Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Fabiana Lucà | + 2424 word(s) | 2424 | 2021-08-03 03:31:02 | | | |
| 2 | Camila Xu | Meta information modification | 2424 | 2021-08-12 05:59:31 | | | | |
| 3 | Camila Xu | Meta information modification | 2424 | 2021-08-12 05:59:55 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Lucà, F. Anticoagulation in Atrial Fibrillation Cardioversion. Encyclopedia. Available online: https://encyclopedia.pub/entry/13051 (accessed on 07 February 2026).
Lucà F. Anticoagulation in Atrial Fibrillation Cardioversion. Encyclopedia. Available at: https://encyclopedia.pub/entry/13051. Accessed February 07, 2026.
Lucà, Fabiana. "Anticoagulation in Atrial Fibrillation Cardioversion" Encyclopedia, https://encyclopedia.pub/entry/13051 (accessed February 07, 2026).
Lucà, F. (2021, August 11). Anticoagulation in Atrial Fibrillation Cardioversion. In Encyclopedia. https://encyclopedia.pub/entry/13051
Lucà, Fabiana. "Anticoagulation in Atrial Fibrillation Cardioversion." Encyclopedia. Web. 11 August, 2021.
Copy Citation
Atrial fibrillation (AF) patient care encompasses different possible management strategies which are classified as rhythm-control therapies, aimed at restoring and maintaining the sinus rhythm, and rate-control therapies, aimed at ensuring an appropriate control of heart rate during AF.
atrial fibrillation
electrical cardioversion
pharmacological cardioversion
non-vitamin K antagonist oral anticoagulants
1. Introduction
Atrial fibrillation (AF) patient care encompasses different possible management strategies which are classified as rhythm-control therapies, aimed at restoring and maintaining the sinus rhythm, and rate-control therapies, aimed at ensuring an appropriate control of heart rate during AF. Although a rhythm-control strategy may have some clinical benefit, it does not seem to offer advantages in terms of mortality or morbidity over a rate-control strategy [1]. Although some observational data [2][3] is in favor of rhythm control strategy, in order to reduce thromboembolic risk in patients with paroxysmal in contrast with persistent AF (due to a reduced risk of stroke in patients with paroxysmal as opposed to persistent AF), randomized clinical trials (RCTs), including the Atrial Fibrillation Follow-up Investigation of Rhythm Management trial, have failed to show significant difference in survival or thromboembolic events related to rhythm control compared to rate control strategy [1]. Notably, thromboembolic risk needs to be evaluated according to the CHA2DS2-VASc score (congestive heart failure, hypertension (1 point for presence of each), age ≥ 75 years (2 points), diabetes mellitus (1 point), stroke/TIA (2 points), vascular disease, age 65 to 74 years, female sex (1 point for presence of each); scores range from 0 to 9), regardless of the adopted strategy.
Nonetheless, rhythm control still represents the preferred strategy, especially in young patients who are symptomatic and in patients with hemodynamic instability.
2. Acute Hemodynamic Instability
Acute hemodynamic instability in AF, due to a rapid ventricular rate (typically >150 bpm or higher in patients compromised by co-morbidities), is characterized by clinical manifestations such as syncope, acute pulmonary edema, myocardial ischemia, symptomatic hypotension, or cardiogenic shock. In those patients an emergency electrical cardioversion has to be promptly performed, and anticoagulation should be started as soon as possible [4].
