
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alessandro Arrigo | + 1750 word(s) | 1750 | 2021-07-31 16:15:54 |
Video Upload Options
The human retina may be affected by two macro groups of diseases, namely maculopathies and retinopathies. Whereas maculopathies are confined to the central part of the retina, bounded by the vascular arcades, retinopathies may extend up to the extreme retinal periphery. These two categories can be further subdivided according to the main features characterizing the disease, thus taking into consideration exudative or atrophic phenomena.
1. Introduction
The management of exudative retinal diseases underwent a revolution due to the introduction of intravitreal treatments. There are two main classes of intravitreal drugs, namely anti-vascular endothelial growth factors (anti-VEGF) and corticosteroids molecules. The clinical course and the outcome of retinal diseases radically changed thanks to the efficacy of these molecules in determining the regression of the exudation and the restoration of the macular profile.
Exudation is an active process, and its nature depends on each specific retinal disease, causing fluid to accumulate within the retina or in the subretinal space. It mainly involves variable amounts of fluid, the major pathogenic features of which are the breakdown of the blood-retinal barrier and increased inflammation [1][2][3]. Retinal diseases can also be characterized by other types of debris, including lipofuscin and lipidic and proteinaceous materials [3][4]. Retinal diseases can also be characterized by the progressive degeneration of inner and outer retinal layers. These atrophic changes may occur independently or in the context of an initial exudative disease [3][5].
Current retinal therapeutic approaches are based on these premises and designed to prompt the exudation to regress, stimulate debris reabsorption or prevent the atrophy from expanding.
2. Retinal Drugs for Exudative Diseases
The prognosis of retinal exudative diseases changed radically after the introduction of intravitreal therapies. While the old laser-based treatments were effective in blocking exudation, they were associated with an extremely poor visual outcome [6][7][8]; nowadays, patients can expect to preserve their quality of life and a good visual function. The current intravitreal therapeutic bullets consist of anti-vascular endothelial growth factor (anti-VEGF) and corticosteroids. The pros of anti-VEGF drugs are their easier management and the low instance of side effects; the cons comprise their limited duration, meaning a large number of injections are required, and their contraindication in patients displaying a high risk of cardiovascular dysfunction. In contrast, the pros of corticosteroids include their longer duration, thus reducing the number of injections administered and their greater anti-inflammatory action. Conversely, corticosteroids are closely associated with an increase in intraocular pressure and a faster progression of cataracts.
3. The Role of Inflammation in the Human Retina
4. Emerging Therapies for Exudative Retinal Diseases
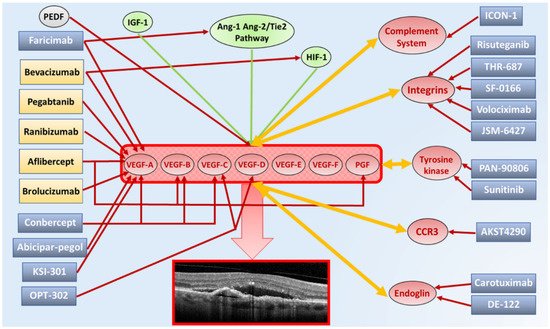
4.1. AKST4290
4.2. Carotuximab
4.3. Complement Inhibitors
4.4. Integrins Inhibitors
4.5. KSI-301
5. Retinal Drugs for Non-Exudative Diseases
5.1. Complement Inhibitors
5.2. Brimonidine
References
- Díaz-Coránguez, M.; Ramos, C.; Antonetti, D.A. The inner blood-retinal barrier: Cellular basis and development. Vision Res. 2017, 139, 123–137.
- Antonetti, D.A.; Klein, R.; Gardner, T.W. Diabetic retinopathy. N. Engl. J. Med. 2012, 366, 1227–1239.
- De Jong, P.T. Age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1474–1485.
- Davoudi, S.; Papavasileiou, E.; Roohipoor, R.; Cho, H.; Kudrimoti, S.; Hancock, H.; Hoadley, S.; Andreoli, C.; Husain, D.; James, M.; et al. Optical coherence tomography characteristics of macular edema and hard exudates and their association with lipid serum levels in type 2 diabetes. Retina 2016, 36, 1622–1629.
