CAPE-incorporated nanoparticles of HAsPBPE were fabricated by the nanoprecipitation method and then the organic solvent was removed by dialysis. CAPE-incorporated HAsPBPE nanoparticles have a small particle size of about 80 or 100 nm and they have a spherical shape. When CAPE-incorporated HAsPBPE nanoparticles were irradiated, nanoparticles became swelled or disintegrated and their morphologies were changed. Furthermore, the CAPE release rate from HAsPBPE nanoparticles were increased according to the radiation dose, indicating that CAPE-incorporated HAsPBPE nanoparticles have radio-sensitivity. CAPE and CAPE-incorporated HAsPBPE nanoparticles appropriately prevented radiation-induced cell death and suppressed intracellular accumulation of reactive oxygen species (ROS). CAPE and CAPE-incorporated HAsPBPE nanoparticles efficiently improved survivability of mice from radiation-induced death and reduced apoptotic cell death.
1. Introduction
Ionizing radiation has been extensively used for therapeutic and diagnostic purposes of human disease
[1][2][3][4][5]. Radiation therapy is regarded as one of the primary treatment options for cancer patients since it is a promising candidate for inoperable cancer and induces apoptosis/necrosis of cancer cells
[1][2][3]. Furthermore, ionizing radiation has been extensively used for medical imaging of human disease since it is still considered as an ideal tool for detection of disease and malignancies
[4][5]. Even though ionizing radiation is widely utilized for diagnosis/therapy of human diseases, repeated or high dose radiation is closely associated with undesirable effects against healthy cells and tissues since ionizing radiation induces oxidative stress in the surrounding field of irradiation
[6][7][8]. Irradiation causes DNA damage and metabolic stress in healthy cells and tissues since it elevates the ROS level not only in disease cells/tissues but also in healthy cells/tissues
[7][8]. To overcome these drawbacks, many scientists have investigated the strategy for radioprotection of healthy cells/tissues from irradiation
[7][8][9][10]. For example, Liu et al. reported that whey hydrolysate peptides have effectiveness in radioprotection of mice from
60Coγ radiation damage and then prolonged survival time of mice
[7]. Otherwise, amifostine is widely used in clinics to attenuate radiation-induced damage
[11][12]. However, the side effects of amifostine are also problematic such as severe skin eruption, hypotension, fever/rash, immune hypersensitivity syndrome, and fatigue, etc.
[13][14][15][16]. For these reasons, antioxidants efficiently attenuate tissue injury from radiation-induced damage
[9][10]. Qin et al. reported that resveratrol inhibits oxidative stress from radiation and then reduces intestinal injury
[9]. Epigallocatechin gallate (EGCG) is known to decrease ROS levels, suppress radiation-induced intestinal injury, and then prolong the survival time of mice
[10].
Caffeic acid phenethyl ester (CAPE), which is a propolis of honeybee hives, has various biological activities such as antioxidants, anti-allergenic, anticarcinogenic, anti-inflammatory, and immunomodulation
[17][18][19][20][21][22]. CAPE has an inhibitory effect against nuclear oxygenation of linoleic acid and arachidonic acid and an effectiveness in the decrease of chemotherapy or radiation therapy-induced damages
[17]. Furthermore, CAPE was considered as a strong antioxidant in the biological system
[23][24][25]. Nasution et al. reported the inhibitory effect against oxidative stress in brain injury
[23]. CAPE also has a protective effect against nephrotoxicity and oxidative kidney damage
[24]. CAPE showed an anti-oxidative effect against the experimental periodontitis model, i.e., it decreases the total oxidant status in gingival tissues and then improves therapeutic potential
[25]. Especially, the pretreatment of CAPE prior to irradiation of rats effectively protects radiation-induced hepatic or brain injury
[26][27]. Chu et al. reported that CAPE administration decreased the serum levels of alanine aminotransferase and aspartate aminotransferase, while the activity of superoxide dismutase and glutathione was increased
[26]. Then, CAPE effectively protected from radiation-induced hepatotoxicity and inhibited hepatocyte apoptosis. The pretreatment of CAPE also protects brain damage from ionizing radiation through the control of total oxidant status and oxidative stress index
[27].
