
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Roberto Panichi | + 1939 word(s) | 1939 | 2020-05-08 11:48:12 | | | |
| 2 | Nora Tang | -7 word(s) | 1932 | 2020-05-26 12:01:57 | | | | |
| 3 | Nora Tang | -22 word(s) | 1910 | 2020-10-30 12:53:44 | | |
Video Upload Options
The estrogen estradiol is a potent neuroactive steroid that may regulate brain structure and function. Although the eff ects of estradiol have been historically associated with gonadal secretion, the discovery that this steroid may be synthesized within the brain has expanded this traditional concept. Indeed, it is accepted that de novo synthesized estradiol in the nervous system (nE2) may modulate several aspects of neuronal physiology, including synaptic transmission and plasticity, thereby influencing a variety of behaviors. These modulations may be on a time scale of minutes via non-classical and often membrane-initiated mechanisms or hours and days by classical actions on gene transcription. Besides the high level, recent investigations in the cerebellum indicate that even a low aromatase expression can be related to the fast nE2 eff ect on brain functioning. These pieces of evidence point to the importance of an on-demand and localized nE2 synthesis to rapidly contribute to regulating synaptic transmission. This review is geared at exploring a new scenario for the impact of estradiol on brain processes as it emerges from the nE2 action on cerebellar neurotransmission and cerebellum-dependent learning.
1. Introduction
Estrogens are part of the neuroactive steroid family that may regulate the structure and function of neural networks via multiple modes and time courses. The most potent estrogen in influencing brain functions is the 17 beta-estradiol that exerts its effect via both classical long term actions on genomic mechanisms and rapid non-classical effects [1][2][3][4][5][6][7]. The estradiol impacts on neural physiology depend on its bioavailability in a brain structure, and it has been shown that, in addition to peripheral synthesis such as in the gonads, the estradiol can be locally produced in the nervous system [8]. The de novo synthesized 17 beta-estradiol in nervous tissues, defined as neurosteroid (nE2), has the same structure and mechanisms of synthesis than the gonadal derived estrogen (Figure 1).
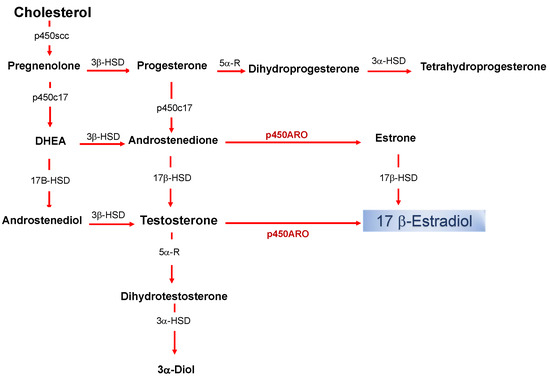
Figure 1. Biosynthetic pathway for neurosteroids in the brain. The arrows indicate biosynthetic pathways of neurosteroids identified in the brain. P450scc, Cytochrome P450 cholesterol side-chain cleavage enzyme; p450c17, cytochrome P450 17a-hydroxylase/C17; DHEA, dehydroepiandrosterone; 17β-HSD, 17beta-hydroxysteroid dehydrogenase; 3β-HSD 3beta-hydroxysteroid dehydrogenase D5–D4 isomerase; 5α-R, 5alfa-reductase; p450ARO, cytochrome P450 aromatase; 3α-HSD 3alfa-hydroxysteroid dehydrogenase D5–D4 isomerase.
The production of nE2 requires an aromatase-dependent conversion of testosterone, which may be either of the peripheral origins or locally synthesized from the precursor cholesterol [9][10][11][12]. Thus, estradiol is no longer merely considered a hormone produced by the ovaries and only related to the control of female sexual maturation and reproduction. Instead, it is now known to have multiple homeostatic roles, and in the nervous system, estradiol controls a variety of processes in males as well as females [2][13][14][15][16]. Via the “classical” genomic mode of action, estradiol interacts with intracellular receptors to influence transcriptional pathways and regulate DNA transcription within minutes (Figure 2) [17].
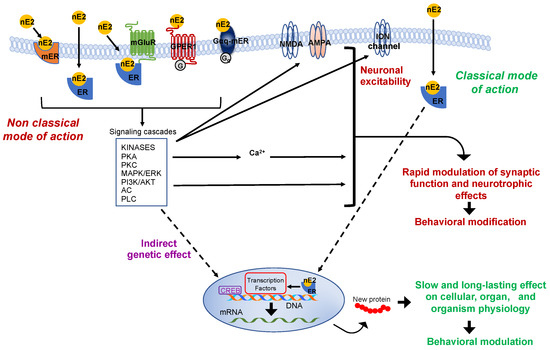
Figure 2. Schematic representation of the classic and non-classic mode of action of the estrogen estradiol. The schema represents possible estradiol effects on neural targets. Both classical and non-classical effects require estrogen receptors activation by estradiol (nE2). In the classic genomic mode of action, nE2 binds to cytoplasmatic estrogen receptors beta or alpha (ERs: ERα, ERβ), receptors dimerize (not shown), and translocate to the nucleus. Once bound specific estrogen response element on the DNA, the dimer possibly recruits transcriptional coregulator to modulates the gene transcription. The results may be a slow and long-lasting effect on cells and, ultimately, on the entire organism. In the non-classical mode of action, nE2 binds cytoplasmatic or membrane-associated estrogen receptors (ERs, GPER-1, Gαq). The binding triggers intracellular signaling cascades involving several kinases (e.g., PCA, Protein kinase A; PKC, Protein kinases C; MAPK, mitogen-activated protein kinase; PI3K, phosphoinositide 3-kinase), adenylyl cyclase (AC), phospholipase C (PLC), and fluctuation in intracellular calcium concentration. Such intracellular signaling may result in the rapid modulation of synaptic function (the schema also shows possible neurotrophic effects) and, ultimately, in behavioral modifications. Finally, in the estrogen-mediated indirect genetic effect, nE2 binding to estrogen receptors induces signaling cascades that might activate transcription factors (e.g., CREB, cAMP response element-binding protein) to regulate the gene transcription. AKT, Protein kinase B; AMPA, ionotropic glutamate receptor; ER, classical estrogen receptor; ERK, extracellular signal-regulated kinase; GPER-1, G protein-coupled transmembrane estrogen receptor-1; Gαq-mER, Gαq-coupled membrane-associated estrogen receptor; mER, membrane-associated classical estrogen receptor; mGluR, metabotropic glutamate receptor; NMDA, ionotropic glutamate receptor.
