
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Tayyaba Hasan | + 2914 word(s) | 2914 | 2021-08-11 07:49:41 | | | |
| 2 | Vivi Li | Meta information modification | 2914 | 2021-08-12 03:54:00 | | |
Video Upload Options
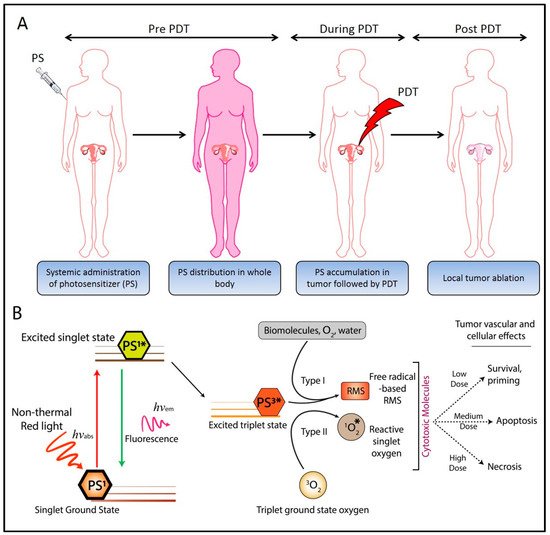
Ovarian cancer (OvCa) is the leading cause of gynecological cancer-related deaths in the United States, with five-year survival rates of 15–20% for stage III cancers and 5% for stage IV cancers. The standard of care for advanced OvCa involves surgical debulking of disseminated disease in the peritoneum followed by chemotherapy. Despite advances in treatment efficacy, the prognosis for advanced stage OvCa patients remains poor and the emergence of chemoresistant disease localized to the peritoneum is the primary cause of death. Therefore, a complementary modality that is agnostic to typical chemo- and radio-resistance mechanisms is urgently needed. Photodynamic therapy (PDT), a photochemistry-based process, is an ideal complement to standard treatments for residual disease. The confinement of the disease in the peritoneal cavity makes it amenable for regionally localized treatment with PDT. PDT involves photochemical generation of cytotoxic reactive molecular species (RMS) by non-toxic photosensitizers (PSs) following exposure to non-harmful visible light, leading to localized cell death. However, due to the complex topology of sensitive organs in the peritoneum, diffuse intra-abdominal PDT induces dose-limiting toxicities due to non-selective accumulation of PSs in both healthy and diseased tissue. In an effort to achieve selective damage to tumorous nodules, targeted PS formulations have shown promise to make PDT a feasible treatment modality in this setting. This targeted strategy involves chemical conjugation of PSs to antibodies, referred to as photoimmunoconjugates (PICs), to target OvCa specific molecular markers leading to enhanced therapeutic outcomes while reducing off-target toxicity.
1. Introduction
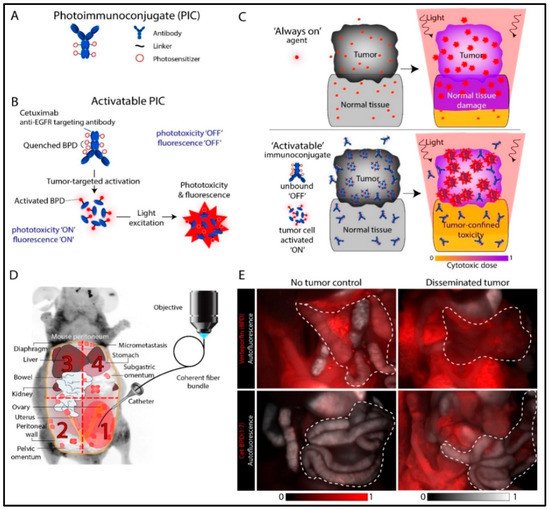
2. Photoimmunoconjugates and Photoimmunotherapy
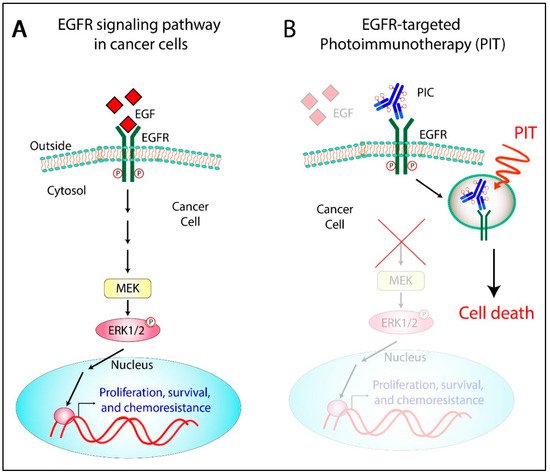
3. The Rationale to Target EGFR for OvCa Photoimmunotherapy
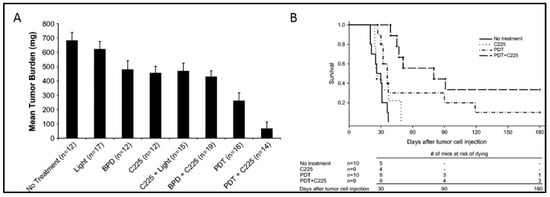
4. PIC-Based Combination Therapies
References
- Christie, E.; Bowtell, D. Acquired chemotherapy resistance in ovarian cancer. Ann. Oncol. 2017, 28, viii13–viii15.