Nano-dimensional vehicles such as nanoparticles, polymeric micelles, liposomes or colloidal carriers have been extensively investigated in the diverse field of biomedical science
[28][29][30][31][32]. Especially, nanoparticles have been frequently considered for stimuli-sensitive drug delivery of bioactive agents, i.e., they can be modified to respond against the stimulus in the biological system such as acidic pH, temperature, magnetic field, oxidative stress, light, and irradiation
[32][33][34][35][36][37]. For example, Choi et al. reported that iron oxide-incorporated lipocomplexes respond to the magnetic field, concentrate around the tumor mass, and then specifically target cancer
[32]. Polymer conjugates having diselenide linkage can be used to target cancer cells and deliver the cancer drug through reactive oxygen species (ROS)-sensitive cleavage of selenide linkage since the tumor microenvironment normally revealed increased redox potential and elevated ROS level
[35]. Choi et al. reported that methoxy poly(ethylene glycol)-b-poly(DL-lactide-co-glycolide) block copolymer nanoparticles having diselenide linkages responded to irradiation and then specifically released ebselen, i.e., they showed that the ebselen release rate was accelerated according to the dose of irradiation
[37]. Furthermore, the antibody decorated lipid nanoparticles incorporating CAPE are preferentially target disease cells with low intrinsic cytotoxicity against normal cells
[38]. Nanoparticles are reasonable candidates for the delivery of bioactive agents to improve biological activity and to reduce genotoxicity
[38][39][40].
2. Current Insights on Radioprotection
Even though ionizing irradiation has been extensively used for diagnosis and therapeutic purposes, risks from radiation damage are always problematic in the human body. Especially, it is known that the ROS level is significantly increased in the field of irradiation and then these induce oxidative stress against cells and tissues
[6][41]. The increased ROS and/or reactive nitrogen species (RNS) level induce metabolic stress and affect the nuclear function of DNA repair mechanism
[41]. Due to these reasons, clinicians use a radioprotectant such as amifostine to prevent radiation-induced cell/tissue damage
[11][12]. However, many scientists have tried to develop novel and non-toxic antioxidants since adverse side effects of amifostine are always problematic in clinical use
[13][14][15][16]. Many kinds of antioxidants have been investigated to prevent radiation-damage and to substitute amifostine
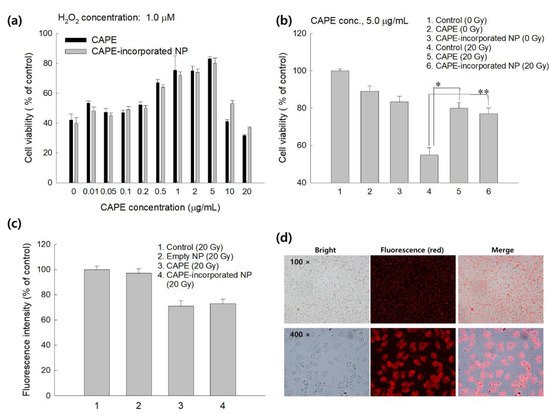
[7][8][9][10][14][15][42][43]. For example, Bai et al. reported that CAPE effectively prevents cellular oxidative stress from radiation by elimination of free radicals and then improves cell viability
[42]. They argued that CAPE properly reduced the intracellular ROS level after radiation of L929 cells. Song et al. also reported that intracellular ROS accumulation induced by H