Two typical estrogen receptor isoforms (ERs: ERα, ERβ) are known to participate in classical influence. However, to produce detectable functional effects on the cellular level or the entire organism, the “classical” mode of action requires post-transcriptional events that take hours to days to occur [7][18][19]. In the brain, faster non-classical and often membrane-initiated mechanisms are observable within a few seconds (for some electrophysiological effects) to minutes after estradiol activated signaling involving intracellular cascades that may include several protein kinases and result in cytoplasmatic fluctuations of calcium concentrat (Figure 2) [19][20][21][22][23][24]. Some of these intracellular signalings could also affect the gene transcription through the so-called “indirect genetic effect” (Figure 2) [25]. However, the essential point considered here is that estradiol may rapidly modulate pathways associated with the regulation of neurotransmission and neuronal activity such as those related to cytoplasmatic protein kinases cascades and calcium fluct (Figure 2) [23][26].
Rapid membrane-initiated effects involve estrogen G-protein-coupled receptors (GPCRs: GPER-1 and Gαq-mER), classical estrogen receptors (ERα, ERβ), and probably other putative receptors (e.g., ER-X) [27][28][29][30][31][32][33][34][35]. It is noteworthy to mention that non-classical estrogen actions on neural circuits are not limited to those that occur via the activation of membrane-associated estradiol pathways, but also include mechanisms that can depend on the estrogen binding of cytoplasmatic receptors with the consequent activation of various intracellular signaling cascades (Figure 2) [36].
It has been established that nE2 may be a potent regulator of the neuronal functions when produced in large amounts [4][37]. However, how generalized nE2 synthesis is within the nervous system and whether some of the nE2 effects can be even mediated by low estradiol production are issues of rising interest in neurophysiology and neuroendocrinology.
Recent evidence showed that besides the high level of synthesis, even alleged minute and localized nE2 production might contribute to rapidly modulating aspects of neuronal physiology like the synaptic transmission and plasticity (Figure 2).
Experiments conducted in the brainstem and cerebellum indicate that despite the supposed low synthesis in the experimental models used, nE2 may contribute to rapidly modulating neurotransmission via dynamic change of its availability at a synaptic level [38][39][40][41][42]. It has already been demonstrated that modifications in local estradiol synthesis may be achieved by changes in aromatase activity. Although possible changes in aromatase activity result from transcriptional control that alters the enzyme concentration, these changes generally occur slowly in a time scale of hours or days. Alternatively, much more rapid changes in the rate of nE2 production may result from post-translational events that potentiate/inhibit the enzyme activity (Figure 3) [38].
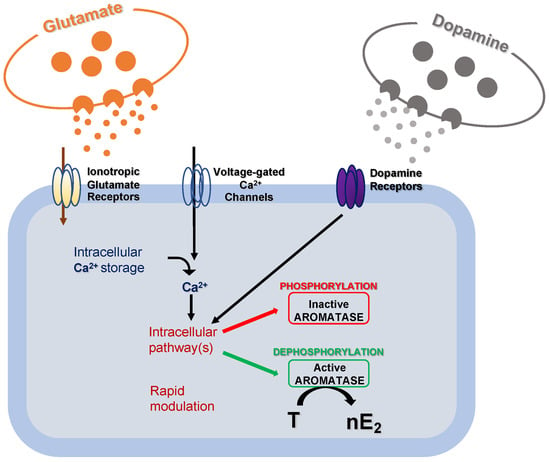
Figure 3. Schematic cartoon representing the post-translational mechanisms modulating aromatase activity. Fluctuations in intracellular calcium (Ca2+) concentration, along with glutamatergic and dopaminergic signals, might rapidly modulate intracellular pathways to regulate aromatase activity via phosphorylation/dephosphorylation processes. As a result, the transformation rate of testosterone (T) into 17 beta-estradiol (nE2) may be rapidly regulated.
Post-translational modifications are common mechanisms involved in the control of protein activity in the brain, like in the case of the regulation of neurotransmitter activity in neurons [43].
The evidence introduced above provides new insight into the effectiveness of nE2 in regulating neuronal activity and indicating that estradiol may play an essential role in the modulation of network functioning, even in brain structures in which, so far, its synthesis has been assumed to be too low to be effective. Here, we review the emerging view from this scenario that expands the traditional significance of nE2 production into a new physiological role and includes recently suggested mechanisms involved in the rapid control of the neural transmission, with particular regard to the synaptic transmission in the cerebellum.
