
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Katie Dunphy | + 2232 word(s) | 2232 | 2021-08-03 08:36:25 |
Video Upload Options
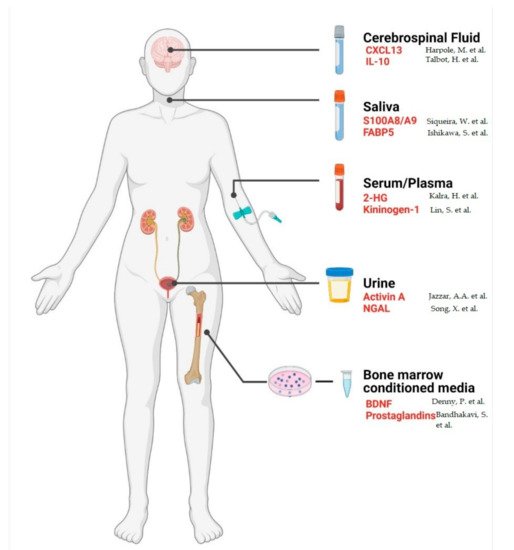
The proteomes of biofluids, including serum, saliva, cerebrospinal fluid, and urine, are highly dynamic with protein abundance fluctuating depending on the physiological and/or pathophysiological context. Improvements in mass-spectrometric technologies have facilitated the in-depth characterisation of biofluid proteomes which are now considered hosts of a wide array of clinically relevant biomarkers. Promising efforts are being made in the field of biomarker diagnostics for haematologic malignancies. Several serum and urine-based biomarkers such as free light chains, β-microglobulin, and lactate dehydrogenase are quantified as part of the clinical assessment of haematological malignancies. However, novel, minimally invasive proteomic markers are required to aid diagnosis and prognosis and to monitor therapeutic response and minimal residual disease.
1. Introduction
| Tissue-Based Proteomics | Biofluid-Based Proteomics | ||
|---|---|---|---|
| Advantages | Disadvantages | Advantages | Disadvantages |
| Direct analysis of proteins from site of disease | Invasive procedure | Non-invasive | Not in direct proximity to the site of disease |
| Facilitates the study of the bone marrow microenvironment | Localised sampling bias due to heterogeneity of the bone marrow microenvironment | Ease of longitudinal sampling | High abundance proteins can hamper detection |
| Gold standard for diagnostic and prognostic applications | High cost | Low cost | |
| Bone marrow biopsies can be painful procedures | Reflective of disease state | ||
2. Blood
2.1. Complexity of the Serum/Plasma Proteome
2.2. Methods for Analysing the Serum/Plasma Proteome
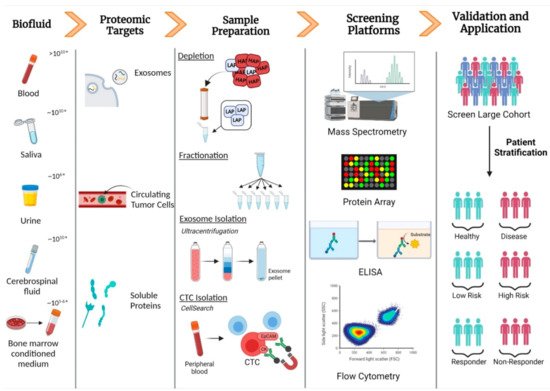
2.3. Detecting Biomarkers in Serum and Plasma
2.4. Serum/Plasma Biomarkers in Haematological Malignancies
| Biofluid | Protein | Type of Blood Cancer | Technology | Clinical Purpose | References |
|---|---|---|---|---|---|
| Serum | Monoclonal immunoglobulin (M-protein) | Multiple myeloma | Serum protein electrophoresis immunofixation electrophoresis | Diagnostic and monitoring disease | [21] |
| Free light chains (Bence Jones proteins) | Multiple myeloma | Immunoturbidimetric and immunonephelometric assays | Diagnostic and monitoring of patients with light-chain disease. | [22] | |
| Βeta 2-microglobulin | Multiple myeloma | Nephelometry immunoturbidimetry | Prognostic | [23][24][25][26][27][28][29][30] | |
| Acute leukaemia | |||||
| Chronic leukaemia | |||||
| Hodgkin’s lymphoma | |||||
| Non-Hodgkin’s lymphoma | |||||
| Lactate dehydrogenase | Multiple myeloma | Enzyme kinetics assay | Prognostic | [31][32][33][34][35] | |
| Acute leukaemia | |||||
| Chronic leukaemia | |||||
| Hodgkin’s lymphoma | |||||
| Non-Hodgkin’s lymphoma | |||||
| Uric acid | Acute myeloid leukaemia | Colorimetric enzyme assay | Prognostic | [36] | |
| Urine | Monoclonal immunoglobulin (M-protein) | Multiple myeloma | Protein electrophoresis Immunofixation electrophoresis |
Diagnostic and monitoring of disease | [37] |
| Free light chains (Bence Jones proteins) | Multiple myeloma | Immunofixation electrophoresis Immunoturbidimetry |
Monitor disease progression and response to therapy | [37] | |
| Cerebrospinal fluid | Βeta 2-microglobulin | Lymphoma | Nephelometry | Indicative of central nervous system (CNS) involvement | [38] |
| Leukaemia |
2.5. Proteomics of Other Biofluids in Haematological Malignancies
Proteomic analyses focused on saliva, bone marrow conditioned media, urine and cerebrospinal fluid in haematological malignancies are discussed in detail in the full version of this review article.