- Jager, R.D.; Mieler, W.F.; Miller, J.W. Age-related macular degeneration. N. Engl. J. Med. 2008, 358, 2606–2617.
- Jost, B.F.; Alexander, M.F.; Maguire, M.G.; Fine, S.L.; Chamberlin, J.A.; Murphy, R.P. Laser treatment for choroidal neovascularization outside randomized clinical trials. Arch. Ophthalmol. 1988, 106, 357–361.
- Shah, C.P.; Chen, C. Review of therapeutic advances in diabetic retinopathy. Ther. Adv. Endocrinol. Metab. 2011, 2, 39–53.
- Stenner, A.M.; Frederiksen, K.H.; Grauslund, J. Is there still a role of macular laser treatment in branch retinal vein occlusion in the era of intravitreal injections? Acta Ophthalmol. 2020, 98, 9–21.
- Hageman, G.S.; Luthert, P.J.; Victor Chong, N.H.; Johnson, L.V.; Anderson, D.H.; Mullins, R.F. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch’s membrane interface in aging and age-related macular degeneration. Prog. Retin. Eye Res. 2001, 20, 705–732.
- Xu, H.; Chen, M.; Forrester, J.V. Para-inflammation in the aging retina. Prog. Retin. Eye Res. 2009, 28, 348–368.
- Tang, J.; Kern, T.S. Inflammation in diabetic retinopathy. Prog. Retin. Eye Res. 2011, 30, 343–358.
- Joussen, A.M.; Murata, T.; Tsujikawa, A.; Kirchhof, B.; Bursell, S.E.; Adamis, A.P. Leukocyte-mediated endothelial cell injury and death in the diabetic retina. Am. J. Pathol. 2001, 158, 147–152.
- Koss, M.J.; Pfister, M.; Rothweiler, F.; Michaelis, M.; Cinatl, J.; Schubert, R.; Koch, F.H. Comparison of cytokine levels from undiluted vitreous of untreated patients with retinal vein occlusion. Acta Ophthalmol. 2012, 90, e98–e103.
- De Smet, M.D.; Taylor, S.R.; Bodaghi, B.; Miserocchi, E.; Murray, P.I.; Pleyer, U.; Zierhut, M.; Barisani-Asenbauer, T.; LeHoang, P.; Lightman, S. Understanding uveitis: The impact of research on visual outcomes. Prog. Retin. Eye Res. 2011, 30, 452–470.
- Olivares-González, L.; Velasco, S.; Campillo, I.; Rodrigo, R. Retinal Inflammation, Cell Death and Inherited Retinal Dystrophies. Int. J. Mol. Sci. 2021, 22, 2096.
- Sharma, N.K.; Prabhakar, S.; Gupta, A.; Singh, R.; Gupta, P.K.; Gupta, P.K.; Anand, A. New biomarker for neovascular age-related macular degeneration: Eotaxin-2. DNA Cell Biol. 2012, 31, 1618–1627.
- Grisanti, S.; Canbek, S.; Kaiserling, E.; Adam, A.; Lafaut, B.; Gelisken, F.; Szurman, P.; Henke-Fahle, S.; Oficjalska-Mlynczak, J.; Bartz-Schmidt, K.U. Expression of endoglin in choroidal neovascularization. Exp. Eye Res. 2004, 78, 207–213.
- Shen, W.; Lee, S.R.; Yam, M.; Zhu, L.; Zhang, T.; Pye, V.; Mathai, A.E.; Shibagaki, K.; Zhang, J.Z.; Matsugi, T.; et al. A Combination Therapy Targeting Endoglin and VEGF-A Prevents Subretinal Fibro-Neovascularization Caused by Induced Müller Cell Disruption. Investig. Ophthalmol. Vis. Sci. 2018, 59, 6075–6088.
- Frederick, P.A.; Kleinman, M.E. The Immune System and AMD. Curr. Ophthalmol. Rep. 2014, 2, 14–19.
- Pan, W.W.; Lin, F.; Fort, P.E. The innate immune system in diabetic retinopathy. Prog. Retin. Eye Res. 2021, 100940.