2. The Effectiveness of the de Novo Synthesized Estradiol in Rapidly Influencing the Neuronal Functioning
It has long been assumed that the impact of nE2 on the regulation of neural functions should be restricted at those brain areas in which the estradiol synthesizing enzyme aromatase is highly expressed and, of course, estrogen receptors are present. According to this assumption, in such areas, nE2 may rapidly reach high concentrations [4][44][45], bind membrane ERs, or activate other non-classical mechanisms to initiate intracellular signaling and acutely influence the neuronal physiology [13][21][33][36][46]. Rapid activation of membrane estrogen pathways by nE2 may be facilitated by the co-localization of aromatase and estrogen receptors in the plasma membrane or their localization at the synaptic level in
neurons of the central nervous system [47][48][49]. Non-classical influences include, but are not limited to, the modulation of synaptic activity, synaptogenesis, and spinogenesis (Figure 2) [23][50][51]. Rapid functional regulation of synaptic activity by estradiol has been found, so far, for the glutamatergic, GABAergic, cholinergic, and dopaminergic systems [27][41][42][52][53][54][55][56][57]. Of course, also classical genomic delayed effects influencing neuronal structure and function can be mediated by nE2 and interact with the non-classical mode of actions [2][13][19]. nE2 may, therefore, regulate over different timescale phenomena such as synaptic transmission, spine density, synaptic connectivity, synaptogenesis, and neurogenesis, that mediate behavioral changes (Figure 2) [13][20][21][27][58][59].
In many vertebrates, brain areas with the highest aromatase expression include hypothalamic preoptic nuclei, the bed nucleus of the stria terminalis, and medial amygdala, typically involved in the control of stress responses, sexual and social behaviors [2][58][59][60] and other structures that, like in the case of the hippocampus and inferior olive, are related to sensorimotor aspects of information processing and learning phenomena [44][61][62][63][64]. Consistently, besides its role in the regulation of reproduction and sexual functions [1][2][65][66][67], nE2 may influence a variety of other processes and behaviors from the mood to learning and memory formation and have a neuroprotective role as well [14][27][50][52][68][69][70][71][72][73][74][75][76].
However, despite a significant interspecific variability, low aromatase expression may be present in other brain areas such as temporal, occipital, and cingulate cortex, putamen, brainstem, and cerebellum of adult animals including rodents and humans [64][77][78][79][80].
Rodents are the most used model to study brain functions. For instance, in the cerebellum of rodents, aromatase is highly expressed only during neonatal life while it is maintained at a constant low level during peripubertal (the period between day 30 and day 45 after birth) and adulthood (post-pubertal) [64][81][82]. Thus, while the role of nE2 in regulating cerebellar development has been investigated [83][84], its effects upon the functioning of the cerebellum in adults (in the following text, the term “adult” refers to a period comprehensive the peri and post-pubertal period) have long been considered irrelevant or ignored.
Nevertheless, the age-related maintenance of a low level of aromatase expression in the cerebellum seems to have a cardinal physiological significance in the contribution of controlling the cerebellar function. Recent reports showed that nE2 synthesis rapidly influences the glutamatergic transmission between the parallel fibers and Purkinje cells in the cerebellar cortex and acutely impact cerebellar dependent behaviors in adult rodents [39][40][72][85]. Furthermore, the high level of estrogen receptors found in the cerebellum of adults is supportive of cerebellar responsiveness even to a minute and localized nE2 synthesis [86][87][88].
The above-reported findings are consistent with previous discoveries indicating an acute modulation of nE2 on glutamatergic synapses in vestibular nuclei, which are assimilable to extra cerebellar nuclei and have, like the cerebellum, a low level of aromatase expression [41][42][64][89][90][91]. Thus, even at low expression, aromatase may play a functional role in allowing the brain to exert dynamic control over local neuronal functions via nE2 synthesis, as may be the case of synaptic transmission and plasticity in the cerebellar circuitry.
References
- C.A. Cornil; Gregory F. Ball; Jacques Balthazart; Rapid control of male typical behaviors by brain-derived estrogens.. Frontiers in Neuroendocrinology 2012, 33, 425-46, 10.1016/j.yfrne.2012.08.003.
- Jacques Balthazart; Michelle Baillien; C.A. Cornil; Gregory F. Ball; Preoptic aromatase modulates male sexual behavior: slow and fast mechanisms of action. Physiology & Behavior 2004, 83, 247-270, 10.1016/s0031-9384(04)00356-7.
- Yasushi Hojo; Gen Murakami; Hideo Mukai; Shimpei Higo; Yusuke Hatanaka; Mari Ogiue-Ikeda; Hirotaka Ishii; Tetsuya Kimoto; Suguru Kawato; Estrogen synthesis in the brain—Role in synaptic plasticity and memory. Molecular and Cellular Endocrinology 2008, 290, 31-43, 10.1016/j.mce.2008.04.017.
- Catherine S Woolley; Acute Effects of Estrogen on Neuronal Physiology. Annual Review of Pharmacology and Toxicology 2007, 47, 657-680, 10.1146/annurev.pharmtox.47.120505.105219.
- K. S. J. Ervin; A. Phan; C. S. Gabor; Elena Choleris; Rapid Oestrogenic Regulation of Social and Nonsocial Learning. Journal of Neuroendocrinology 2013, 25, 1116-1132, 10.1111/jne.12079.
- Bradley M. Cooke; Catherine S. Woolley; Gonadal hormone modulation of dendrites in the mammalian CNS. Journal of Neurobiology 2005, 64, 34-46, 10.1002/neu.20143.
- Pamela J. McMillan; Daniel M. Dorsa; Estrogen actions in the central nervous system. Current Opinion in Endocrinology & Diabetes 1999, 6, 33, 10.1097/00060793-199902000-00006.
- Anne Tm Konkle; Margaret M. McCarthy; Developmental time course of estradiol, testosterone, and dihydrotestosterone levels in discrete regions of male and female rat brain.. Endocrinology 2010, 152, 223-35, 10.1210/en.2010-0607.