References
- Macklin, A.; Khan, S.; Kislinger, T. Recent advances in mass spectrometry based clinical proteomics: Applications to cancer research. Clin. Proteom. 2020, 17.
- Yang, X.-L.; Shi, Y.; Zhang, D.-D.; Xin, R.; Deng, J.; Wu, T.-M.; Wang, H.-M.; Wang, P.-Y.; Liu, J.-B.; Li, W.; et al. Quantitative proteomics characterization of cancer biomarkers and treatment. Mol. Ther.-Oncolytics 2021, 21, 255–263.
- Marrugo-Ramírez, J.; Mir, M.; Samitier, J. Blood-Based Cancer Biomarkers in Liquid Biopsy: A Promising Non-Invasive Alternative to Tissue Biopsy. Int. J. Mol. Sci. 2018, 19, 2877.
- Pietrowska, M.; Wlosowicz, A.; Gawin, M.; Widlak, P. MS-Based Proteomic Analysis of Serum and Plasma: Problem of High Abundant Components and Lights and Shadows of Albumin Removal. Adv. Exp. Med. Biol. 2019, 1073, 57–76.
- Yu, Z.; Kastenmüller, G.; He, Y.; Belcredi, P.; Möller, G.; Prehn, C.; Mendes, J.; Wahl, S.; Roemisch-Margl, W.; Ceglarek, U.; et al. Differences between Human Plasma and Serum Metabolite Profiles. PLoS ONE 2011, 6, e21230.
- O’Connell, G.C.; Alder, M.; Webel, A.R.; Moore, S.M. Neuro biomarker levels measured with high-sensitivity digital ELISA differ between serum and plasma. Bioanalysis 2019, 11, 2087–2094.
- O’Neal, W.K.; Anderson, W.; Basta, P.V.; Carretta, E.E.; Doerschuk, C.M.; Barr, R.G.; Bleecker, E.R.; Christenson, S.A.; Curtis, J.L.; Han, M.K.; et al. Comparison of serum, EDTA plasma and P100 plasma for luminex-based biomarker multiplex assays in patients with chronic obstructive pulmonary disease in the SPIROMICS study. J. Transl. Med. 2014, 12, 9.
- Mannello, F. Serum or plasma samples? The “Cinderella” role of blood collection procedures: Preanalytical methodological issues influence the release and activity of circulating matrix metalloproteinases and their tissue inhibitors, hampering diag-nostic trueness and leading to misinterpretation. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 611–614.
- Kim, H.S. Blood Glucose Measurement: Is Serum Equal to Plasma? Diabetes Metab. J. 2016, 40, 365–366.
- Ekdahl, K.N.; Norberg, D.; Bengtsson, A.A.; Sturfelt, G.; Nilsson, U.R.; Nilsson, B. Use of Serum or Buffer-Changed EDTA-Plasma in a Rapid, Inexpensive, and Easy-To-Perform Hemolytic Complement Assay for Differential Diagnosis of Systemic Lupus Erythematosus and Monitoring of Patients with the Disease. Clin. Vaccine Immunol. 2007, 14, 549–555.
- Chan, K.C.; Lucas, D.A.; Hise, D.; Schaefer, C.F.; Xiao, Z.; Janini, G.M.; Buetow, K.H.; Issaq, H.J.; Veenstra, T.D.; Conrads, T.P. Analysis of the human serum proteome. Clin. Proteom. 2004, 1, 101–225.