2O
2 stimulation was effectively inhibited by the pretreatment of CAPE
[44]. They argued that H
2O
2-induced upregulation of TNF-α and COX-2 expression can be suppressed by the treatment of CAPE with a dose/time dependent manner. Moreover, we showed that CAPE-incorporated nanoparticles as well as CAPE effectively suppressed intracellular ROS accumulation induced by the irradiation of cells. These behaviors must be correlated with higher viability of cells as shown in
Figure 1b,c. Furthermore, CAPE and CAPE-incorporated nanoparticles efficiently prevented H
2O
2-induced cell death, i.e., the viability of cells treated with CAPE or CAPE-incorporated nanoparticles was almost two times higher than the control (
Figure 1a). CAPE or CAPE-incorporated nanoparticles against H
2O
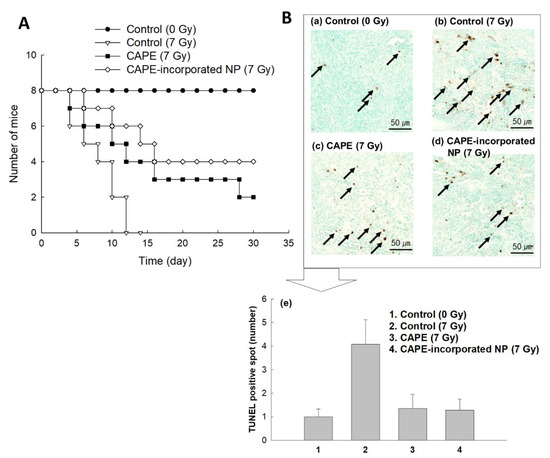
2-induced cell death were dose-dependently protected cells. Furthermore, they also showed positive effects against survivability of mice from irradiation. Especially, CAPE-incorporated nanoparticles showed extended survivability of mice after irradiation at 7 Gy compared to CAPE itself (
Figure 2). These results indicated that CAPE-incorporated nanoparticles have a superior activity against radiation-induced injury. Actually, CAPE-incorporated nanoparticles appropriately reduced apoptotic cell death and improved tissue morphologies as shown in
Figure 5. CAPE and CAPE-incorporated nanoparticles are a great potential for preventing radiation-induced injury. Yoncheva et al. reported that CAPE-incorporated polymeric micelles exhibited a superior protective effect against H
2O
2-induced oxidative stress compared to the free CAPE treatment
[39]. Furthermore, CAPE-loaded poly(DL-lactide-co-glycolide) nanoparticles are more effective in the reduction of mutagenicity than the free CAPE itself
[40]. We also prepared CAPE-incorporated nanoparticles using HAsPBPE conjugates since HA is a biocompatible and water-soluble biopolymer, and TbEA-PBPE moiety acts as a hydrophobic moiety in polymer backbone
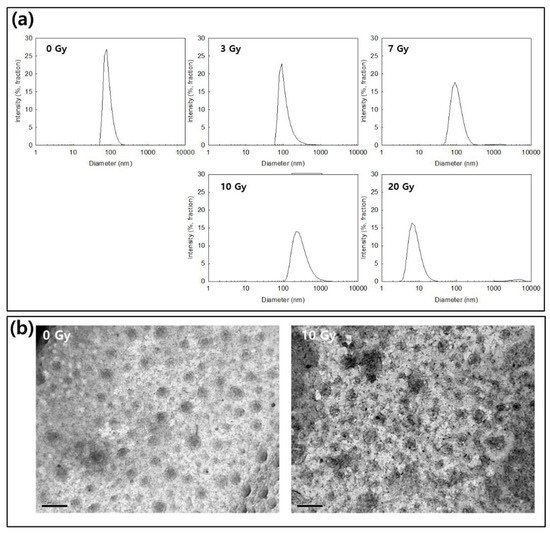
[45]. Then, HAsPBPE conjugates may form core-shell type nanoparticles, i.e., HA acts as an outershell of the nanoparticles while TbEA-PBPE is composed of the hydrophobic inner-core of the nanoparticles. Nanoparticles showed spherical morphologies with small particle sizes less than 100 nm in the average diameter as shown in
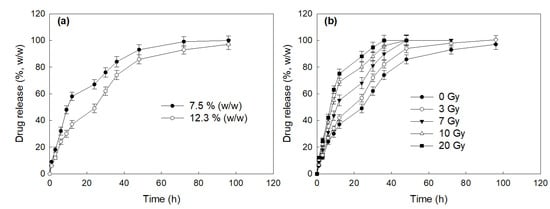
Figure 3. In the drug release experiment, nanoparticles showed a burst release behavior until 12 h and then was released continuously until 96 h, indicating that the CAPE existing near the surface of the nanoparticles was released rapidly and, after that, the CAPE in the core of the nanoparticles was released more slowly. In our previous reports, the burst release of CAPE from nanoparticles was also observed in the initial phase of drug release study followed by a sustained release pattern
[46].