- Sodi, A.; Passerini, I.; Bacherini, D.; Boni, L.; Palchetti, S.; Murro, V.; Caporossi, O.; Mucciolo, D.P.; Franco, F.; Vannozzi, L.; et al. CFH Y402H polymorphism in Italian patients with age-related macular degeneration, retinitis pigmentosa, and Stargardt disease. Ophthalm. Genet. 2018, 39, 699–705.
- Williams, M.A.; McKay, G.J.; Chakravarthy, U. Complement inhibitors for age-related macular degeneration. Cochrane Database Syst. Rev. 2014, 2014, CD009300.
- Park, D.H.; Connor, K.M.; Lambris, J.D. The Challenges and Promise of Complement Therapeutics for Ocular Diseases. Front. Immunol. 2019, 10, 1007.
- Wu, J.; Sun, X. Complement system and age-related macular degeneration: Drugs and challenges. Drug Des. Devel. Ther. 2019, 13, 2413–2425.
- Li, M.; Sakaguchi, D.S. Inhibition of integrin-mediated adhesion and signaling disrupts retinal development. Dev. Biol. 2004, 275, 202–214.
- Bhatwadekar, A.D.; Kansara, V.; Luo, Q.; Ciulla, T. Anti-integrin therapy for retinovascular diseases. Exp. Opin. Investig. Drugs 2020, 29, 935–945.
- Boyer, D.S.; Schmidt-Erfurth, U.; van Lookeren Campagne, M.; Henry, E.C.; Brittain, C. The pathophysiology of geographic atrophy secondary to age-related macular degeneration and the complement pathway as a therapeutic target. Retina 2017, 37, 819–835.
- Boon, C.J.; den Hollander, A.I.; Hoyng, C.B.; Cremers, F.P.; Klevering, B.J.; Keunen, J.E. The spectrum of retinal dystrophies caused by mutations in the peripherin/RDS gene. Prog. Retin. Eye Res. 2008, 27, 213–235.
- Bird, A.C. Retinal photoreceptor dystrophies. Am. J. Ophthalmol. 1995, 119, 543–562.
- Parodi, M.B.; Zucchiatti, I.; Cicinelli, M.V.; Cascavilla, M.L.; Bandello, F. Nutritional supplementation in age-related macular degeneration. Retina 2016, 36, 1119–1125.
- Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch. Ophthalmol. 2001, 119, 1417–1436.
- Group, A.R.; Chew, E.Y.; Clemons, T.; SanGiovanni, J.P.; Danis, R.; Domalpally, A.; McBee, W.; Sperduto, R.; Ferris, F.L. The Age-Related Eye Disease Study 2 (AREDS2): Study design and baseline characteristics (AREDS2 report number 1). Ophthalmology 2012, 119, 2282–2289.
- Brito-García, N.; Del Pino-Sedeño, T.; Trujillo-Martín, M.M.; Coco, R.M.; Rodríguez de la Rúa, E.; Del Cura-González, I.; Serrano-Aguilar, P. Effectiveness and safety of nutritional supplements in the treatment of hereditary retinal dystrophies: A systematic review. Eye 2017, 31, 273–285.
- Liao, D.S.; Grossi, F.V.; El Mehdi, D.; Gerber, M.R.; Brown, D.M.; Heier, J.S.; Wykoff, C.C.; Singerman, L.J.; Abraham, P.; Grassmann, F.; et al. Complement C3 Inhibitor Pegcetacoplan for Geographic Atrophy Secondary to Age-Related Macular Degeneration: A Randomized Phase 2 Trial. Ophthalmology 2020, 127, 186–195.
- Saylor, M.; McLoon, L.K.; Harrison, A.R.; Lee, M.S. Experimental and clinical evidence for brimonidine as an optic nerve and retinal neuroprotective agent: An evidence-based review. Arch. Ophthalmol. 2009, 127, 402–406.
- Nizari, S.; Guo, L.; Davis, B.M.; Normando, E.M.; Galvao, J.; Turner, L.A.; Bizrah, M.; Dehabadi, M.; Tian, K.; Cordeiro, M.F. Non-amyloidogenic effects of α2 adrenergic agonists: Implications for brimonidine-mediated neuroprotection. Cell Death Dis. 2016, 7, e2514.