- Pieper, R.; Gatlin, C.L.; Makusky, A.J.; Russo, P.S.; Schatz, C.R.; Miller, S.S.; Su, Q.; McGrath, A.M.; Estock, M.A.; Parmar, P.P.; et al. The human serum proteome: Display of nearly 3700 chromatographically separated protein spots on two-dimensional electrophoresis gels and identification of 325 distinct proteins. Proteomics 2003, 3, 1345–1364.
- Veenstra, T.D.; Conrads, T.P.; Hood, B.L.; Avellino, A.M.; Ellenbogen, R.G.; Morrison, R.S. Biomarkers: Mining the Biofluid Proteome. Mol. Cell. Proteom. 2005, 4, 409–418.
- Anderson, N.L.; Anderson, N.G. The Human Plasma Proteome: History, character, and diagnostic prospects. Mol. Cell. Proteom. 2002, 1, 845–867.
- Lee, P.Y.; Osman, J.; Low, T.Y.; Jamal, R. Plasma/serum proteomics: Depletion strategies for reducing high-abundance proteins for biomarker discovery. Bioanalysis 2019, 11, 1799–1812.
- Duan, X.; Yarmush, D.; Berthiaume, F.; Jayaraman, A.; Yarmush, M.L. Immunodepletion of albumin for two-dimensional gel detection of new mouse acute-phase protein and other plasma proteins. Proteomics 2005, 5, 3991–4000.
- Tsang, J.C.; Lo, Y.D. Circulating nucleic acids in plasma/serum. Pathology 2007, 39, 197–207.
- Dementeva, N.; Kokova, D.; Mayboroda, O. Current Methods of the Circulating Tumor Cells (CTC) Analysis: A Brief Overview. Curr. Pharm. Des. 2017, 23, 4726–4728.
- Yu, S.; Cao, H.; Shen, B.; Feng, J. Tumor-derived exosomes in cancer progression and treatment failure. Oncotarget 2015, 6, 37151–37168.
- Hocking, J.; Mithraprabhu, S.; Kalff, A.; Spencer, A. Liquid biopsies for liquid tumors: Emerging potential of circulating free nucleic acid evaluation for the management of hematologic malignancies. Cancer Biol. Med. 2016, 13, 215–225.
- Willrich, M.A.; Murray, D.L.; Kyle, R.A. Laboratory testing for monoclonal gammopathies: Focus on monoclonal gammopathy of undetermined significance and smoldering multiple myeloma. Clin. Biochem. 2018, 51, 38–47.
- Messiaen, M.A.-S.; De Sloovere, M.M.M.W.; Claus, P.-E.; Vercammen, M.; Van Hoovels, M.L.; Heylen, M.O.; Debrabandere, M.J.; Vanpoucke, M.H.; De Smet, D. Performance Evaluation of Serum Free Light Chain Analysis: Nephelometry vs Turbidimetry, Monoclonal vs Polyclonal Reagents. Am. J. Clin. Pathol. 2017, 147, 611–622.
- Bernard, A.M.; Vyskocil, A.; Lauwerys, R.R. Determination of beta 2-microglobulin in human urine and serum by latex im-munoassay. Clin. Chem. 1981, 27, 832–837.
- Lievens, M.M.; Woestyn, S.; De Nayer, P.; Collet-Cassart, D. Measurement of ß2-Microglobulin in Serum by a Particle-Enhanced Nephelometric Immunoassay. Clin. Chem. Lab. Med. 1991, 29, 401–404.
- Chng, W.J.; on behalf of the International Myeloma Working Group; Dispenzieri, A.; Chim, C.; Fonseca, R.; Goldschmidt, H.; Lentzsch, S.; Munshi, N.C.; Palumbo, A.; Miguel, J.S.; et al. IMWG consensus on risk stratification in multiple myeloma. Leukemia 2013, 28, 269–277.
- Tsimberidou, A.M.; Kantarjian, H.M.; Wen, S.; O’Brien, S.; Cortes, J.; Wierda, W.G.; Koller, C.; Pierce, S.; Brandt, M.; Freireich, E.J.; et al. The Prognostic Significance of Serum β2 Microglobulin Levels in Acute Myeloid Leukemia and Prognostic Scores Predicting Survival: Analysis of 1,180 Patients. Clin. Cancer Res. 2008, 14, 721–730.
- Kantarjian, H.M.; Smith, T.; Estey, E.; Polyzos, A.; O’Brien, S.; Pierce, S.; Beran, M.; Feldman, E.; Keating, M.J. Prognostic significance of elevated serum β2-microglobulin levels in adult acute lymphocytic leukemia. Am. J. Med. 1992, 93, 599–604.