- Yasushi Hojo; Taka-Aki Hattori; Taihei Enami; Aizo Furukawa; Kumiko Suzuki; Hirotaka Ishii; Hideo Mukai; John H. Morrison; William G. M. Janssen; Shiro Kominami; Nobuhiro Harada; Tetsuya Kimoto; Suguru Kawato; Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017α and P450 aromatase localized in neurons. Proceedings of the National Academy of Sciences 2003, 101, 865-870, 10.1073/pnas.2630225100.
- E E Baulieu; Neurosteroids: of the nervous system, by the nervous system, for the nervous system.. Recent Progress in Hormone Research 1997, 52, 1–32.
- Nathalie A. Compagnone; Synthia H. Mellon; Neurosteroids: Biosynthesis and Function of These Novel Neuromodulators. Frontiers in Neuroendocrinology 2000, 21, 1-56, 10.1006/frne.1999.0188.
- Tetsuya Kimoto; Tomokazu Tsurugizawa; Yoichiro Ohta; Jun’Ya Makino; Hiro-Omi Tamura; Yasushi Hojo; Norio Takata; Suguru Kawato; Neurosteroid Synthesis by Cytochrome P450-Containing Systems Localized in the Rat Brain Hippocampal Neurons: N-Methyl-D-Aspartate and Calcium-Dependent Synthesis. Endocrinology 2001, 142, 3578-3589, 10.1210/en.142.8.3578.
- C.A. Cornil; Gregory F. Ball; Jacques Balthazart; The dual action of estrogen hypothesis.. Trends in Neurosciences 2015, 38, 408-16, 10.1016/j.tins.2015.05.004.
- Cheryl S. Rosenfeld; Dusti A. Shay; Victoria J. Vieira-Potter; Cognitive Effects of Aromatase and Possible Role in Memory Disorders. Frontiers in Endocrinology 2018, 9, , 10.3389/fendo.2018.00610.
- John A Morris; Cynthia L Jordan; S. Marc Breedlove; Sexual differentiation of the vertebrate nervous system. Nature Neuroscience 2004, 7, 1034-1039, 10.1038/nn1325.
- Paula Duarte-Guterman; Shunya Yagi; Carmen Chow; Liisa A.M. Galea; Hippocampal learning, memory, and neurogenesis: Effects of sex and estrogens across the lifespan in adults. Hormones and Behavior 2015, 74, 37-52, 10.1016/j.yhbeh.2015.05.024.
- S. Durant; D. Duval; F. Homo-Delarche; Non genomic effects of glucocorticoids: and potentiation of the action of isoproterenol on mouse thymocytes. Journal of Steroid Biochemistry 1986, 25, 1, 10.1016/0022-4731(86)90463-2.
- Julie Jacob; K.S. Sebastian; Sony Devassy; Lakshmi Priyadarsini; Mohamed Febin Farook; A. Shameem; Deepa Mathew; S. Sreeja; Raghava Varman Thampan; Membrane estrogen receptors: Genomic actions and post transcriptional regulation. Molecular and Cellular Endocrinology 2006, 246, 34-41, 10.1016/j.mce.2005.11.015.
- Nandini Vasudevan; Nald W. Pfaff; Non-genomic actions of estrogens and their interaction with genomic actions in the brain. Frontiers in Neuroendocrinology 2008, 29, 238-257, 10.1016/j.yfrne.2007.08.003.
- Jacques Balthazart; Elena Choleris; Luke Remage-Healey; Steroids and the brain: 50 years of research, conceptual shifts and the ascent of non-classical and membrane-initiated actions. Hormones and Behavior 2018, 99, 1-8, 10.1016/j.yhbeh.2018.01.002.
- Lauren Rudolph; C.A. Cornil; Melinda A. Mittelman-Smith; Jennifer R. Rainville; Luke Remage-Healey; Kevin Sinchak; Paul Micevych; Actions of Steroids: New Neurotransmitters. The Journal of Neuroscience 2016, 36, 11449-11458, 10.1523/JNEUROSCI.2473-16.2016.
- Oline K. Rønnekleiv; Martin J. Kelly; Membrane-Initiated Effects of Estradiol in the Central Nervous System. Hormones, Brain and Behavior 2017, null, 1-22, 10.1016/b978-0-12-803592-4.00043-2.
- Oberlander, J.G.; Woolley, C.S. 17beta-estradiol acutely potentiates glutamatergic synaptic transmission in the hippocampus through distinct mechanisms in males and females. J. Neurosci. 2016, 36, 2677–2690. [Google Scholar] [CrossRef]
- M J Kelly; R L Moss; C A Dudley; Differential sensitivity of preoptic-septal neurons to microelectrophoresed estrogen during the estrous cycle.. Brain Research 1976, 114, 152–157.
- Mittelman-Smith, M.A.; Rudolph, L.M.; Mohr, M.A.; Micevych, P.E. Rodent models of non-classical progesterone action regulating ovulation. Front. Endocrinol. 2017, 8.
- De-Juan Wang; L.-D. Su; Yanan Wang; Ng Yang; Cheng-Long Sun; Lin Zhou; Xin-Xin Wang; Ying Shen; Long-Term Potentiation at Cerebellar Parallel Fiber–Purkinje Cell Synapses Requires Presynaptic and Postsynaptic Signaling Cascades. The Journal of Neuroscience 2014, 34, 2355-2364, 10.1523/JNEUROSCI.4064-13.2014.