Electrical or pharmacological cardioversion (CV) is the cornerstone of the rhythm management strategy. Electrical cardioversion (ECV) is not only recommended in hemodynamically unstable patients but is also the preferred strategy in stable patients when AF duration is prolonged, whereas pharmacological cardioversion (PCV) may be preferred in recent onset AF [4]. In stable patients, pharmacological and electrical cardioversion can both be suitable options to perform. Electrical cardioversion is more effective, although sedation is needed [4]. Of note, pre-treatment with AADs can improve the efficacy of elective electrical cardioversion. Irrespective of the CV method employed, reestablishment of sinus rhythm confers a substantial possibility of peri-cardioversion thromboembolism, with a stroke rate enclosed by 5 and 7% in non-anticoagulated patients [1][2][3][4][5]. The risk of stroke or systemic embolism (SSE) is higher forthwith next to CV, with 82% of cases occurring within 72 h and 98% of accidents manifesting within 10 days [4]. This risk is related to the potential embolization of previous thrombus from the atrial appendage after recovery of effective atrial contractility. Furthermore, the mechanism of CV might support novel thrombus development as a result of transitory atrial stunning. For these reasons, current European Society of Cardiology (ESC) guidelines recommend not less than 3 weeks of adequate anticoagulation before CV, accompanied by minimum of 4 weeks of anticoagulation after the procedure in patients with AF duration > 48 h (or unknown) irrespective of CHA2DS2-VASc score or transesophageal echocardiography (TEE) done to exclude left atrial thrombi [4].
In the last 50 years, a great deal of clinical experience has been accumulated on the use of VKAs for anticoagulation in AF. However, this treatment has never been validated in large RCT. To prevent thromboembolic complications, the availability of non-vitamin K antagonist oral anticoagulants (NOACs) represents a big step forward thanks to their more predictable therapeutic effect and more favorable hemorrhagic risk profile [6]. The advantage of a rapid and predictable action onset of these drugs is particularly useful in the peri-cardioversion setting. Indeed, in this specific setting the longer and variable time needed to achieve an effective anticoagulation with VKAs often requires the use of a heparin bridge and a delayed CV. NOACs’ use in AF cardioversion is epidemiologically relevant in clinical practice [7][8].
In the present paper we review NOACs’ use in AF patients undergoing CV, with a focus on safety and efficacy findings. Furthermore, on the basis of current evidence we share pragmatic thoughts about NOAC using in different peri-cardioversion scenarios. As far as we are concerned, the most recent ESC guidelines should be considered a useful guide in order to avoid fraught roads with danger and tricky situations as has been summarized in Figure 1.
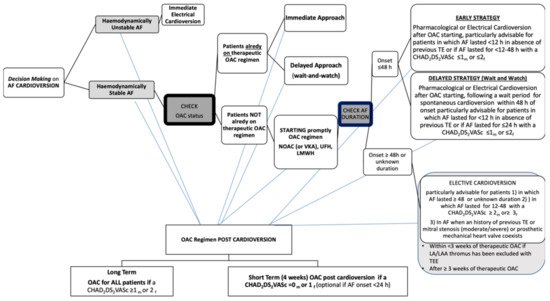
Figure 1. Decision making on atrial fibrillation cardioversion according to 2020 ESC Guidelines. AF, atrial fibrillation; OAC, oral anticoagulant; NOAC, non-vitamin K antagonist oral anticoagulant;VKA, vitamin K antagonist; UFH, unfractionated heparin; LMWH, low-molecular-weight heparin; TE, thromboembolism; h, hour; CHA2DS2-VASc, congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, stroke, vascular disease, age 65–74 years, sex category (female); m, male; f, female; LA, left atrium; LAA, left atrial appendage; TEE, transesophageal echocardiography.
3. Electrical Cardioversion in Emergency
Recent-onset episodes of AF are among the most usual arrhythmias that physicians deal with, constituting almost 35% of hospital accesses due to arrhythmias [9]. The prevalence of AF is enhancing owing to the increase of the age of population. Despite an almost stable relative rate of hospitalization, the emergency department admissions for AF are increasing [10].
The first step in the management of AF patients consists to assess if the patient is hemodynamically stable or is presenting symptoms. In hemodynamically unstable AF patients, an emergency ECV should be carried out promptly. The steps to follow for emergency management of AF are prevention of thromboembolism and hemodynamic stabilization, followed by symptom relief. Synchronized direct current ECV is the favored practice in severely hemodynamically unstable patients with hypotension, acute coronary syndrome, or pulmonary edema due to new-onset AF [11]. Although ECV is connected with a high initial success percentage (68–98%), and it can be effective for solving an acute compromised situation [12], it is not a risk-free procedure. Moreover, long-term maintenance of sinus rhythm is not guaranteed, and a relapse of AF after ECV is associated with a poor outcome [13]. As highlighted in the more recent guidelines for the management of AF, defibrillator devices with biphasic waveforms are more effective than monophasic ones, and an anterior–posterior electrode position should be preferred over antero–lateral, as the ECV procedure seems to be safer and more successful [4].