- Rodriguez, J.; Cortes, J.; Talpaz, M.; O’Brien, S.; Smith, T.L.; Rios, M.B.; Kantarjian, H. Serum beta-2 microglobulin levels are a significant prognostic factor in Philadelphia chromosome-positive chronic myelogenous leukemia. Clin. Cancer Res. 2000, 6, 147–152.
- Wu, L.; Wang, T.; Gui, W.; Lin, H.; Xie, K.; Wang, H.; Gao, T.; Zhang, X.; Liu, L.; Han, T.; et al. Prognostic Significance of Serum Beta-2 Microglobulin in Patients with Non-Hodgkin Lymphoma. Oncology 2014, 87, 40–47.
- Vassilakopoulos, T.P.; Nadali, G.; Angelopoulou, M.K.; Siakantaris, M.P.; Dimopoulou, M.N.; Kontopidou, F.N.; Karkantaris, C.; Kokoris, S.I.; Kyrtsonis, M.C.; Tsaftaridis, P.; et al. The prognostic significance of beta(2)-microglobulin in patients with Hodgkin’s lymphoma. Haematologica 2002, 87, 701–708; discussion 708.
- Teke, H.; Başak, M.; Teke, D.; Kanbay, M. Serum Level of Lactate Dehydrogenase is a Useful Clinical Marker to Monitor Progressive Multiple Myeloma Diseases: A Case Report. Turk. J. Haematol. 2014, 31, 84–87.
- D’Angelo, G.; Giardini, C.; Calvano, D. Clinical significance of the determination of lactate dehydrogenase in acute leukemia and non-Hodgkin’s lymphoma. Minerva Med. 1989, 80, 549–552.
- Patel, P.; Adhvaryu, S.G.; Balar, D.B. Serum lactate dehydrogenase and its isoenzymes in leukemia patients: Possible role in diagnosis and treatment monitoring. Neoplasma 1994, 41, 55–59.
- Endrizzi, L.; Fiorentino, M.V.; Salvagno, L.; Segati, R.; Pappagallo, G.L.; Fosser, V. Serum lactate dehydrogenase (LDH) as a prognostic index for non-Hodgkin’s lymphoma. Eur. J. Cancer Clin. Oncol. 1982, 18, 945–949.
- García, R.; Hernández, J.; Caballero; González, M.; Galende, J.; Del Cañizo, M.; Vázquez, L.; Miguel, J.S. Serum lactate dehydrogenase level as a prognostic factor in Hodgkin’s disease. Br. J. Cancer 1993, 68, 1227–1231.
- Yamauchi, T.; Negoro, E.; Lee, S.; Takai, M.; Matsuda, Y.; Takagi, K.; Kishi, S.; Tai, K.; Hosono, N.; Tasaki, T.; et al. A high serum uric acid level is associated with poor prognosis in patients with acute myeloid leukemia. Anticancer Res. 2013, 33, 3947–3951.
- Singh, G. Serum and Urine Protein Electrophoresis and Serum-Free Light Chain Assays in the Diagnosis and Monitoring of Monoclonal Gammopathies. J. Appl. Lab. Med. 2020, 5, 1358–1371.
- Jeffery, G.M.; Frampton, C.M.; Legge, H.M.; Hart, D.N.J.; Jeffery, M.G. Cerebrospinal fluid B2-microglobulin levels in meningeal involvement by malignancy. Pathology 1990, 22, 20–23.
- Chanukuppa, V.; Taware, R.; Taunk, K.; Chatterjee, T.; Sharma, S.; Somasundaram, V.; Rashid, F.; Malakar, D.; Santra, M.K.; Rapole, S. Proteomic Alterations in Multiple Myeloma: A Comprehensive Study Using Bone Marrow Interstitial Fluid and Serum Samples. Front. Oncol. 2021, 10.
- Bai, J.; Yang, Y.; Wang, J.; Zhang, L.; Wang, F.; He, A. Variability of serum novel serum peptide biomarkers correlates with the disease states of multiple myeloma. Clin. Proteom. 2019, 16, 17.
- Yen, K.E.; Bittinger, M.A.; Su, S.M.; Fantin, V.R. Cancer-associated IDH mutations: Biomarker and therapeutic opportunities. Oncogene 2010, 29, 6409–6417.