- Saleh Zahedi Asl; Mohammad Khaksari; Ali Siahposht Khachki; Nader Shahrokhi; Shahla Nourizade; Contribution of estrogen receptors alpha and beta in the brain response to traumatic brain injury. Journal of Neurosurgery 2013, 119, 353-361, 10.3171/2013.4.jns121636.
- John Kuo; Naheed Hamid; Galyna Bondar; Eric R. Prossnitz; Paul Micevych; Membrane estrogen receptors stimulate intracellular calcium release and progesterone synthesis in hypothalamic astrocytes.. The Journal of Neuroscience 2010, 30, 12950-7, 10.1523/JNEUROSCI.1158-10.2010.
- Georgina G J Hazell; Song T. Yao; James Roper; Eric R. Prossnitz; Anne-Marie O'carroll; Stephen J. Lolait; Localisation of GPR30, a novel G protein-coupled oestrogen receptor, suggests multiple functions in rodent brain and peripheral tissues. Journal of Endocrinology 2009, 202, 223-236, 10.1677/JOE-09-0066.
- Ellis R. Levin; Cellular Functions of the Plasma Membrane Estrogen Receptor. Trends in Endocrinology & Metabolism 1999, 10, 374-377, 10.1016/s1043-2760(99)00192-7.
- Michael Meaney; Toran-Allerand Cd; Guan X; MacLusky Nj; Horvath Tl; Diano S; Singh M; Connolly Es; Nethrapalli Is; Tinnikov Aa; Faculty of 1000 evaluation for ER-X: a novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury.. F1000 - Post-publication peer review of the biomedical literature 2002, 22, , 10.3410/f.1010518.156657.
- C.Dominique Toran-Allerand; Estrogen and the Brain: Beyond ER-α, ER-β, and 17β-Estradiol. Annals of the New York Academy of Sciences 2005, 1052, 136-144, 10.1196/annals.1347.009.
- Jian Qiu; Oline K. Rønnekleiv; Martin J. Kelly; Modulation of hypothalamic neuronal activity through a novel G-protein-coupled estrogen membrane receptor.. Steroids 2008, 73, 985-91, 10.1016/j.steroids.2007.11.008.
- Mahnaz Razandi; Ali Pedram; Geoffrey L. Greene; Ellis R. Levin; Cell Membrane and Nuclear Estrogen Receptors (ERs) Originate from a Single Transcript: Studies of ER? and ER? Expressed in Chinese Hamster Ovary Cells. Molecular Endocrinology 1999, 13, 307-319, 10.1210/mend.13.2.0239.
- Soltysik, K.; Czekaj, P. Membrane estrogen receptors—Is it an alternative way of estrogen action? J. Physiol. Pharmacol. 2013, 64, 129–142.
- Ellis R. Levin; Stephen R Hammes; Nuclear receptors outside the nucleus: extranuclear signalling by steroid receptors. Nature Reviews Molecular Cell Biology 2016, 17, 783-797, 10.1038/nrm.2016.122.
- Roberto Cosimo Melcangi; G.C. Panzica; Luis M. García-Segura; Neuroactive steroids: focus on human brain. Neuroscience 2011, 191, 1-5, 10.1016/j.neuroscience.2011.06.024.
- Charlier, T.D.; Cornil, C.A.; Patte-Mensah, C.; Meyer, L.; Mensah-Nyagan, A.G.; Balthazart, J. Local modulation of steroid action: Rapid control of enzymatic activity. Front. Neurosci. 2015, 9, 83.
- Valerie L Hedges; Gang Chen; Lei Yu; Amanda A Krentzel; Joseph R Starrett; Jing-Ning Zhu; Piratheepan Suntharalingam; Luke Remage-Healey; Jian-Jun Wang; Timothy J Ebner; Paul G Mermelstein; Local Estrogen Synthesis Regulates Parallel Fiber–Purkinje Cell Neurotransmission Within the Cerebellar Cortex. Endocrinology 2018, 159, 1328-1338, 10.1210/en.2018-00039.
- Cristina V. Dieni; Aldo Ferraresi; Jacqueline Sullivan; Sivarosa Grassi; Vito E. Pettorossi; Roberto Panichi; Acute inhibition of estradiol synthesis impacts vestibulo-ocular reflex adaptation and cerebellar long-term potentiation in male rats. Anatomy and Embryology 2017, 223, 837-850, 10.1007/s00429-017-1514-z.
- S. Grassi; Adele Frondaroli; Cristina V. Dieni; Mariangela Scarduzio; Vito E. Pettorossi; Long-Term Potentiation in the Rat Medial Vestibular Nuclei Depends on Locally Synthesized 17β-Estradiol. The Journal of Neuroscience 2009, 29, 10779-10783, 10.1523/JNEUROSCI.1697-09.2009.
- Mariangela Scarduzio; Roberto Panichi; Vito Enrico Pettorossi; Silvarosa Grassi; Synaptic Long-Term Potentiation and Depression in the Rat Medial Vestibular Nuclei Depend on Neural Activation of Estrogenic and Androgenic Signals. PLOS ONE 2013, 8, e80792, 10.1371/journal.pone.0080792.
- Bo-Shiun Chen; Katherine W. Roche; Regulation of NMDA receptors by phosphorylation. Neuropharmacology 2007, 53, 362-8, 10.1016/j.neuropharm.2007.05.018.
- Yasushi Hojo; Shimpei Higo; Hirotaka Ishii; Yuuki Ooishi; Hideo Mukai; Gen Murakami; Toshihiro Kominami; Tetsuya Kimoto; Seijiro Honma; Nald Poirier; Suguru Kawato; Comparison between Hippocampus-Synthesized and Circulation-Derived Sex Steroids in the Hippocampus. Endocrinology 2009, 150, 5106-5112, 10.1210/en.2009-0305.
