
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kenshin Takemura | + 1943 word(s) | 1943 | 2021-08-06 13:42:38 | | | |
| 2 | Conner Chen | Meta information modification | 1943 | 2021-09-22 04:11:12 | | |
Video Upload Options
SPR and LSPR-based virus sensors amplify the signal response of samples at extremely low concentrations, which is otherwise difficult to read and analyze using conventional methods. Commercially available virus detection systems, except PCR, are easy to use and can process multiple samples but have considerable sensitivity limitations, resulting in false negatives. The detection methods based on SPR and LSPR require only virus capturing at the sensor site, rendering the methods simple and highly sensitive.
1. Introduction
The current COVID-19 pandemic caused by SARS-CoV-2 has revealed how highly infectious emerging viruses spread in modern society and the gravity of social consequences such infections can cause [1][2][3]. Viral infections can spread rapidly, especially via droplet transmission, as in the case of SARS-CoV-2 and similar viruses [4]. Nowadays, where people travel around the world with much ease, viruses capable of spreading via droplet transmission are one of the most dangerous causes of infectious diseases. Outbreaks of emerging viruses have been attributed mainly to adaptive outbreaks [5][6]. Genetic mutations that allow infection of previously uninfected hosts can lead to explosive pandemics [7]. These facts imply that emerging viral outbreaks and pandemics will continue to occur with high transmissibility. One way to prevent or control such pandemics is to develop a virus detection technology that enables the prevention of the global spread of viruses.
Polymerase chain reaction (PCR)-based detection methods form the gold standard methods for virus detection [8]. However, the cost of equipment, the need for skilled technicians, and the time required for detection are their limitations [9]. For the SARS-CoV-2 pandemic, the problem was almost solved by expanding the number of sites with PCR facilities and active research and development worldwide [10][11][12]. In recent years, the shortage of skilled personnel, which is the most important limitation of PCR, is also being solved by automating the PCR system to detect the extracted viral RNA [13][14]. Therefore, next-generation technologies should have a reliable single-step detection of the target virus and require minimum time for detection.
On the surface of a solid material such as a metal with a free charge, the surface charge (mostly electrons) oscillates collectively due to light irradiation. This phenomenon is called surface plasmon resonance (SPR). [15]. SPR-induced surface charge oscillations are coupled with electromagnetic waves under certain conditions. The quantum of these oscillations is described as the surface plasmon polariton (SPP), and the excitation of SPP is an essential step in SPR biosensors [16][17][18]. SPR significantly increases the surface sensitivity of the substrates to react with the target material in certain spectroscopic measurement techniques, as characterized by the conditions of resonance between the irradiated light and the substrate [19][20]. The increased sensitivity of SPR has also been applied in virus detection techniques [21][22][23][24][25]. Compared to SPR on an individual substrate such as a thin metal film, the plasmon phenomenon similarly produced by irradiating light on metal nanoparticles is described as localized SPR (LSPR) [26]. The plasmon phenomenon generated on the nanoparticle surface generates a strong electric field in the vicinity of the nanoparticles [27]. Within this plasmon region, the interaction of light with molecules and other types of fluorescent nanomaterials is enhanced [28]. Furthermore, when nanoparticles that form an electric field are in close proximity to each other or bind together, a significantly enhanced electric field is formed between the particles, and in this electric field, Raman scattering of certain chemical species is electromagnetically enhanced [29]. Surface-enhanced infrared absorption (SEIRA) has also been reported as an LSPR-based application [30][31]. The potential for application in biosensing is high, because infrared absorption can be induced in the infrared region by adjusting the shape and size of the nanoparticles. [32]. The plasmon resonance effect can be applied to various biosensing fields because it produces a strong optical response and signal enhancement at the micro/nanoscale.
2. Current Advancements in SPR-Based Sensor to Detect Virus Particles
In order to effectively utilize the properties of SPR, a method to specifically bind the virus to the metal film is necessary. Antibodies are suitable as trapping materials because they can be modified based on the substrate using chemical cross-linking, and by selecting a structure with high specificity, a highly selective sensor can be easily constructed [33].
