49]. The consideration of using targeted biological agents in tumours that lack RAS/BRAF mutations in left-sided CRC. Research has shown that the site of the primary tumour influences the effectiveness of anti-epidermal growth factor receptor (EGFR) agents; we avoid the use of an anti-EGFR agent in right-sided primary tumours, even if RAS/BRAF wild type [25][50]. Evidence from the EPOC trial highlights that in 272 patients with resectable hepatic metastases of KRAS wild-type mCRC were given FOLFOX with or without cetuximab, pre-operatively and post-operatively for 12 weeks, and it is clear that the addition of cetuximab was associated with significantly worse progression-free survival (PFS) (15.5 versus 22.2 months) [22][51][52].
References
- Saridaki, Z.; Souglakos, J. Genetic Alterations in Colorectal Cancer in Older Patients. In Management of Colorectal Cancers in Older People; Papamichael, D., Audisio, R., Eds.; Springer: London, UK, 2013; pp. 9–20.
- Rivlin, N.; Brosh, R.; Oren, M.; Rotter, V. Mutations in the p53 Tumor Suppressor Gene: Important Milestones at the Various Steps of Tumorigenesis. Genes Cancer 2011, 2, 466–474.
- Dhirendra, K.S.; Dwight, V.N.; McCormick, F. RAS Proteins and Their Regulators in Human Disease. Cell 2017, 170, 17–33.
- Guo, Y.; Pan, W.; Liu, S.; Shen, Z.; Xu, Y.; Hu, L. ERK/MAPK signalling pathway and tumorigenesis (Review). Exp. Ther. Med. 2020, 19, 1997–2007.
- Cheng, Y.; Tian, H. Current Development Status of MEK Inhibitors. Molecules 2017, 22, 1551.
- Maverakis, E.; Tran, K.; Cheng, M.; Mitra, A.; Ogawa, H.; Shi, V.; Olney, L.; Kloxin, A. MEK inhibitors and their potential in the treatment of advanced melanoma: The advantages of combination therapy. Drug Des. Dev. Ther. 2015, 10, 43–52.
- Samir, B.; Arnab, G. Colorectal Liver Metastasis: Current Concepts. Indian J. Surg. 2020, 1–10.
- Martin, J.; Petrillo, A.; Smyth, E.C.; Shaida, N.; Khwaja, S.; Cheow, H.K.; Duckworth, A.; Heister, P.; Praseedom, R.; Jah, A.; et al. Colorectal liver metastases: Current management and future perspectives. World J. Clin. Oncol. 2020, 11, 761–808.
- Voizard, N.; Cerny, M.; Assad, A.; Billiard, J.-S.; Olivié, D.; Perreault, P.; Kielar, A.; Do, R.K.G.; Yokoo, T.; Sirlin, C.B.; et al. Assessment of hepatocellular carcinoma treatment response with LI-RADS: A pictorial review. Insights Imaging 2019, 10, 1–22.
- Tamandl, D.; Mang, T.; Ba-Ssalamah, A. Imaging of colorectal cancer—The clue to individualized treatment. Innov. Surg. Sci. 2020, 3, 3–15.
- Venook, A.P.; Curley, S.A. Management of potentially resectable colorectal cancer liver metastases. World J. Gastrointest. Surg. 2020, 5, 138.
- Chow, F.C.-L.; Chok, K.S.-H. Colorectal liver metastases: An update on multidisciplinary approach. World J. Hepatol. 2019, 11, 150–172.
- Adam, R.; de Gramont, A.; Figueras, J.; Kokudo, N.; Kunstlinger, F.; Loyer, E.; Poston, G.; Rougier, P.; Rubbia-Brandt, L.; Sobrero, A.; et al. Managing synchronous liver metastases from colorectal cancer: A multidisciplinary international consensus. Cancer Treat. Rev. 2015, 41, 729–741.
- Dhir, M.; Sasson, A.R. Surgical Management of Liver Metastases from Colorectal Cancer. J. Oncol. Pr. 2016, 12, 33–39.
- Ono, K.; Abe, T.; Oshita, A.; Sumi, Y.; Yano, T.; Okuda, H.; Kurayoshi, M.; Kobayashi, T.; Ohdan, H.; Noriyuki, T.; et al. Efficacy of upfront hepatectomy without neoadjuvant chemotherapy for resectable colorectal liver metastasis. World J. Surg. Oncol. 2021, 19, 1–8.
- Paulatto, L.; Burgio, M.D.; Sartoris, R.; Beaufrère, A.; Cauchy, F.; Paradis, V.; Vilgrain, V.; Ronot, M. Colorectal liver metastases: Radiopathological correlation. Insights Imaging 2020, 11, 1–19.
- Stevenson, H.L.; Prats, M.M.; Sasatomi, E. Chemotherapy-induced Sinusoidal Injury (CSI) score: A novel histologic assess-ment of chemotherapy-related hepatic sinusoidal injury in patients with colorectal liver metastasis. BMC Cancer 2017, 17, 1–11.