- Hideo Mukai; Tetsuya Kimoto; Yasushi Hojo; Suguru Kawato; Gen Murakami; Shimpei Higo; Yusuke Hatanaka; Mari Ogiue-Ikeda; Modulation of synaptic plasticity by brain estrogen in the hippocampus. Biochimica et Biophysica Acta (BBA) - General Subjects 2010, 1800, 1030-1044, 10.1016/j.bbagen.2009.11.002.
- Cornil, C.A.; Ball, G.F.; Balthazart, J. Functional significance of the rapid regulation of brain estrogen action: Where do the estrogens come from? Brain Res. 2006, 1126, 2–26.
- Storman, E.M.; Liu, N.J.; Wessendorf, M.W.; Gintzler, A.R. Physical linkage of estrogen receptor alpha and aromatase in rat: Oligocrine and endocrine actions of CNS-produced estrogens. Endocrinology 2018, 159, 2683–2697.
- Oliver Kretz; Lars Fester; Uwe Wehrenberg; Lepu Zhou; Silke Brauckmann; Shanting Zhao; Janine Prange-Kiel; Thomas Naumann; Hubertus Jarry; Michael Frotscher; Gabriele M. Rune; Hippocampal Synapses Depend on Hippocampal Estrogen Synthesis. The Journal of Neuroscience 2004, 24, 5913-5921, 10.1523/JNEUROSCI.5186-03.2004.
- David J. Bailey; Yekaterina V. Makeyeva; Elizabeth R. Paitel; Alyssa L. Pedersen; Angel T. Hon; Jordan A. Gunderson; Colin J. Saldanha; Hippocampal Aromatization Modulates Spatial Memory and Characteristics of the Synaptic Membrane in the Male Zebra Finch. Endocrinology 2017, 158, 852-859, 10.1210/en.2016-1692.
- I. Azcoitia; Maria-Angeles Arevalo; Luis M. García-Segura; Neural-derived estradiol regulates brain plasticity. Journal of Chemical Neuroanatomy 2018, 89, 53-59, 10.1016/j.jchemneu.2017.04.004.
- Katherine J. Sellers; Filippo Erli; Pooja Raval; Iain A. Watson; Ding Chen; Deepak P. Srivastava; Rapid modulation of synaptogenesis and spinogenesis by 17β-estradiol in primary cortical neurons. Frontiers in Cellular Neuroscience 2015, 9, , 10.3389/fncel.2015.00137.
- María Elvira Brocca; Luis M. García-Segura; Non-reproductive Functions of Aromatase in the Central Nervous System Under Physiological and Pathological Conditions. Cellular and Molecular Neurobiology 2018, 39, 473-481, 10.1007/s10571-018-0607-4.
- Liisa A. Tremere; Jin Kwon Jeong; Raphael Pinaud; Estradiol Shapes Auditory Processing in the Adult Brain by Regulating Inhibitory Transmission and Plasticity-Associated Gene Expression. The Journal of Neuroscience 2009, 29, 5949-5963, 10.1523/JNEUROSCI.0774-09.2009.
- Vito Enrico Pettorossi; Michela Di Mauro; Mariangela Scarduzio; Roberto Panichi; Alessandro Tozzi; Paolo Calabresi; Silvarosa Grassi; Modulatory role of androgenic and estrogenic neurosteroids in determining the direction of synaptic plasticity in the CA1 hippocampal region of male rats. Physiological Reports 2013, 1, e00185, 10.1002/phy2.185.
- Tozzi, A.; de Iure, A.; Tantucci, M.; Durante, V.; Quiroga-Varela, A.; Giampa, C.; Di Mauro, M.; Mazzocchetti, P.; Costa, C.; Di Filippo, M.; et al. Endogenous 17beta-estradiol is required for activity-dependent long-term potentiation in the striatum: Interaction with the dopaminergic system. Front. Cell. Neurosci. 2015, 9, 192. [Google Scholar] [CrossRef]
- Michela Di Mauro; Alessandro Tozzi; Paolo Calabresi; Vito Enrico Pettorossi; Silvarosa Grassi; Neo-synthesis of estrogenic or androgenic neurosteroids determine whether long-term potentiation or depression is induced in hippocampus of male rat. Frontiers in Cellular Neuroscience 2015, 9, 241, 10.3389/fncel.2015.00376.
- P G Mermelstein; J B Becker; D J Surmeier; Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor.. The Journal of Neuroscience 1996, 16, , null.
- Charles E. Roselli; Linda E. Horton; John A. Resko; Distribution and Regulation of Aromatase Activity in the Rat Hypothalamus and Limbic System*. Endocrinology 1985, 117, 2471-2477, 10.1210/endo-117-6-2471.
- Jennifer M. Lymer; Paul A.S. Sheppard; Talya Kuun; Andrea Blackman; Nilay Jani; Sahnon Mahbub; Elena Choleris; Estrogens and their receptors in the medial amygdala rapidly facilitate social recognition in female mice. Psychoneuroendocrinology 2018, 89, 30-38, 10.1016/j.psyneuen.2017.12.021.
- Junyi Li; Robert B. Gibbs; Detection of estradiol in rat brain tissues: Contribution of local versus systemic production. Psychoneuroendocrinology 2019, 102, 84-94, 10.1016/j.psyneuen.2018.11.037.
- S. Wirth; Single Neurons in the Monkey Hippocampus and Learning of New Associations. Science 2003, 300, 1578-1581, 10.1126/science.1084324.
- T. Liu; D. Xu; J. Ashe; K. Bushara; Specificity of inferior olive response to stimulus timing.. Journal of Neurophysiology 2008, 100, 1557-61, 10.1152/jn.00961.2007.