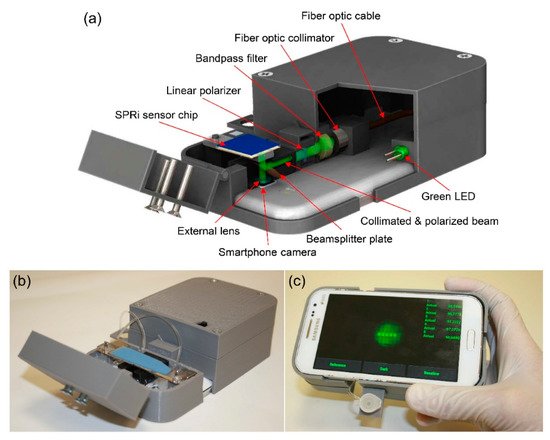
The research focus in recent years on virus detection technologies has often been on sensitivity and speed, and for on-site detection technologies, whether the system can be handheld or wireless and employs reusable methods [46][47][48][49][50]. As for the research on virus detection technology using SPR, the major points required in the future are not high sensitivity and rapidity, but thin film formation and suitable construction systems to enhance the practicality and applicability.
3. LSPR Phenomenon on Nanoscale Systems and Application of LSPR to Virus Sensing
After LSPR, an enhancement of electrolysis and light quenching occurs in the vicinity of the nanoparticles, based on the size, shape, and material used [51][52][53]. Therefore, the size and shape of the nanoparticles need to be precisely controlled to obtain the desired optical response. The most fundamental application of optical signal response by LSPR is the change in the peak absorption wavelength due to the local refractive index change on the nanoparticle surface (plasmon peak shift) and the fluorescence enhancement effect caused by using near-field light.
The near-field light formed near the noble metal nanoparticles by LSPR enhances the interaction of the light with the fluorescent material present at a certain distance in the region [54][55][56]. The resulting significant fluorescence enhancement effect of the fluorophores is widely used in the development of highly sensitive sensing techniques, as the effect enables the emittance of signal responses even from small amounts of sample. These observations render LSPR a potential signal amplification method for virus sensing [57].
Kim et al. designed a structure in which a virus was sandwiched between two different sizes of AuNP on an AuNP-laden substrate [58]. In this method, two gold nanoparticles in close proximity repel each other in a plasmon resonance state in the presence of the virus, resulting in a stronger peak shift effect than that in the non-sandwich state. The signal enhancement effect of the sandwich structure has been demonstrated, and a 100-fold increase in the sensitivity has been successfully achieved compared to the virus captured by AuNP-laden substrate only. Furthermore, the sensitivity of the sandwich structure differed depending on the size of the gold nanoparticles on the secondary antibody site. The smaller the particle, the stronger the signal response, even at low sample concentrations. This work demonstrated that particle size has a significant effect on the use of LSPR for developing application-based systems.
The LSPR-based fluorescence virus sensor should have a controlled nanoscale distance between the plasmon particles and fluorescence material. In the case of antigen–antibody reaction, forming a thin film on the plasmon particles beforehand to control the nanometer distance is effective [59]. A typical example of functionalization of nanomaterials is the self-assembled monolayer of the layer-by-layer method [60][61][62]. Both methods essentially require a thin film of desired thickness and the modification of antibodies and aptamers. When virus-specific DNA/RNA is used as a probe, the length of the sequence during hybridization can be easily adjusted. Therefore, it is only necessary to functionalize the probe directly on the plasmon particle [63][64]. The development of virus detection technology based on the LSPR fluorescence enhancement effect is challenging because it requires precise control of the nanoparticles’ size and particle distance to detect viruses with higher sensitivity. However, developing a simple and reproducible control method will be a necessary element for the next generation of LSPR detection technology, as all the reported studies have succeeded in detecting viruses with high sensitivity.
4. Conclusions
SPR and LSPR-based virus sensors amplify the signal response of samples at extremely low concentrations, which is otherwise difficult to read and analyze using conventional methods. Commercially available virus detection systems, except PCR, are easy to use and can process multiple samples but have considerable sensitivity limitations, resulting in false negatives. The detection methods based on SPR and LSPR require only virus capturing at the sensor site, rendering the methods simple and highly sensitive. To achieve high sensitivity, the structure and thickness of the thin metal film must be optimized for the SPR-based sensor, and the size and shape of the plasmon particles must be controlled for the LSPR-based sensor. In addition, the SPR-based sensors are witnessing advancements with respect to reusability and miniaturization. As for LSPR, it is easy to construct a simple system to observe the optical response effectively. Collectively, studies on SPR and LSPR-based virus sensing research have already demonstrated sufficient sensitivity. In particular, LSPR is more advantageous for miniaturization because it requires only a light source and a detector as the device configuration. Therefore, as with the SPR and LSPR system, more practical developmental research will continue to progress in the future. The next-generation virus detection technology should combine simplicity and rapidity with high sensitivity.