Moreover, maximum fixed-energy electrical CV was more effective than an energy-escalation strategy in achieving sinus rhythm based on current data. It has been claimed that an initial synchronized shock at maximum defibrillator output (360 J) was a reasonable approach without an increasing in adverse events [14]. What is particularly noticeable is that an initial energy setting of 360 J resulted more efficiently than traditional approach, particularly when the duration of AF is longer [15].
Patients with the Wolff–Parkinson–White syndrome suffering from acute AF require special precautions in their management. Due to the prolonged atrial conduction WPW, characterized by a longer maximal atrial conduction delay and wider conduction delay zone, the atrial vulnerability to develop of AF is greater [16]. Ventricular fibrillation could be caused by rapid atrioventricular conduction over the accessory conduction pathway. Therefore, in a patient with Wolff–Parkinson–White syndrome, drugs that block atrioventricular node conduction (digoxin, β-blockers, and calcium channel blockers) are not indicated since they are ineffective on the accessory pathways so they could trigger ventricular fibrillation. In emergency setting immediate CV should not be delayed in order to perform adequate anticoagulation. In this situation, intravenous heparin or low molecular weight heparin (LMWH) should be administered before CV [17].
After cardioversion a long term OAC strategy (OAC for all patients if a CHAD2DS2VASc ≥ 1men or ≥2female) or short term OAC strategy (4 weeks OAC if a CHAD2DS2VASc = 0men or ≥1female (optional if AF onset < 24 h) is needed [4].
3.1. Electrical Cardioversion in Patients with AF Which Occurred within Less Than 48 h
In the absence of hemodynamic compromise, the management of AF is guided by symptoms and its duration. Synchronized direct ECV should be the elective procedure to be followed by physicians, as it re-establishes faster and more efficiently than PCV, and involves a briefer hospitalization [18]. Of note, pre-treatment with antiarrhythmic drugs can improve the efficacy of ECV.
Recently, the RACE7 ACWAS trial [19], demonstrated that a wait-and-watch approach with rate control medication only and CV within 48 h of symptom onset was as safe as, and non-inferior to, immediate CV of paroxysmal AF, which is likely to terminate spontaneously within 24 h.
In clinical practice, there is clear evidence of a lack regarding the need for anticoagulation when AF has occurred within 48 h in naïve patients with a very low risk of stroke. AF itself independently increases stroke risk by 5-fold, which represents one of the leading causes of morbidity and death in AF patients [20]. The annual risk of stroke in those patients is greater than 20%. It is important to recognize that in absence of additional clinical stroke risk, assessed by CHA2DS2-VASc score, AF patients without anticoagulant therapy have an ischemic stroke rate of 0.43% per year. Conversely in those with 1 additional point in the CHA2DS2-VASc score the risk range from 1.18% to 3.50% per year. Another commonly held claim is that an increased risk of stroke and systemic thromboembolism in AF is, in a certain way, linked to a persistent prothrombotic state, as demonstrated by the increasing of platelet activation, thrombin formation, and inflammation with a reduction fibrinolysis process and by the endothelial dysfunction both. Unsurprisingly, what is particularly noticeable is that even young and very low-risk patients with AF have prothrombotic alterations, and the so-called prothrombotic fibrin clot phenotype could be often present. Głowicki and coworkers showed that a prothrombotic pathway might involve an increasing risk among patients with AF with the CHA2DS2-VASc score of 1 despite the sex [21].