- Pfeiffer, P.; Gruenberger, T.; Glynne-Jones, R. Synchronous liver metastases in patients with rectal cancer: Can we establish which treatment first? Ther. Adv. Med. Oncol. 2018, 10, 1–10.
- Al Bandar, M.H.; Kim, N.K. Current status and future perspectives on treatment of liver metastasis in colorectal cancer (Re-view). Oncol. Rep. 2017, 37, 2553–2564.
- Benoist, S.; Brouquet, A.; Penna, C.; Julié, C.; Hajjam, M.E.; Chagnon, S.; Mitry, E.; Rougier, P.; Nordlinger, B. Complete response of colorectal liver metastases after chemotherapy: Does it mean cure? J. Clin. Oncol. 2006, 24, 3939–3945.
- Symonds, L.K.; Cohen, S.A. Use of perioperative chemotherapy in colorectal cancer metastatic to the liver. Gastroenterol. Rep. 2019, 7, 301–311.
- Primrose, J.; Falk, S.; Finch-Jones, M.; Valle, J.; O’Reilly, D.; Siriwardena, A.; Hornbuckle, J.; Peterson, M.; Rees, M.; Iveson, T.; et al. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metas-tasis (New EPOC): Long-term results of a multicentre, randomized, controlled, phase 3 trial. Lancet Oncol. 2020, 21, 398–411.
- Wensink, E.; Bond, M.; Kucukkose, E.; May, A.; Vink, G.; Koopman, M.; Kranenburg, O.; Roodhart, J. A review of the sensitivity of metastatic colorectal cancer patients with deficient mismatch repair to stand-ard-of-care chemotherapy and monoclonal antibodies, with recommendations for future research. Cancer Treat. Rev. 2021, 95, 102174.
- Poston, G.; Adam, R.; Byrne, B.; Esser, R.; Malik, H.; Wasan, H.; Xu, J. The role of cetuximab in converting initially unresectable colorectal cancer liver metastases for resection. Eur. J. Surg. Oncol. 2017, 43, 2001–2011.
- Xie, Y.-H.; Chen, Y.-X.; Fang, J.-Y. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct. Target. Ther. 2020, 5, 1–30.
- Ichida, H.; Mise, Y.; Ito, H.; Ishizawa, T.; Inoue, Y.; Takahashi, Y.; Shinozaki, E.; Yamaguchi, K.; Saiura, A. Optimal indication criteria for neoadjuvant chemotherapy in patients with resectable colorectal liver metas-tases. World J. Surg. Oncol. 2019, 17, 1–9.
- De Greef, K.; Rolfo, C.; Russo, A.; Chapelle, T.; Bronte, G.; Passiglia, F.; Coelho, A.; Papadimitriou, K.; Peeters, M. Multisciplinary management of patients with liver metastasis from colorectal cancer. World J. Gastroenterol. 2016, 22, 7215–7225.
- Sabanathan, D.; Eslick, G.D.; Shannon, J. Use of Neoadjuvant Chemotherapy Plus Molecular Targeted Therapy in Colorectal Liver Metastases: A Systematic Review and Meta-analysis. Clin. Color. Cancer 2016, 15, e141–e147.
- Villaruz, L.C.; Socinski, M.A. The clinical viewpoint: Definitions, limitations of RECIST, practical considerations of measure-ment. Clin. Cancer Res. 2013, 19, 2629–2636.
- Aykan, N.F.; Özatlı, T. Objective response rate assessment in oncology: Current situation and future expectations. World J. Clin. Oncol. 2020, 11, 53–73.
- Mahvi, D.A.; Liu, R.; Grinstaff, M.W.; Colson, Y.L.; Raut, C.P. Local Cancer Recurrence: The Realities, Challenges, and Opportunities for New Therapies. CA A Cancer J. Clin. 2018, 68, 488–505.
- Tohme, S.; Simmons, R.L.; Tsung, A. Surgery for Cancer: A Trigger for Metastases. Cancer Res. 2017, 77, 1548–1552.
- Engstrand, J.; Nilsson, H.; Strömberg, C.; Jonas, E.; Freedman, J. Colorectal cancer liver metastases—A population-based study on incidence, management and survival. BMC Cancer 2018, 18, 1–11.
- Vatandoust, S.; Price, T.J.; Karapetis, C. Colorectal cancer: Metastases to a single organ. World J. Gastroenterol. 2015, 21, 11767–11776.
- Lam, V.W.T.; Laurence, J.M.; Pang, T.; Johnston, E.; Hollands, M.J.; Pleass, H.C.C.; Richardson, A.J. A systematic review of a liver-first approach in patients with colorectal cancer and synchronous colorec-tal liver metastases. HPB 2014, 16, 101–108.