References
- Chowell, G.; Mizumoto, K. The COVID-19 pandemic in the USA: What might we expect? Lancet 2020, 395, 1093–1094.
- Sarkar, K.; Khajanchi, S.; Nieto, J.J. Modeling and forecasting the COVID-19 pandemic in India. Chaos Solitons Fractals 2020, 139, 110049.
- Lodigiani, C.; Iapichino, G.; Carenzo, L.; Cecconi, M.; Ferrazzi, P.; Sebastian, T.; Kucher, N.; Studt, J.-D.; Sacco, C.; Alexia, B. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb. Res. 2020, 191, 9–14.
- Dbouk, T.; Drikakis, D. On coughing and airborne droplet transmission to humans. Phys. Fluids 2020, 32, 053310.
- Klontz, K.C.; Hynes, N.A.; Gunn, R.A.; Wilder, M.H.; Harmon, M.W.; Kendal, A.P. An outbreak of influenza A/Taiwan/1/86 (H1N1) infections at a naval base and its association with airplane travel. Am. J. Epidemiol. 1989, 129, 341–348.
- Sasaki, T.; Higa, Y.; Bertuso, A.G.; Isawa, H.; Takasaki, T.; Minakawa, N.; Sawabe, K. Susceptibility of Indigenous and Transplanted Mosquito spp. in Japan to Dengue Virus. Jpn. J. Infect. Dis. 2015, 68, 425–427.
- Starr, T.N.; Greaney, A.J.; Addetia, A.; Hannon, W.W.; Choudhary, M.C.; Dingens, A.S.; Li, J.Z.; Bloom, J.D. Prospective mapping of viral mutations that escape antibodies used to treat COVID-19. Science 2021, 371, 850–854.
- Atmar, R.L.; Neill, F.H.; Romalde, J.L.; Le Guyader, F.; Woodley, C.M.; Metcalf, T.G.; Estes, M.K. Detection of Norwalk virus and hepatitis A virus in shellfish tissues with the PCR. Appl. Environ. Microbiol. 1995, 61, 3014–3018.
- Watzinger, F.; Ebner, K.; Lion, T. Detection and monitoring of virus infections by real-time PCR. Mol. Asp. Med. 2006, 27, 254–298.
- Khandurina, J.; McKnight, T.E.; Jacobson, S.C.; Waters, L.C.; Foote, R.S.; Ramsey, J.M. Integrated system for rapid PCR-based DNA analysis in microfluidic devices. Anal. Chem. 2000, 72, 2995–3000.
- Lanciotti, R.S.; Kerst, A.J.; Nasci, R.S.; Godsey, M.S.; Mitchell, C.J.; Savage, H.M.; Komar, N.; Panella, N.A.; Allen, B.C.; Volpe, K.E. Rapid detection of West Nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J. Clin. Microbiol. 2000, 38, 4066–4071.
- Jothikumar, N.; Cromeans, T.L.; Robertson, B.H.; Meng, X.; Hill, V.R. A broadly reactive one-step real-time RT-PCR assay for rapid and sensitive detection of hepatitis E virus. J. Virol. Methods 2006, 131, 65–71.
- Nörz, D.; Fischer, N.; Schultze, A.; Kluge, S.; Mayer-Runge, U.; Aepfelbacher, M.; Pfefferle, S.; Lütgehetmann, M. Clinical evaluation of a SARS-CoV-2 RT-PCR assay on a fully automated system for rapid on-demand testing in the hospital setting. J. Clin. Virol. 2020, 128, 104390.
- Emery, C.; Relich, R.; Davis, T.; Young, S.; Sims, M.; Boyanton, B., Jr. Multicenter evaluation of NeuMoDx group B Streptococcus assay on the NeuMoDx 288 molecular system. J. Clin. Microbiol. 2019, 57, e01324-18.
- Pockrand, I.; Swalen, J.; Gordon Ii, J.; Philpott, M. Surface plasmon spectroscopy of organic monolayer assemblies. Surf. Sci. 1978, 74, 237–244.