Patients with acute onset AF were traditionally considered at lower risk of thrombo-embolic events, related to the shorter time for atrial thrombus formation [22]. However, CV has an inherent risk of stroke in non-anticoagulated patients, which is lowered by anticoagulant drugs administration [4]. Nonetheless, those patients with new onset AF and stroke risk factors take probably more advantages of using oral anticoagulant (OAC). The most common risk stratification score currently utilized is the CHA2DS2-VASc [23]. A CHA2DS2-VASc score of 1 or more for men, and 2 or more for women is considered the cut off value for starting oral anticoagulation, in order to protect patients from stroke events. The incidence of stroke and thrombo-embolic events varies significantly in patients with CHA2DS2-VASc scores of 1 or 2. Of note, an age of more than 65 years is associated with an increased risk of stroke, and it also potentiates other risk factors, such as heart failure and sex [24].
Regarding the decision whether to start an OAC for a new-onset AF in naïve patients, several studies showed that starting pre-cardioversion anticoagulation in all patients with AF episodes of less than 24 h, or even 12 h, would afford even greater safety [25][26]. In the Fin CV (Finnish Cardioversion) Study, a large multicenter retrospective cohort trial exploring the occurrence and risk factors of thromboembolic complications after CV in acute AF [27], 7660 cardioversions were carried out in 3143 consecutive patients with AF that occurred within 48 h. The overwhelming preponderance (88%) of CVs were ECVs. Embolic complications at 30 days were reported after 5116 effective CVs in 2481 patients with neither oral anticoagulation nor peri-procedural heparin therapy. Thirty-eight embolic events occurred (0.7% of efficacious procedures; 95% confidence interval (CI): 0.5% to 1.0%) after a follow-up of 1 month, and 31 of these were strokes. Logistic regression analyses revealed that age, female sex, heart failure, and diabetes were independent predictors of embolic events. Moreover, with the coexistence of multiple risk factors, the risk results in an extremely elevated risk (approximately 10%), that is notably higher than after elective CV of AF with conventional anticoagulation. Based upon these results, the authors affirmed that for certain subgroups of patients with a new onset AF, the risk of a stroke becomes notable and a pre/post CV anticoagulant approach is preferable. Indeed, according to the more recent guidelines for managing AF, a pre-cardioversion anticoagulation therapy is now recommended for all patients, irrespective of risk factors for stroke (Class IIa B). The same thing is valid for the four-week post-procedural anticoagulant therapy, both in case of PCV or ECV [4].
3.2. Electrical Cardioversion in AF Which Lasted for Longer Than 48 h
In patients with hemodynamic stability and an AF which lasted for longer than 48 h or of unknown time of onset, ECV should be planned after an appropriate anticoagulation therapy, either with 4 consecutive weeks of warfarin with weekly therapeutic INR (2–3) or 4 weeks of NOACs without any interruption. Alternatively, a TEE to exclude left atrial appendage thrombus (LAAT) could also be performed followed by an immediate ECV and a subsequent oral anticoagulation for at least 4 weeks [28]. A window time of 48 h of AF is generally considered the boundary beyond which LAAT may organize, and CV-caused atrial stunning is likely to occur [29]. As previously reported, thromboembolic events are also frequent in the first month after CV [27].
Although it could seem generally better to reinstate a sinus rhythm in all patients with persistent AF, all studies that have evaluated rhythm control in comparison with rate control (with appropriate anticoagulation) have resulted in neutral outcomes [30][31].
Factors which might facilitate an attempt at rhythm control should be evaluated [4].
The left atrial volume index (LAVI) could represent an effective marker in predicting the maintenance of SR contributing to the identify patients in which CV is likely to be successful. A cut-off 55 mL/m2 has been proposed [32]. Another factor which should be taken into account is the left atrial diameter since it seems to be associated with AF recurrence after cardioversion if it is larger than 44 mm [33]. Conversely, it could be that a heterogeneous electrical activation of the LA appears to be related to AF recurrence. An advanced interatrial block (aIAB), P wave duration > 120 ms, and biphasic P waves in the inferior leads have been recognized as independent predictors of AF recurrence [34].