- Cooke, S.L.; Brenton, J.D. Evolution of platinum resistance in high-grade serous ovarian cancer. Lancet Oncol. 2011, 12, 1169–1174.
- Jelovac, D.; Armstrong, D.K. Recent progress in the diagnosis and treatment of ovarian cancer. CA Cancer J. Clin. 2011, 61, 183–203.
- Cortez, A.J.; Tudrej, P.; Kujawa, K.A.; Lisowska, K.M. Advances in ovarian cancer therapy. Cancer Chemother. Pharmacol. 2018, 81, 17–38.
- Brackmann, M.; Stasenko, M.; Uppal, S.; Erba, J.; Reynolds, R.K.; McLean, K. Comparison of first-line chemotherapy regimens for ovarian carcinosarcoma: A single institution case series and review of the literature. BMC Cancer 2018, 18, 172.
- Kelland, L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer 2007, 7, 573.
- Yap, T.A.; Carden, C.P.; Kaye, S.B. Beyond chemotherapy: Targeted therapies in ovarian cancer. Nat. Rev. Cancer 2009, 9, 167.
- Hendren, S.K.; Hahn, S.M.; Spitz, F.R.; Bauer, T.W.; Rubin, S.C.; Zhu, T.; Glatstein, E.; Fraker, D.L. Phase II trial of debulking surgery and photodynamic therapy for disseminated intraperitoneal tumors. Ann. Surg. Oncol. 2001, 8, 65–71.
- Sindelar, W.F.; DeLaney, T.F.; Tochner, Z.; Thomas, G.F.; Dachoswki, L.J.; Smith, P.D.; Friauf, W.S.; Cole, J.W.; Glatstein, E. Technique of photodynamic therapy for disseminated intraperitoneal malignant neoplasms: Phase I study. Arch. Surg. 1991, 126, 318–324.
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic therapy. JNCI J. Natl. Cancer Inst. 1998, 90, 889–905.
- Delaney, T.F.; Sindelar, W.F.; Tochner, Z.; Smith, P.D.; Friauf, W.S.; Thomas, G.; Dachowski, L.; Cole, J.W.; Steinberg, S.M.; Glatstein, E. Phase I study of debulking surgery and photodynamic therapy for disseminated intraperitoneal tumors. Int. J. Radiat. Oncol. Biol. Phys. 1993, 25, 445–457.
- Wierrani, F.; Fiedler, D.; Grin, W.; Henry, M.; Dienes, E.; Gharehbaghi, K.; Krammer, B.; Grünberger, W. Clinical effect of meso-tetrahydroxyphenylchlorine based photodynamic therapy in recurrent carcinoma of the ovary: Preliminary results. BJOG Int. J. Obstet. Gynaecol. 1997, 104, 376–378.
- Tochner, Z.; Mitchell, J.; Hoekstra, H.; Smith, P.; DeLuca, A.; Barnes, M.; Harrington, F.; Manyak, M.; Russo, D.; Russo, A. Photodynamic therapy of the canine peritoneum: Normal tissue response to intraperitoneal and intravenous photofrin followed by 630 nm light. Lasers Surg. Med. 1991, 11, 158–164.