- Barnes, W.L. Surface plasmon-polariton length scales: A route to sub-wavelength optics. J. Opt. A Pure Appl. Opt. 2006, 8, S87.
- Weeber, J.-C.; Krenn, J.R.; Dereux, A.; Lamprecht, B.; Lacroute, Y.; Goudonnet, J.-P. Near-field observation of surface plasmon polariton propagation on thin metal stripes. Phys. Rev. B 2001, 64, 045411.
- Wang, B.; Wang, G.P. Surface plasmon polariton propagation in nanoscale metal gap waveguides. Opt. Lett. 2004, 29, 1992–1994.
- Lahav, A.; Auslender, M.; Abdulhalim, I. Sensitivity enhancement of guided-wave surface-plasmon resonance sensors. Opt. Lett. 2008, 33, 2539–2541.
- Hoa, X.D.; Kirk, A.; Tabrizian, M. Towards integrated and sensitive surface plasmon resonance biosensors: A review of recent progress. Biosens. Bioelectron. 2007, 23, 151–160.
- Boltovets, P.; Snopok, B.; Boyko, V.; Shevchenko, T.; Dyachenko, N.; Shirshov, Y.M. Detection of plant viruses using a surface plasmon resonance via complexing with specific antibodies. J. Virol. Methods 2004, 121, 101–106.
- Baac, H.; Hajós, J.P.; Lee, J.; Kim, D.; Kim, S.J.; Shuler, M.L. Antibody-based surface plasmon resonance detection of intact viral pathogen. Biotechnol. Bioeng. 2006, 94, 815–819.
- Lei, Y.; Chen, H.; Dai, H.; Zeng, Z.; Lin, Y.; Zhou, F.; Pang, D. Electroless-plated gold films for sensitive surface plasmon resonance detection of white spot syndrome virus. Biosens. Bioelectron. 2008, 23, 1200–1207.
- Chinowsky, T.M.; Soelberg, S.D.; Baker, P.; Swanson, N.R.; Kauffman, P.; Mactutis, A.; Grow, M.S.; Atmar, R.; Yee, S.S.; Furlong, C.E. PorTable 24-analyte surface plasmon resonance instruments for rapid, versatile biodetection. Biosens. Bioelectron. 2007, 22, 2268–2275.
- Suenaga, E.; Mizuno, H.; Kumar, P.K. Influenza virus surveillance using surface plasmon resonance. Virulence 2012, 3, 464–470.
- Hutter, E.; Fendler, J.H. Exploitation of localized surface plasmon resonance. Adv. Mater. 2004, 16, 1685–1706.
- Willets, K.A.; Van Duyne, R.P. Localized surface plasmon resonance spectroscopy and sensing. Annu. Rev. Phys. Chem. 2007, 58, 267–297.
- Stranik, O.; McEvoy, H.; McDonagh, C.; MacCraith, B. Plasmonic enhancement of fluorescence for sensor applications. Sens. Actuators B Chem. 2005, 107, 148–153.
- Stiles, P.L.; Dieringer, J.A.; Shah, N.C.; Van Duyne, R.P. Surface-enhanced Raman spectroscopy. Annu. Rev. Anal. Chem. 2008, 1, 601–626.
- Osawa, M. Surface-enhanced infrared absorption. Near-Field Opt. Surf. Plasmon Polaritons 2001, 81, 163–187.
- Kundu, J.; Le, F.; Nordlander, P.; Halas, N.J. Surface enhanced infrared absorption (SEIRA) spectroscopy on nanoshell aggregate substrates. Chem. Phys. Lett. 2008, 452, 115–119.
- Hartstein, A.; Kirtley, J.; Tsang, J. Enhancement of the infrared absorption from molecular monolayers with thin metal overlayers. Phys. Rev. Lett. 1980, 45, 201.
- Iqbal, S.S.; Mayo, M.W.; Bruno, J.G.; Bronk, B.V.; Batt, C.A.; Chambers, J.P. A review of molecular recognition technologies for detection of biological threat agents. Biosens. Bioelectron. 2000, 15, 549–578.
- Ahmed, S.R.; Kim, J.; Suzuki, T.; Lee, J.; Park, E.Y. Enhanced catalytic activity of gold nanoparticle-carbon nanotube hybrids for influenza virus detection. Biosens. Bioelectron. 2016, 85, 503–508.