- Paschka, P.; Schlenk, R.F.; Gaidzik, V.I.; Habdank, M.; Krönke, J.; Bullinger, L.; Späth, D.; Kayser, S.; Zucknick, M.; Götze, K.; et al. IDH1 and IDH2 Mutations Are Frequent Genetic Alterations in Acute Myeloid Leukemia and Confer Adverse Prognosis in Cytogenetically Normal Acute Myeloid Leukemia With NPM1 Mutation Without FLT3 Internal Tandem Duplication. J. Clin. Oncol. 2010, 28, 3636–3643.
- Tefferi, A.; Lasho, T.L.; Abdel-Wahab, O.; Guglielmelli, P.; Patel, J.; Caramazza, D.; Pieri, L.; Finke, C.M.; Kilpivaara, O.; Wadleigh, M.; et al. IDH1 and IDH2 mutation studies in 1473 patients with chronic-, fibrotic- or blast-phase essential thrombocythemia, polycythemia vera or myelofibrosis. Leukemia 2010, 24, 1302–1309.
- Heuser, M.; Palmisiano, N.; Mantzaris, I.; Mims, A.; DiNardo, C.; Silverman, L.R.; Wang, E.S.; Fiedler, W.; Baldus, C.; Schwind, S.; et al. Safety and efficacy of BAY1436032 in IDH1-mutant AML: Phase I study results. Leukemia 2020, 34, 2903–2913.
- Bories, P.-N.; Nakib, S.; Cynober, L.; Alary, A.S.; Coude, M.-M.; Chevillon, F.; Tamburini, J.; Birsen, R.; Kosmider, O.; Bouscary, D. Establishing assay-specific 97.5th percentile upper reference limit for serum D-2-hydroxyglutarate for the management of patients with acute myeloid leukemia. Clin. Chem. Lab. Med. 2018, 57, e57–e59.
- Poinsignon, V.; Mercier, L.; Nakabayashi, K.; David, M.; Lalli, A.; Penard-Lacronique, V.; Quivoron, C.; Saada, V.; DE Botton, S.; Broutin, S.; et al. Quantitation of isocitrate dehydrogenase (IDH)-induced D and L enantiomers of 2-hydroxyglutaric acid in biological fluids by a fully validated liquid tandem mass spectrometry method, suitable for clinical applications. J. Chromatogr. B 2016, 1022, 290–297.
- Yu, R.; Zhang, J.; Zang, Y.; Zeng, L.; Zuo, W.; Bai, Y.; Liu, Y.; Sun, K.; Liu, Y. iTRAQ-based quantitative protein expression profiling of biomarkers in childhood B-cell and T-cell acute lymphoblastic leukemia. Cancer Manag. Res. 2019, 11, 7047–7063.
- Kárai, B.; Gyurina, K.; Ujfalusi, A.; Sędek, Ł.; Barna, G.; Jáksó, P.; Svec, P.; Szánthó, E.; Nagy, A.C.; Müller, J.; et al. Expression Patterns of Coagulation Factor XIII Subunit A on Leukemic Lymphoblasts Correlate with Clinical Outcome and Genetic Subtypes in Childhood B-cell Progenitor Acute Lymphoblastic Leukemia. Cancers 2020, 12, 2264.
- Cavalcante, M.D.S.; Romero, J.C.T.; Lobo, M.D.P.; Moreno, F.B.M.B.; Bezerra, L.P.; Lima, D.S.; Matos, J.C.; Moreira, R.D.A.; Monteiro-Moreira, A.C.D.O. A panel of glycoproteins as candidate biomarkers for early diagnosis and treatment evaluation of B-cell acute lymphoblastic leukemia. Biomark. Res. 2016, 4, 1–8.
- Kearney, P.; Boniface, J.J.; Price, N.; Hood, L. The building blocks of successful translation of proteomics to the clinic. Curr. Opin. Biotechnol. 2018, 51, 123–129.
- Harshman, S.; Canella, A.; Ciarlariello, P.D.; Agarwal, K.; Branson, O.E.; Rocci, A.; Cordero, H.; Phelps, M.A.; Hade, E.; Dubovsky, J.A.; et al. Proteomic characterization of circulating extracellular vesicles identifies novel serum myeloma associated markers. J. Proteom. 2016, 136, 89–98.
- Prieto, D.; Sotelo, N.S.; Seija, N.; Sernbo, S.; Abreu, C.; Durán, R.; Gil, M.; Sicco, E.; Irigoin, V.; Oliver, A.; et al. S100-A9 protein in exosomes from chronic lymphocytic leukemia cells promotes NF-κB activity during disease progression. Blood 2017, 130, 777–788.