- Goff, B.; Hermanto, U.; Rumbaugh, J.; Blake, J.; Bamberg, M.; Hasan, T. Photoimmunotherapy and biodistribution with an OC125-chlorin immunoconjugate in an in vivo murine ovarian cancer model. Br. J. Cancer 1994, 70, 474.
- Duska, L.R.; Hamblin, M.R.; Miller, J.L.; Hasan, T. Combination photoimmunotherapy and cisplatin: Effects on human ovarian cancer ex vivo. J. Natl. Cancer Inst. 1999, 91, 1557–1563.
- Molpus, K.L.; Hamblin, M.R.; Rizvi, I.; Hasan, T. Intraperitoneal Photoimmunotherapy of Ovarian Carcinoma Xenografts in Nude Mice Using Charged Photoimmunoconjugates. Gynecol. Oncol. 2000, 76, 397–404.
- Hamblin, M.R.; Miller, J.L.; Hasan, T. Effect of charge on the interaction of site-specific photoimmunoconjugates with human ovarian cancer cells. Cancer Res. 1996, 56, 5205–5210.
- Tochner, Z.A.; Hahn, S.; Glatstein, E. Photoimmunotherapy and ovarian cancer: An improbable fiction or a palpable hit? J. Natl. Cancer Inst. 1999, 91, 1526–1527.
- Duska, L.; Hamblin, M.; Bamberg, M.; Hasan, T. Biodistribution of charged F (ab’) 2 photoimmunoconjugates in a xenograft model of ovarian cancer. Br. J. Cancer 1997, 75, 837.
- Goff, B.A.; Bamberg, M.; Hasan, T. Photoimmunotherapy of human ovarian carcinoma cells ex vivo. Cancer Res. 1991, 51, 4762–4767.
- DeWitt, J.M.; Sandrasegaran, K.; O’Neil, B.; House, M.G.; Zyromski, N.J.; Sehdev, A.; Perkins, S.M.; Flynn, J.; McCranor, L.; Shahda, S. Phase 1 study of EUS-guided photodynamic therapy for locally advanced pancreatic cancer. Gastrointest. Endosc. 2019, 89, 390–398.
- Huggett, M.T.; Jermyn, M.; Gillams, A.; Illing, R.; Mosse, S.; Novelli, M.; Kent, E.; Bown, S.; Hasan, T.; Pogue, B. Phase I/II study of verteporfin photodynamic therapy in locally advanced pancreatic cancer. Br. J. Cancer 2014, 110, 1698.
- Nath, S.; Obaid, G.; Hasan, T. The Course of Immune Stimulation by Photodynamic Therapy: Bridging fundamentals of photochemically-induced Immunogenic Cell Death to the Enrichment of T Cell Repertoire. Photochem. Photobiol. 2019.
- Schmidt, S.; Wagner, U.; Oehr, P.; Krebs, D. Clinical use of photodynamic therapy in gynecologic tumor patients—Antibody-targeted photodynamic laser therapy as a new oncologic treatment procedure. Zent. Fur Gynakol. 1992, 114, 307–311.
- Schmidt, S.; Wagner, U.; Schultes, B.; Oehr, P.; Decleer, W.; Ertmer, W.; Lubaschowski, H.; Biersack, H.J.; Krebs, D. [Photodynamic laser therapy with antibody-bound dyes. A new procedure in therapy of gynecologic malignancies]. Fortschr. Der Med. 1992, 110, 298–301.
- Löning, M.; Diddens, H.; Küpker, W.; Diedrich, K.; Hüttmann, G. Laparoscopic fluorescence detection of ovarian carcinoma metastases using 5-aminolevulinic acid-induced protoporphyrin IX. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2004, 100, 1650–1656.
- del Carmen, M.G.; Rizvi, I.; Chang, Y.; Moor, A.C.; Oliva, E.; Sherwood, M.; Pogue, B.; Hasan, T. Synergism of epidermal growth factor receptor-targeted immunotherapy with photodynamic treatment of ovarian cancer in vivo. J Natl Cancer Inst 2005, 97, 1516–1524.