- Tokuno, O.; Fujiwara, M.; Nakajoh, Y.; Yamanouchi, S.; Adachi, M.; Ikeda, A.; Kitayama, S.; Takahashi, T.; Kase, T.; Kinoshita, S. Comparison of detection sensitivity in rapid-diagnosis influenza virus kits. Kansenshogaku Zasshi. J. Jpn. Associ. Infect. Dis. 2009, 83, 525–533.
- Sun, J.; Lei, X.; Wang, W.; Liu, Y.; Liang, P.; Bao, H.; Wang, Q.; Guo, Y.; Yang, J.; Yan, Z. Development and evaluation of a paramagnetic nanoparticle based immunochromatographic strip for specific detection of 2009 H1N1 influenza virus. J. Nanosci. Nanotechnol. 2013, 13, 1684–1690.
- Chiu, N.-F.; Fan, S.-Y.; Yang, C.-D.; Huang, T.-Y. Carboxyl-functionalized graphene oxide composites as SPR biosensors with enhanced sensitivity for immunoaffinity detection. Biosens. Bioelectron. 2017, 89, 370–376.
- Wu, L.; Jia, Y.; Jiang, L.; Guo, J.; Dai, X.; Xiang, Y.; Fan, D. Sensitivity improved SPR biosensor based on the MoS2/graphene–aluminum hybrid structure. J. Light. Technol. 2016, 35, 82–87.
- Kumar, R.; Kushwaha, A.S.; Srivastava, M.; Mishra, H.; Srivastava, S. Enhancement in sensitivity of graphene-based zinc oxide assisted bimetallic surface plasmon resonance (SPR) biosensor. J. Appl. Phys. 2018, 124, 1–10.
- Bai, H.; Wang, R.; Hargis, B.; Lu, H.; Li, Y. A SPR aptasensor for detection of avian influenza virus H5N1. Sensors 2012, 12, 12506–12518.
- Nguyen, V.-T.; Seo, H.B.; Kim, B.C.; Kim, S.K.; Song, C.-S.; Gu, M.B. Highly sensitive sandwich-type SPR based detection of whole H5Nx viruses using a pair of aptamers. Biosens. Bioelectron. 2016, 86, 293–300.
- Kumar, P.K. Monitoring intact viruses using aptamers. Biosensors 2016, 6, 40.
- Vindas, K.; Leroy, L.; Garrigue, P.; Voci, S.; Livache, T.; Arbault, S.; Sojic, N.; Buhot, A.; Engel, E. Highly parallel remote SPR detection of DNA hybridization by micropillar optical arrays. Anal. Bioanal. Chem. 2019, 411, 2249–2259.
- Jordan, C.E.; Frutos, A.G.; Thiel, A.J.; Corn, R.M. Surface plasmon resonance imaging measurements of DNA hybridization adsorption and streptavidin/DNA multilayer formation at chemically modified gold surfaces. Anal. Chem. 1997, 69, 4939–4947.
- Guner, H.; Ozgur, E.; Kokturk, G.; Celik, M.; Esen, E.; Topal, A.E.; Ayas, S.; Uludag, Y.; Elbuken, C.; Dana, A. A smartphone based surface plasmon resonance imaging (SPRi) platform for on-site biodetection. Sens. Actuators B Chem. 2017, 239, 571–577.
- Raziq, A.; Kidakova, A.; Boroznjak, R.; Reut, J.; Öpik, A.; Syritski, V. Development of a portable MIP-based electrochemical sensor for detection of SARS-CoV-2 antigen. Biosens. Bioelectron. 2021, 178, 113029.
- Wang, B.; Li, B.; Huang, H.; Yang, S.; Jian, D.; Liu, J.; Yan, K.; Shan, Y.; Wang, S.; Liu, F. Sensitive antibody fluorescence immunosorbent assay (SAFIA) for rapid on-site detection on avian influenza virus H9N2 antibody. Anal. Chim. Acta 2021, 1164, 338524.
- Barauna, V.G.; Singh, M.N.; Barbosa, L.L.; Marcarini, W.D.; Vassallo, P.F.; Mill, J.G.; Ribeiro-Rodrigues, R.; Campos, L.C.; Warnke, P.H.; Martin, F.L. Ultrarapid On-Site Detection of SARS-CoV-2 Infection Using Simple ATR-FTIR Spectroscopy and an Analysis Algorithm: High Sensitivity and Specificity. Anal. Chem. 2021, 93, 2950–2958.