However, in the case of undated AF, not only an adequate anticoagulation, but also proper control of the heart rate is required. Beta-blockers, digoxin, diltiazem, or verapamil are recommended to control heart rate in AF patients with left ventricle ejection fraction (LVEF) ≥ 40%, while calcium antagonists should be avoided in the case of left ventricular dysfunction.
References
- Wyse, D.G.; Waldo, A.L.; Di Marco, J.P.; Domanski, M.J.; Rosenberg, Y.; Schron, E.B.; Kellen, J.C.; Greene, H.L.; Mickel, M.C.; Dalquist, J.; et al. A Comparison of Rate Control and Rhythm Control in Patients with Atrial Fibrillation. N. Engl. J. Med. 2002, 347, 1825–1833.
- Ganesan, A.; Chew, D.P.; Hartshorne, T.; Selvanayagam, J.B.; Aylward, P.; Sanders, P.; McGavigan, A.D. The impact of atrial fibrillation type on the risk of thromboembolism, mortality, and bleeding: A systematic review and meta-analysis. Eur. Heart J. 2016, 37, 1591–1602.
- Steinberg, B.A.; Hellkamp, A.S.; Lokhnygina, Y.; Patel, M.R.; Breithardt, G.; Hankey, G.; Becker, R.C.; Singer, D.E.; Halperin, J.L.; Hacke, W.; et al. Higher risk of death and stroke in patients with persistent vs. paroxysmal atrial fibrillation: Results from the ROCKET-AF Trial. Eur. Heart J. 2015, 36, 288–296.
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collabora-tion with the European Association of Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 42, 373–489.
- Hansen, M.L.; Jepsen, R.M.H.G.; Olesen, J.B.; Ruwald, M.H.; Karasoy, D.; Gislason, G.; Hansen, J.; Køber, L.; Husted, S.; Torp-Pedersen, C. Thromboembolic risk in 16 274 atrial fibrillation patients undergoing direct current cardioversion with and without oral anticoagulant therapy. Europace 2015, 17, 18–23.
- Ruff, C.T.; Giugliano, R.; Braunwald, E.; Hoffman, E.B.; Deenadayalu, N.; Ezekowitz, M.D.; Camm, A.J.; Weitz, J.; Lewis, B.S.; Parkhomenko, A.; et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: A meta-analysis of randomised trials. Lancet 2014, 383, 955–962.
- Papp, J.; Zima, E.; Bover, R.; Karaliute, R.; Rossi, A.; Szymanski, C.; Troccoli, R.; Schneider, J.; Fagerland, M.W.; Camm, A.J.; et al. Changes in oral anticoagulation for elective cardioversion: Results from a European cardioversion registry. Eur. Heart J. Cardiovasc. Pharmacother. 2017, 3, 147–150.
- Steffel, J.; Collins, R.; Ant, M.; Cornu, P.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; Rowell, N.; et al. 2021 European Heart Rhythm Association Practical Guide on the Use of Non-Vitamin K Antagonist Oral Anticoagulants in Patients with Atrial Fibrillation. Europace 2021.
- Connors, S.; Dorian, P. Management of supraventricular tachycardia in the emergency department. Can. J. Cardiol. 1997, 13, 19A–24A.
- McDonald, A.J.; Pelletier, A.J.; Ellinor, P.; Camargo, C.A. Increasing US Emergency Department Visit Rates and Subsequent Hospital Admissions for Atrial Fibrillation from 1993 to 2004. Ann. Emerg. Med. 2008, 51, 58–65.
- Mittal, S.; Ayati, S.; Stein, K.M.; Schwartzman, D.; Cavlovich, D.; Tchou, P.J.; Markowitz, S.M.; Slotwiner, D.J.; Scheiner, M.A.; Lerman, B.B. Transthoracic Cardioversion of Atrial Fibrillation: Comparison of Rectilinear Biphasic Versus Damped Sine Wave Monophasic Shocks. Circulation 2000, 101, 1282–1287.