- Obaid, G.; Spring, B.Q.; Bano, S.; Hasan, T. Activatable clinical fluorophore-quencher antibody pairs as dual molecular probes for the enhanced specificity of image-guided surgery. J. Biomed. Opt. 2017, 22, 121607.
- Urano, Y.; Asanuma, D.; Hama, Y.; Koyama, Y.; Barrett, T.; Kamiya, M.; Nagano, T.; Watanabe, T.; Hasegawa, A.; Choyke, P.L. Selective molecular imaging of viable cancer cells with pH-activatable fluorescence probes. Nat. Med. 2009, 15, 104.
- Spring, B.Q.; Abu-Yousif, A.O.; Palanisami, A.; Rizvi, I.; Zheng, X.; Mai, Z.; Anbil, S.; Sears, R.B.; Mensah, L.B.; Goldschmidt, R.; et al. Selective treatment and monitoring of disseminated cancer micrometastases in vivo using dual-function, activatable immunoconjugates. Proc. Natl. Acad. Sci. USA 2014, 111, E933–E942.
- Patterson, M.S.; Wilson, B.C.; Graff, R. In vivo tests of the concept of photodynamic threshold dose in normal rat liver photosensitized by aluminum chlorosulphonated phthalocyanine. Photochem. Photobiol. 1990, 51, 343–349.
- McGuire, W.P.; Hoskins, W.J.; Brady, M.F.; Kucera, P.R.; Partridge, E.E.; Look, K.Y.; Clarke-Pearson, D.L.; Davidson, M. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. New Engl. J. Med. 1996, 334, 1–6.
- Folli, S.; Westermann, P.; Braichotte, D.; Pèlegrin, A.; Wagnières, G.; van den Bergh, H.; Mach, J.-P. Antibody-indocyanin conjugates for immunophotodetection of human squamous cell carcinoma in nude mice. Cancer Res. 1994, 54, 2643–2649.
- Rosenthal, E.L.; Warram, J.M.; De Boer, E.; Chung, T.K.; Korb, M.L.; Brandwein-Gensler, M.; Strong, T.V.; Schmalbach, C.E.; Morlandt, A.B.; Agarwal, G. Safety and tumor specificity of cetuximab-IRDye800 for surgical navigation in head and neck cancer. Clin. Cancer Res. 2015, 21, 3658–3666.
- Nagaya, T.; Nakamura, Y.A.; Choyke, P.L.; Kobayashi, H. Fluorescence-guided surgery. Front. Oncol. 2017, 7, 314.
- COGLIATI, T.; BRUSA, P.; CANEVARI, S.; CALDERA, M. Preparation and Biological Characterization of Conjugates Consisting of Ricin and a Tumor-Specific Non-Internalizing. Anticancer Res. 1991, 11, 417–422.
- Ozzello, L.; De Rosa, C.; Blank, E.; Cantell, K.; Ceriani, R.; Habif, D. The use of natural interferon alpha conjugated to a monoclonal antibody anti mammary epithelial mucin (Mc5) for the treatment of human breast cancer xenografts. Breast Cancer Res. Treat. 1993, 25, 265–276.
- Alam, F.; Soloway, A.H.; Barth, R.F.; Mafune, N.; Adams, D.M.; Knoth, W.H. Boron neutron capture therapy: Linkage of a boronated macromolecule to monoclonal antibodies directed against tumor-associated antigens. J. Med. Chem. 1989, 32, 2326–2330.
- Barth, R.F.; Adams, D.M.; Soloway, A.H.; Alam, F.; Darby, M.V. Boronated starburst dendrimer-monoclonal antibody immunoconjugates: Evaluation as a potential delivery system for neutron capture therapy. Bioconj. Chem. 1994, 5, 58–66.
- Oseroff, A.R.; Ohuoha, D.; Hasan, T.; Bommer, J.C.; Yarmush, M.L. Antibody-targeted photolysis: Selective photodestruction of human T-cell leukemia cells using monoclonal antibody-chlorin e6 conjugates. Proc. Natl. Acad. Sci. 1986, 83, 8744–8748.