- Rodriguez-Manzano, J.; Malpartida-Cardenas, K.; Moser, N.; Pennisi, I.; Cavuto, M.; Miglietta, L.; Moniri, A.; Penn, R.; Satta, G.; Randell, P. Handheld point-of-care system for rapid detection of SARS-CoV-2 extracted RNA in under 20 min. ACS Cent. Sci. 2021, 7, 307–317.
- Suenaga, E.; Mizuno, H.; Penmetcha, K.K. Monitoring influenza hemagglutinin and glycan interactions using surface plasmon resonance. Biosens. Bioelectron. 2012, 32, 195–201.
- Mayer, K.M.; Hafner, J.H. Localized surface plasmon resonance sensors. Chem. Rev. 2011, 111, 3828–3857.
- Yockell-Lelièvre, H.; Lussier, F.; Masson, J.-F. Influence of the particle shape and density of self-assembled gold nanoparticle sensors on LSPR and SERS. J. Phys. Chem. C 2015, 119, 28577–28585.
- Zoric, I.; Zach, M.; Kasemo, B.; Langhammer, C. Gold, platinum, and aluminum nanodisk plasmons: Material independence, subradiance, and damping mechanisms. ACS Nano 2011, 5, 2535–2546.
- Shen, Y.; He, T.; Wang, W.; Zhan, Y.; Hu, X.; Yuan, B.; Zhou, X. Fluorescence enhancement on silver nanoplates at the single-and sub-nanoparticle level. Nanoscale 2015, 7, 20132–20141.
- Schmelzeisen, M.; Zhao, Y.; Klapper, M.; Müllen, K.; Kreiter, M. Fluorescence enhancement from individual plasmonic gap resonances. ACS Nano 2010, 4, 3309–3317.
- Bardhan, R.; Grady, N.K.; Cole, J.R.; Joshi, A.; Halas, N.J. Fluorescence enhancement by Au nanostructures: Nanoshells and nanorods. ACS Nano 2009, 3, 744–752.
- Adegoke, O.; Morita, M.; Kato, T.; Ito, M.; Suzuki, T.; Park, E.Y. Localized surface plasmon resonance-mediated fluorescence signals in plasmonic nanoparticle-quantum dot hybrids for ultrasensitive Zika virus RNA detection via hairpin hybridization assays. Biosens. Bioelectron. 2017, 94, 513–522.
- Kim, J.; Oh, S.Y.; Shukla, S.; Hong, S.B.; Heo, N.S.; Bajpai, V.K.; Chun, H.S.; Jo, C.-H.; Choi, B.G.; Huh, Y.S. Heteroassembled gold nanoparticles with sandwich-immunoassay LSPR chip format for rapid and sensitive detection of hepatitis B virus surface antigen (HBsAg). Biosens. Bioelectron. 2018, 107, 118–122.
- Malikova, N.; Pastoriza-Santos, I.; Schierhorn, M.; Kotov, N.A.; Liz-Marzán, L.M. Layer-by-layer assembled mixed spherical and planar gold nanoparticles: Control of interparticle interactions. Langmuir 2002, 18, 3694–3697.
- Sharma, A.; Matharu, Z.; Sumana, G.; Solanki, P.R.; Kim, C.; Malhotra, B. Antibody immobilized cysteamine functionalized-gold nanoparticles for aflatoxin detection. Thin Solid Film. 2010, 519, 1213–1218.
- Santhanam, V.; Liu, J.; Agarwal, R.; Andres, R.P. Self-assembly of uniform monolayer arrays of nanoparticles. Langmuir 2003, 19, 7881–7887.
- Srivastava, S.; Kotov, N.A. Composite layer-by-layer (LBL) assembly with inorganic nanoparticles and nanowires. Acc. Chem. Res. 2008, 41, 1831–1841.
- Dutta Chowdhury, A.; Ganganboina, A.B.; Nasrin, F.; Takemura, K.; Doong, R.-A.; Utomo, D.I.S.; Lee, J.; Khoris, I.M.; Park, E.Y. Femtomolar detection of dengue virus DNA with serotype identification ability. Anal. Chem. 2018, 90, 12464–12474.
- Mei, Z.; Tang, L. Surface-plasmon-coupled fluorescence enhancement based on ordered gold nanorod array biochip for ultrasensitive DNA analysis. Anal. Chem. 2017, 89, 633–639.