- Stacey L. Reeber; Joshua J. White; Nicholas A. George-Jones; Roy V. Sillitoe; Architecture and development of olivocerebellar circuit topography. Frontiers in Neural Circuits 2013, 6, , 10.3389/fncir.2012.00115.
- Nino Tabatadze; Satoru M. Sato; Catherine S. Woolley; Quantitative Analysis of Long-Form Aromatase mRNA in the Male and Female Rat Brain. PLOS ONE 2014, 9, e100628, 10.1371/journal.pone.0100628.
- Correction: Seredynski et al., Neuroestrogens Rapidly Regulate Sexual Motivation But Not Performance. The Journal of Neuroscience 2013, 33, 4623-4623, 10.1523/JNEUROSCI.0316-13.2013.
- Geert De Groof; Jacques Balthazart; C.A. Cornil; Annemie Van Der Linden; Topography and Lateralized Effect of Acute Aromatase Inhibition on Auditory Processing in a Seasonal Songbird. The Journal of Neuroscience 2017, 37, 4243-4254, 10.1523/JNEUROSCI.1961-16.2017.
- C.A. Cornil; Mélanie Taziaux; Michelle Baillien; Gregory F. Ball; Jacques Balthazart; Rapid effects of aromatase inhibition on male reproductive behaviors in Japanese quail.. Hormones and Behavior 2005, 49, 45-67, 10.1016/j.yhbeh.2005.05.003.
- Amanda Sierra; I. Azcoitia; Luis M. García-Segura; Endogenous estrogen formation is neuroprotective in model of cerebellar ataxia.. Endocrine 2003, 21, 43-52, 10.1385/endo:21:1:43.
- Quanguang Zhang; Ruimin Wang; Hui Tang; Yan Dong; Alice Chan; Gangadhara Reddy Sareddy; Ratna K. Vadlamudi; Darrell W. Brann; Brain-derived estrogen exerts anti-inflammatory and neuroprotective actions in the rat hippocampus.. Molecular and Cellular Endocrinology 2014, 389, 84-91, 10.1016/j.mce.2013.12.019.
- Victoria Luine; Estradiol: Mediator of memories, spine density and cognitive resilience to stress in female rodents.. The Journal of Steroid Biochemistry and Molecular Biology 2015, 160, 189-95, 10.1016/j.jsbmb.2015.07.022.
- PhD Marcia Ratner; Vidhya Kumaresan; David Farb; Neurosteroid Actions in Memory and Neurologic/Neuropsychiatric Disorders.. Frontiers in Endocrinology 2019, 10, 169, 10.3389/fendo.2019.00169.
- Cristina V. Dieni; Jacqueline Sullivan; Mario Faralli; Samuele Contemori; Andrea Biscarini; Vito E. Pettorossi; Roberto Panichi; 17 beta-estradiol synthesis modulates cerebellar dependent motor memory formation in adult male rats. Neurobiology of Learning and Memory 2018, 155, 276-286, 10.1016/j.nlm.2018.08.011.
- Bronwyn Graham; Mohammed R. Milad; Inhibition of estradiol synthesis impairs fear extinction in male rats. Learning & Memory 2014, 21, 347-350, 10.1101/lm.034926.114.
- Luke Remage-Healey; Stephanie Dong; Nigel T. Maidment; Barney A. Schlinger; Presynaptic control of rapid estrogen fluctuations in the songbird auditory forebrain.. The Journal of Neuroscience 2011, 31, 10034-8, 10.1523/JNEUROSCI.0566-11.2011.
- Elizabeth B Engler-Chiurazzi; Candice M Brown; J.M. Povroznik; James W. Simpkins; Estrogens as neuroprotectants: Estrogenic actions in the context of cognitive aging and brain injury.. Progress in Neurobiology 2016, 157, 188-211, 10.1016/j.pneurobio.2015.12.008.
- Yuko Hara; Elizabeth M. Waters; Bruce S. McEwen; John H. Morrison; Estrogen Effects on Cognitive and Synaptic Health Over the Lifecourse.. Physiological Reviews 2015, 95, 785-807, 10.1152/physrev.00036.2014.
- Anat Biegon; Sung Won Kim; David L. Alexoff; Millard Jayne; Pauline Carter; Barbara Hubbard; Payton King; Jean Logan; Lisa Muench; Deborah Pareto; David Schlyer; Colleen Shea; Frank Telang; Gene-Jack Wang; Youwen Xu; Joanna S. Fowler; Unique distribution of aromatase in the human brain: In vivo studies with PET and [N-methyl-11C]vorozole. Synapse 2010, 64, 801-807, 10.1002/syn.20791.
- I. Azcoitia; J.G. Yague; Luis M. García-Segura; Estradiol synthesis within the human brain. Neuroscience 2011, 191, 139-147, 10.1016/j.neuroscience.2011.02.012.
- Esteban Lavaque; Aurora Mayen; I. Azcoitia; Manuel Tena-Sempere; Luis M. García-Segura; Sex differences, developmental changes, response to injury and cAMP regulation of the mRNA levels of steroidogenic acute regulatory protein, cytochrome p450scc, and aromatase in the olivocerebellar system. Journal of Neurobiology 2006, 66, 308-318, 10.1002/neu.20221.
- Y Takahashi; Y Fukabori; Y Kaburagi; H Yamanaka; A Kanbegawa; S Honma; [The regulation system of brain aromatase activity; effects of androgens on hypothalamic aromatase in the male rat].. Folia Endocrinologica Japonica 1986, 62, 192–200.