- Steele, J.K.; Liu, D.; Stammers, A.T.; Whitney, S.; Levy, J.G. Suppressor deletion therapy: Selective elimination of T suppressor cells in vivo using a hematoporphyrin conjugated monoclonal antibody permits animals to reject syngeneic tumor cells. Cancer Immunol. Immunother. 1988, 26, 125–131.
- Mew, D.; Lum, V.; Wat, C.; Towers, G.; Sun, C.C.; Walter, R.; Wright, W.; Berns, M.; Levy, J. Ability of specific monoclonal antibodies and conventional antisera conjugated to hematoporphyrin to label and kill selected cell lines subsequent to light activation. Cancer Res. 1985, 45, 4380–4386.
- Goff, B.A.; Bachor, R.; Kollias, N.; Hasan, T. Effects of photodynamic therapy with topical application of 5-aminolevulinic acid on normal skin of hairless guinea pigs. J. Photochem. Photobiol. B Biol. 1992, 15, 239–251.
- Hasan, T.; Lin, C.; Lin, A. Laser-induced selective cytotoxicity using monoclonal antibody-chromophore conjugates. Prog. Clin. Biol. Res. 1989, 288, 471.
- Hasan, T.; Lin, A.; Yarmush, D.; Oseroff, A.; Yarmush, M. Monoclonal antibody-chromophore conjugates as selective phototoxins. J. Control. Release 1989, 10, 107–117.
- Hasan, T.; Sherwood, M.; Anderson, T.; Bamberg, M.; Flotte, T.J.; Zurawski, V.R. Cellular response of ovarian carcinoma cells to antibody-photosensitizer-mediated injury. In Proceedings of the Photodynamic Therapy: Mechanisms II; pp. 136–144. Available online: https://spie.org/Publications/Proceedings/Volume/1203?SSO=1 (accessed on 26 November 2019).
- Vrouenraets, M.B.; Visser, G.W.; Stewart, F.A.; Stigter, M.; Oppelaar, H.; Postmus, P.E.; Snow, G.B.; van Dongen, G.A. Development of meta-tetrahydroxyphenylchlorin-monoclonal antibody conjugates for photoimmunotherapy. Cancer Res. 1999, 59, 1505–1513.
- Jiang, F.N.; Liu, D.J.; Neyndorff, H.; Chester, M.; Jiang, S.-y.; Levy, J.G. Photodynamic killing of human squamous cell carcinoma cells using a monoclonal antibody-photosensitizer conjugate. JNCI J. Natl. Cancer Inst. 1991, 83, 1218–1225.
- Krinick, N.; Sun, Y.; Joyner, D.; Spikes, J.; Straight, R.; Kopeček, J. A polymeric drug delivery system for the simultaneous delivery of drugs activatable by enzymes and/or light. J. Biomater. Sci. Polym. Ed. 1994, 5, 303–324.
- Lu, X.-M.; Fischman, A.; Stevens, E.; Lee, T.; Strong, L.; Tompkins, R.; Yarmush, M. Sn-chlorin e6 antibacterial immunoconjugates. An in vitro and in vivo analysis. J. Immunol. Methods 1992, 156, 85–99.
- Morgan, J.; Gray, A.; Huehns, E. Specific targeting and toxicity of sulphonated aluminium phthalocyanine photosensitised liposomes directed to cells by monoclonal antibody in vitro. Br. J. Cancer 1989, 59, 366.
- Sandland, J.; Boyle, R.W. Photosensitizer Antibody–Drug Conjugates: Past, Present, and Future. Bioconj. Chem. 2019, 30, 975–993.
- Wang, S.; Hüttmann, G.M.; Rudnitzki, F.; Diddens-Tschoeke, H.; Zhang, Z.; Rahmanzadeh, R. Indocyanine green as effective antibody conjugate for intracellular molecular targeted photodynamic therapy. J. Biomed. Opt. 2016, 21, 078001.
- Goff, B.A.; Blake, J.; Bamberg, M.P.; Hasan, T. Treatment of ovarian cancer with photodynamic therapy and immunoconjugates in a murine ovarian cancer model. Br. J. Cancer 1996, 74, 1194.
- Pogrebniak, H.; Matthews, W.; Black, C.; Russo, A.; Mitchell, J.; Smith, P.; Roth, J.; Pass, H. Targetted phototherapy with sensitizer-monoclonal antibody conjugate and light. Surg. Oncol. 1993, 2, 31–42.