- Sahlmann, C.-O.; Homayounfar, K.; Niessner, M.; Dyczkowski, J.; Conradi, L.-C.; Braulke, F.; Meller, B.; Beißbarth, T.; Ghadimi, B.M.; Meller, J.; et al. Repeated adjuvant anti-CEA radioimmunotherapy after resection of colorectal liver metastases: Safety, feasibility, and long-term efficacy results of a prospective phase 2 study. Cancer 2017, 123, 638–649.
- Creasy, J.M.; Sadot, E.; Koerkamp, B.G.; Chou, J.F.; Gonen, M.; Kemeny, N.E.; Balachandran, V.P.; Kingham, T.P.; DeMatteo, R.P.; Allen, P.J.; et al. Actual 10-year survival following hepatic resection of colorectal liver metastases: What factors preclude cure? Surgery 2018, 163, 1238–1244.
- Wang, L.-J.; Wang, H.-W.; Jin, K.-M.; Li, J.; Xing, B.-C. Comparison of sequential, delayed and simultaneous resection strategies for synchronous colorectal liver metastases. BMC Surg. 2020, 20, 1–9.
- Du Pasquier, C.; Roulin, D.; Bize, P.; Sempoux, C.; Rebecchini, C.; Montemurro, M.; Schäfer, M.; Halkic, N.; Demartines, N. Tumor response and outcome after reverse treatment for patients with synchronous colorectal liver metastasis: A cohort study. BMC Surg. 2020, 20, 78.
- Feo, L.; Polcino, M.; Nash, G.M. Resection of the Primary Tumor in Stage IV Colorectal Cancer: When Is It Necessary? Surg. Clin. N. Am. 2017, 97, 657–669.
- Kuipers, E.J.; Grady, W.M.; Lieberman, D.; Seufferlein, T.; Sung, J.J.; Boelens, P.G.; van de Velde, C.J.H.T.W. Colorectal Cancer. Nat. Rev. Dis. Prim. 2015, 176, 100–106.
- Basso, M.; Dadduzio, V.; Ardito, F.; Lombardi, P.; Strippoli, A.; Vellone, M.; Orlandi, A.; Rossi, S.; Cerchiaro, E.; Cassano, A.; et al. Conversion Chemotherapy for Technically Unresectable Colorectal Liver Metastases. Medicine 2016, 95, e3722.
- Poultsides, G.A.; Bao, F.; Servais, E.L.; Hernandez-Boussard, T.; DeMatteo, R.P.; Allen, P.J.; Fong, Y.; Kemeny, N.E.; Saltz, L.B.; Klimstra, D.S.; et al. Pathologic response to pre-operative chemotherapy in colorectal liver metastases: Fibrosis, not necrosis, predicts outcome. Ann. Surg. Oncol. 2012, 19, 2797–2804.
- Langella, S.; Ardito, F.; Russolillo, N.; Panettieri, E.; Perotti, S.; Mele, C.; Giuliante, F.; Ferrero, A. Intraoperative Ultrasound Staging for Colorectal Liver Metastases in the Era of Liver-Specific Magnetic Resonance Imaging: Is It Still Worthwhile? J. Oncol. 2019, 2019, 1–8.
- Joo, I. The role of intraoperative ultrasonography in the diagnosis and management of focal hepatic lesions. Ultrasonography 2015, 34, 246–257.
- McKeown, E.; Nelson, D.; Johnson, E.K.; Maykel, J.A.; Stojadinovic, A.; Nissan, A.; Avital, I.; Brücher, B.; Steele, S.R. Current Approaches and Challenges for Monitoring Treatment Response in Colon and Rectal Cancer. J. Cancer 2014, 5, 31–43.
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; van Krieken, J.; Aderka, D.; Aguilar, E.A.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422.
- De’Angelis, N.; Baldini, C.; Brustia, R.; Pessaux, P.; Sommacale, D.; Laurent, A.; Le Roy, B.; Tacher, V.; Kobeiter, H.; Luciani, A.; et al. Surgical and regional treatments for colorectal cancer metastases in older patients: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0230914.
- Ikoma, N.; Raghav, K.; Chang, G. An Update on Randomized Clinical Trials in Metastatic Colorectal Carcinoma. Surg. Oncol. Clin. N. Am. 2017, 26, 667–687.
- Yokota, T. Are KRAS/BRAF Mutations Potent Prognostic and/or Predictive Biomarkers in Colorectal Cancers? Anticancer Agents Med. Chem. 2012, 12, 163–171.
- Yang, Y.F.; Wang, G.Y.; He, J.L.; Wu, F.P.; Zhang, Y.N. Overall survival of patients with KRAS wild-type tumor treated with FOLFOX/FORFIRI±cetuximab as the first-line treatment for metastatic colorectal cancer A meta-analysis. Medicine 2017, 96, 2–7.
- Chan, G.; Chee, C.E. Perioperative Chemotherapy for Liver Metastasis of Colorectal Cancer. Cancers 2020, 12, 3535.
More
Information
Subjects:
Immunology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
3 times
(View History)
Update Date:
19 Aug 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