- Arisa Munetomo; Yasushi Hojo; Shimpei Higo; Asami Kato; Kotaro Yoshida; Takuji Shirasawa; Takahiko Shimizu; Anna Barron; Tetsuya Kimoto; Suguru Kawato; Aging-induced changes in sex-steroidogenic enzymes and sex-steroid receptors in the cortex, hypothalamus and cerebellum. The Journal of Physiological Sciences 2015, 65, 253-263, 10.1007/s12576-015-0363-x.
- Hirotaka Sakamoto; Yukio Mezaki; Hanako Shikimi; Kazuyoshi Ukena; Kazuyoshi Tsutsui; Dendritic Growth and Spine Formation in Response to Estrogen in the Developing Purkinje Cell. Endocrinology 2003, 144, 4466-4477, 10.1210/en.2003-0307.
- Kazuyoshi Tsutsui; Biosynthesis, mode of action and functional significance of neurosteroids in the developing Purkinje cell. The Journal of Steroid Biochemistry and Molecular Biology 2006, 102, 187-194, 10.1016/j.jsbmb.2006.09.015.
- Kazuyoshi Tsutsui; Neurosteroid Biosynthesis and Action During Cerebellar Development. The Cerebellum 2012, 11, 414-415, 10.1007/s12311-011-0341-7.
- Valerie L. Hedges; Timothy J. Ebner; Robert L. Meisel; Paul G. Mermelstein; The cerebellum as a target for estrogen action.. Frontiers in Neuroendocrinology 2012, 33, 403-11, 10.1016/j.yfrne.2012.08.005.
- Yayoi Ikeda; Akiko Nagai; Differential expression of the estrogen receptors alpha and beta during postnatal development of the rat cerebellum. Brain Research 2006, 1083, 39-49, 10.1016/j.brainres.2006.02.025.
- Sudha Warrier Mitra; Elena Hoskin; Joel Yudkovitz; Lisset Pear; Hilary A Wilkinson; Shinji Hayashi; Donald W. Pfaff; Sonoko Ogawa; Susan P. Rohrer; James M. Schaeffer; Bruce S. McEwen; Stephen E. Alves; Immunolocalization of Estrogen Receptor ? in the Mouse Brain: Comparison with Estrogen Receptor ?. Endocrinology 2003, 144, 2055-2067, 10.1210/en.2002-221069.
- Richard H Price Jr.; Robert J Handa; Expression of estrogen receptor-beta protein and mRNA in the cerebellum of the rat. Neuroscience Letters 2000, 288, 115-118, 10.1016/s0304-3940(00)01221-0.
- Grassi, S.; Scarduzio, M.; Panichi, R.; Dall’Aglio, C.; Boiti, C.; Pettorossi, V.E. Opposite long-term synaptic effects of 17beta-estradiol and 5alpha-dihydrotestosterone and localization of their receptors in the medial vestibular nucleus of rats. Brain Res. Bull. 2013, 97, 1–7.
- Grassi, S.; Frondaroli, A.; Scarduzio, M.; Dutia, M.B.; Dieni, C.; Pettorossi, V.E. Effects of 17beta-estradiol on glutamate synaptic transmission and neuronal excitability in the rat medial vestibular nuclei. Neuroscience 2010, 165, 1100–1114.
- Horvath; Wikler; Aromatase in Developing Sensory Sytems of the Rat Brain. Journal of Neuroendocrinology 2001, 11, 77-84, 10.1046/j.1365-2826.1999.00285.x.
- Grassi, S.; Frondaroli, A.; Scarduzio, M.; Dutia, M.B.; Dieni, C.; Pettorossi, V.E. Effects of 17beta-estradiol on glutamate synaptic transmission and neuronal excitability in the rat medial vestibular nuclei. Neuroscience 2010, 165, 1100–1114.
- Horvath; Wikler; Aromatase in Developing Sensory Sytems of the Rat Brain. Journal of Neuroendocrinology 2001, 11, 77-84, 10.1046/j.1365-2826.1999.00285.x.
- Liisa A. Tremere; Jin Kwon Jeong; Raphael Pinaud; Estradiol Shapes Auditory Processing in the Adult Brain by Regulating Inhibitory Transmission and Plasticity-Associated Gene Expression. The Journal of Neuroscience 2009, 29, 5949-5963, 10.1523/JNEUROSCI.0774-09.2009.
- Vito Enrico Pettorossi; Michela Di Mauro; Mariangela Scarduzio; Roberto Panichi; Alessandro Tozzi; Paolo Calabresi; Silvarosa Grassi; Modulatory role of androgenic and estrogenic neurosteroids in determining the direction of synaptic plasticity in the CA1 hippocampal region of male rats. Physiological Reports 2013, 1, e00185, 10.1002/phy2.185.
- Tozzi, A.; de Iure, A.; Tantucci, M.; Durante, V.; Quiroga-Varela, A.; Giampa, C.; Di Mauro, M.; Mazzocchetti, P.; Costa, C.; Di Filippo, M.; et al. Endogenous 17beta-estradiol is required for activity-dependent long-term potentiation in the striatum: Interaction with the dopaminergic system. Front. Cell. Neurosci. 2015, 9, 192. [Google Scholar] [CrossRef]
- Michela Di Mauro; Alessandro Tozzi; Paolo Calabresi; Vito Enrico Pettorossi; Silvarosa Grassi; Neo-synthesis of estrogenic or androgenic neurosteroids determine whether long-term potentiation or depression is induced in hippocampus of male rat. Frontiers in Cellular Neuroscience 2015, 9, 241, 10.3389/fncel.2015.00376.
- P G Mermelstein; J B Becker; D J Surmeier; Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor.. The Journal of Neuroscience 1996, 16, , null.