- Toso, E.; Blandino, A.; Sardi, D.; Battaglia, A.; Garberoglio, L.; Miceli, S.; Azzaro, G.; Capello, A.L.; Gaita, F. Electrical Cardioversion of Persistent Atrial Fibrillation: Acute and Long-Term Results Stratified According to Arrhythmia Duration. Pacing Clin. Electrophysiol. 2012, 35, 1126–1134.
- Elesber, A.A.; Rosales, A.G.; Herges, R.M.; Shen, W.-K.; Moon, B.S.; Malouf, J.F.; Ammash, N.M.; Somers, V.; Hodge, D.O.; Gersh, B.J.; et al. Relapse and mortality following cardioversion of new-onset vs. recurrent atrial fibrillation and atrial flutter in the elderly. Eur. Heart J. 2006, 27, 854–860.
- Schmidt, A.S.; Lauridsen, K.G.; Torp, P.; Bach, L.F.; Rickers, H.; Løfgren, B. Maximum-fixed energy shocks for cardioverting atrial fibrillation. Eur. Heart J. 2019, 41, 626–631.
- Gallagher, M.M.; Guo, X.-H.; Poloniecki, J.D.; Yap, Y.G.; Ward, D.; Camm, A. Initial energy setting, outcome and efficiency in direct current cardioversion of atrial fibrillation and flutter. J. Am. Coll. Cardiol. 2001, 38, 1498–1504.
- Hamada, T.; Hiraki, T.; Ikeda, H.; Kubara, I.; Yoshida, T.; Ohga, M.; Imaizumi, T. Mechanisms for Atrial Fibrillation in Patients with Wolff-Parkinson-White Syndrome. J. Cardiovasc. Electrophysiol. 2002, 13, 223–229.
- Van Gelder, I.C.; Hagens, V.E.; Bosker, H.A.; Kingma, J.H.; Kamp, O.; Kingma, T.; Said, S.A.; Darmanata, J.I.; Timmermans, A.J.M.; Tijssen, J.G.P.; et al. A Comparison of Rate Control and Rhythm Control in Patients with Recurrent Persistent Atrial Fibrillation. N. Engl. J. Med. 2002, 347, 1834–1840.
- Dankner, R.; Shahar, A.; Novikov, I.; Agmon, U.; Ziv, A.; Hod, H. Treatment of Stable Atrial Fibrillation in the Emergency Department: A Population-Based Comparison of Electrical Direct-Current versus Pharmacological Cardioversion or Conservative Management. Cardiology 2009, 112, 270–278.
- Pluymaekers, N.A.; Dudink, E.A.; Luermans, J.G.; Meeder, J.G.; Lenderink, T.; Widdershoven, J.; Bucx, J.J.; Rienstra, M.; Kamp, O.; Van Opstal, J.M.; et al. Early or Delayed Cardioversion in Recent-Onset Atrial Fibrillation. N. Engl. J. Med. 2019, 380, 1499–1508.
- Lin, H.-J.; Wolf, P.A.; Kelly-Hayes, M.; Beiser, A.; Kase, C.S.; Benjamin, E.; D’Agostino, R.B. Stroke Severity in Atrial Fibrillation. Stroke 1996, 27, 1760–1764.
- Głowicki, B.; Matusik, P.T.; Plens, K.; Undas, A. Prothrombotic State in Atrial Fibrillation Patients With One Additional Risk Factor of the CHA2DS2-VASc Score (Beyond Sex). Can. J. Cardiol. 2019, 35, 634–643.
- Klein, A.L.; Grimm, R.A.; Murray, R.D.; Apperson-Hansen, C.; Asinger, R.W.; Black, I.W.; Davidoff, R.; Erbel, R.; Halperin, J.L.; Orsinelli, D.; et al. Use of Transesophageal Echocardiography to Guide Cardioversion in Patients with Atrial Fibrillation. N. Engl. J. Med. 2001, 344, 1411–1420.
- Olesen, J.B.; Lip, G.Y.H.; Hansen, M.L.; Hansen, P.R.; Tolstrup, J.S.; Lindhardsen, J.; Selmer, C.; Ahlehoff, O.; Olsen, A.-M.S.; Gislason, G.; et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: Nationwide cohort study. BMJ 2011, 342, d124.