- Del Governatore, M.; Hamblin, M.R.; Shea, C.R.; Rizvi, I.; Molpus, K.G.; Tanabe, K.K.; Hasan, T. Experimental photoimmunotherapy of hepatic metastases of colorectal cancer with a 17.1 A chlorine6 immunoconjugate. Cancer Res. 2000, 60, 4200–4205.
- Fan, Z.; Mendelsohn, J. Therapeutic application of anti-growth factor receptor antibodies. Curr. Opin. Oncol. 1998, 10, 67–73.
- Mendelsohn, J. Use of an antibody to target geldanamycin; Oxford University Press: Oxford, UK, 2000.
- Mendelsohn, J. Jeremiah Metzger Lecture. Targeted cancer therapy. Trans. Am. Clin. Climatol. Assoc. 2000, 111, 95.
- Mendelsohn, J. Blockade of receptors for growth factors: An anticancer therapy—the fourth annual Joseph, H. Burchenal American Association for Cancer Research Clinical Research Award Lecture. Clin. Cancer Res. 2000, 6, 747–753.
- Ciardiello, F.; Tortora, G. A novel approach in the treatment of cancer: Targeting the epidermal growth factor receptor. Clin. Cancer Res. 2001, 7, 2958–2970.
- Baselga, J.; Swain, S.M. Novel anticancer targets: Revisiting ERBB2 and discovering ERBB3. Nat. Rev. Cancer 2009, 9, 463.
- Wells, A. Tumor invasion: Role of growth factor-induced cell motility. Adv. Cancer Res. 1999, 78, 31–101.
- Teplinsky, E.; Muggia, F. EGFR and HER2: Is there a role in ovarian cancer? Transl. Cancer Res 2015, 4.
- Gui, T.; Shen, K. The epidermal growth factor receptor as a therapeutic target in epithelial ovarian cancer. Cancer Epidemiol. 2012, 36, 490–496.
- Abu-Yousif, A.O.; Moor, A.C.; Zheng, X.; Savellano, M.D.; Yu, W.; Selbo, P.K.; Hasan, T. Epidermal growth factor receptor-targeted photosensitizer selectively inhibits EGFR signaling and induces targeted phototoxicity in ovarian cancer cells. Cancer Lett. 2012, 321, 120–127.
- Bhuvaneswari, R.; Gan, Y.Y.; Soo, K.C.; Olivo, M. Targeting EGFR with photodynamic therapy in combination with Erbitux enhances in vivo bladder tumor response. Mol. Cancer 2009, 8, 94.
- Ahmad, N.; Kalka, K.; Mukhtar, H. In vitro and in vivo inhibition of epidermal growth factor receptor-tyrosine kinase pathway by photodynamic therapy. Oncogene 2001, 20, 2314.
- Hongrapipat, J.; Kopecková, P.; Liu, J.; Prakongpan, S.; Kopecek, J. Combination chemotherapy and photodynamic therapy with Fab′ fragment targeted HPMA copolymer conjugates in human ovarian carcinoma cells. Mol. Pharm. 2008, 5, 696–709.
- Rizvi, I.; Dinh, T.A.; Yu, W.; Chang, Y.; Sherwood, M.E.; Hasan, T. Photoimmunotherapy and irradiance modulation reduce chemotherapy cycles and toxicity in a murine model for ovarian carcinomatosis: Perspective and results. Israel J. Chem. 2012, 52, 776–787.
- Celli, J.P.; Solban, N.; Liang, A.; Pereira, S.P.; Hasan, T. Verteporfin-based photodynamic therapy overcomes gemcitabine insensitivity in a panel of pancreatic cancer cell lines. Lasers Surg. Med. 2011, 43, 565–574.
- Huang, H.-C.; Mallidi, S.; Liu, J.; Chiang, C.-T.; Mai, Z.; Goldschmidt, R.; Ebrahim-Zadeh, N.; Rizvi, I.; Hasan, T. Photodynamic therapy synergizes with irinotecan to overcome compensatory mechanisms and improve treatment outcomes in pancreatic cancer. Cancer Res. 2016, 76, 1066–1077.