- Namburi, S.; Broxmeyer, H.E.; Hong, C.-S.; Whiteside, T.L.; Boyiadzis, M. DPP4+ exosomes in AML patients’ plasma suppress proliferation of hematopoietic progenitor cells. Leukemia 2020, 35, 1925–1932.
- Szczepanski, M.J.; Szajnik, M.; Welsh, A.; Whiteside, T.L.; Boyiadzis, M. Blast-derived microvesicles in sera from patients with acute myeloid leukemia suppress natural killer cell function via membrane-associated transforming growth factor-beta1. Haematologica 2011, 96, 1302–1309.
- Hong, C.-S.; Sharma, P.; Yerneni, S.S.; Simms, P.; Jackson, E.K.; Whiteside, T.L.; Boyiadzis, M. Circulating exosomes carrying an immunosuppressive cargo interfere with cellular immunotherapy in acute myeloid leukemia. Sci. Rep. 2017, 7, 4684.
- Gonsalves, W.I.; Rajkumar, S.V.; Dispenzieri, A.; Dingli, D.; Timm, M.M.; Morice, W.G.; Lacy, M.Q.; Buadi, F.K.; Go, R.S.; Leung, N.; et al. Quantification of circulating clonal plasma cells via multiparametric flow cytometry identifies patients with smoldering multiple myeloma at high risk of progression. Leukemia 2016, 31, 130–135.
- Flores-Montero, J.; Sanoja-Flores, L.; Paiva, B.D.L.; Puig, N.; García-Sánchez, O.; Böttcher, S.; Van Der Velden, V.H.J.; Pérez-Morán, J.-J.; Vidriales, M.-B.; Garcia-Sanz, R.; et al. Next Generation Flow for highly sensitive and standardized detection of minimal residual disease in multiple myeloma. Leukemia 2017, 31, 2094–2103.
- Zhang, L.; Beasley, S.; Prigozhina, N.L.; Higgins, R.; Ikeda, S.; Lee, F.Y.; Marrinucci, D.; Jia, S. Detection and Characterization of Circulating Tumour Cells in Multiple Myeloma. J. Circ. Biomarkers 2016, 5, 10.
- Costa, L.J.; Derman, B.A.; Bal, S.; Sidana, S.; Chhabra, S.; Silbermann, R.; Ye, J.C.; Cook, G.; Cornell, R.F.; Holstein, S.A.; et al. International harmonization in performing and reporting minimal residual disease assessment in multiple myeloma trials. Leukemia 2020, 35, 18–30.
- Kumar, S.; Paiva, B.D.L.; Anderson, K.C.; Durie, B.; Landgren, O.; Moreau, P.; Munshi, N.; Lonial, S.; Bladé, J.; Mateos, M.-V.; et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016, 17, e328–e346.
- Ching, T.; Duncan, M.E.; Newman-Eerkes, T.; McWhorter, M.M.E.; Tracy, J.M.; Steen, M.S.; Brown, R.P.; Venkatasubbarao, S.; Akers, N.K.; Vignali, M.; et al. Analytical evaluation of the clonoSEQ Assay for establishing measurable (minimal) residual disease in acute lymphoblastic leukemia, chronic lymphocytic leukemia, and multiple myeloma. BMC Cancer 2020, 20, 612.
- Bergen, H.R., 3rd; Dasari, S.; Dispenzieri, A.; Mills, J.R.; Ramirez-Alvarado, M.; Tschumper, R.C.; Jelinek, D.F.; Barnidge, D.R.; Murray, D.L. Clonotypic Light Chain Peptides Identified for Monitoring Minimal Residual Disease in Multiple Myeloma without Bone Marrow Aspiration. Clin. Chem. 2016, 62, 243–251.
- Martins, C.O.; Huet, S.; Yi, S.S.; Ritorto, M.S.; Landgren, O.; Dogan, A.; Chapman, J.R. Mass Spectrometry–Based Method Targeting Ig Variable Regions for Assessment of Minimal Residual Disease in Multiple Myeloma. J. Mol. Diagn. 2020, 22, 901–911.
- Murray, D.L.; Puig, N.; Kristinsson, S.; Usmani, S.Z.; Dispenzieri, A.; Bianchi, G.; Kumar, S.; Chng, W.J.; Hajek, R.; Paiva, B.; et al. Mass spectrometry for the evaluation of monoclonal proteins in multiple myeloma and related disorders: An International Myeloma Working Group Mass Spectrometry Committee Report. Blood Cancer J. 2021, 11, 24.