- Friberg, L.; Skeppholm, M.; Terént, A. Benefit of Anticoagulation Unlikely in Patients with Atrial Fibrillation and a CHA2DS2-VASc Score of 1. J. Am. Coll. Cardiol. 2015, 65, 225–232.
- Nuotio, I.; Hartikainen, J.E.K.; Grönberg, T.; Biancari, F.; Airaksinen, J. Time to Cardioversion for Acute Atrial Fibrillation and Thromboembolic Complications. JAMA 2014, 312, 647–649.
- Van Gelder, I.C.; Hemels, M.E. The progressive nature of atrial fibrillation: A rationale for early restoration and maintenance of sinus rhythm. Europace 2006, 8, 943–949.
- Airaksinen, K.E.J.; Grönberg, T.; Nuotio, I.; Nikkinen, M.; Ylitalo, A.; Biancari, F.; Hartikainen, J.E.K. Thromboembolic Complications after Cardioversion of Acute Atrial Fibrillation: The FinCV (Finnish CardioVersion) Study. J. Am. Coll. Cardiol. 2013, 62, 1187–1192.
- January, C.T.; Wann, L.S.; Alpert, J.S.; Calkins, H.; Cigarroa, J.E.; Cleveland, J.C.; Conti, J.B.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014, 130, 2071–2104.
- Silverman, D.I.; Manning, W.J. Role of Echocardiography in Patients Undergoing Elective Cardioversion of Atrial Fibrillation. Circulation 1998, 98, 479–486.
- Chatterjee, S.; Sardar, P.; Lichstein, E.; Mukherjee, D.; Aikat, S. Pharmacologic Rate versus Rhythm-Control Strategies in Atrial Fibrillation: An Updated Comprehensive Review and Meta-Analysis. Pacing Clin. Electrophysiol. 2013, 36, 122–133.
- De Denus, S.; Sanoski, C.A.; Carlsson, J.; Opolski, G.; Spinler, S.A. Rate vs Rhythm Control in Patients With Atrial Fibrillation. Arch. Intern. Med. 2005, 165, 258–262.
- Toufan, M.; Kazemi, B.; Molazadeh, N. The significance of the left atrial volume index in prediction of atrial fibrillation recurrence after electrical cardioversion. J. Cardiovasc. Thorac. Res. 2017, 9, 54–59.
- Efremidis, M.; Alexanian, I.P.; Oikonomou, D.; Manolatos, D.; Letsas, K.P.; Pappas, L.K.; Gavrielatos, G.; Vadiaka, M.; Mihas, C.C.; Filippatos, G.S.; et al. Predictors of atrial fibrillation recurrence in patients with long-lasting atrial fibrillation. Can. J. Cardiol. 2009, 25, e119–e124.
- Baranchuk, A.; Yeung, C. Advanced interatrial block predicts atrial fibrillation recurrence across different populations: Learning Bayés syndrome. Int. J. Cardiol. 2018, 272, 221–222.
- Kirchhof, P.; Andresen, D.; Bosch, R.; Borggrefe, M.; Meinertz, T.; Parade, U.; Ravens, U.; Samol, A.; Steinbeck, G.; Treszl, A.; et al. Short-term versus long-term antiarrhythmic drug treatment after cardioversion of atrial fibrillation (Flec-SL): A prospective, randomised, open-label, blinded endpoint assessment trial. Lancet 2012, 380, 238–246.
- Singh, B.N.; Singh, S.N.; Reda, D.J.; Tang, X.C.; Lopez, B.; Harris, C.L.; Fletcher, R.D.; Sharma, S.C.; Atwood, J.E.; Jacobson, A.K.; et al. Amiodarone versus Sotalol for Atrial Fibrillation. N. Engl. J. Med. 2005, 352, 1861–1872.
More
Information
Subjects:
Cardiac & Cardiovascular Systems
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
3 times
(View History)
Update Date:
12 Aug 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