Figure 1. (a) The effect of CAPE or CAPE-incorporated HAsPBPE nanoparticles on the viability of L929 cells in the presence of H2O2. (b) The effect of irradiation on the viability of L929 cells. (c) Intracellular ROS level pretreated with CAPE or CAPE-incorporated HAsPBPE nanoparticles and irradiation. Cell were exposed to 20 Gy radiation. (d) Fluorescence observation of cellular uptake of Ce6-incorporated HAsPBPE nanoparticles. Ce6-incorporated HAsPBPE nanoparticles (final concentration of Ce6: 2 μg/mL. Cells were treated with nanoparticles for 2 h). *, **: p < 0.01.
Figure 2. (
A) The effect of CAPE or CAPE-incorporated HAsPBPE nanoparticles on the survivability of mice
. BALb/C mice were i.v. injected CAPE or CAPE-incorporated HAsPBPE nanoparticles 1 h before irradiation. The CAPE dose was 10 mg/kg. (
B) TUNEL staining of spleen sections. TUNEL staining of spleen sections demonstrated the protective effect of CAPE or CAPE-incorporated HAsPBPE nanoparticles after irradiation. (
a) Control group (0 Gy); (
b) control (7 Gy): The spleen histology of the irradiation group showed decreased TUNEL-positive cells (black arrow); (
c) CAPE (7 Gy); (
d) CAPE-incorporated HAsPBPE nanoparticles group (7 Gy). (
e) Changes of TUNEL-positive staining of apoptotic cells (dark spot). Average ± SD from ten images. Apoptotic spot was calculated from ten captured areas as shown in
Figures S1 and S2. Statistical analysis: 1:3, 1:4,
p < 0.15; 2:3, 2:4,
p < 0.1.
Figure 3. (a) The effect of irradiation on the changes of the particle size of CAPE-incorportaed HAsPBPE nanoparticles. (b) The effect of irradiation on the morphological changes of CAPE-incorportaed HAsPBPE nanoparticles (bar = 200 nm).
Nanoparticulate-based delivery systems for bioactive agents are suitable for eliminating disease cells such as cancer stem cells and ROS
[47][48]. For example, polymeric nanoparticles decorated with specific ligands or antibodies have the potential to target and reject cancer stem cells
[47]. Furthermore, nanocarriers incorporating the dual or multi drug delivery strategy have a great potential to diminish ROS production in disease cells and then reduce oxidative damage in mitochondria
[48]. Then, these dual/multi drug deliveries using nanocarriers may provide a synergistic effect in the improvement of therapeutic index of neuroregenerative strategy
[48]. Our concept for radioprotection is to develop radio-sensitive nanomedicine for the sustained and/or radio-responsive release of CAPE. Since CAPE itself is a lipophilic agent and is hardly dissolved in an aqueous solution, nanoparticles can solve these problems, i.e., nanoparticles enable us to dissolve CAPE in an aqueous solution
[28]. Furthermore, nanoparticles can be modified to be sensitive to physicochemical stimulus such as radiation, pH, ROS, magnetic field, and light
[32][33][34][35][36][37]. HAsPBPE nanoparticles can respond to ROS since the PBPE moiety and thiol group in TbEA in the polymer backbone are known to have ROS-sensitivity and these moieties are able to be decomposed in the oxidative stress
[45].
Actually, CAPE-incorporated nanoparticles of HAsPBPE were disintegrated in the presence of H2O2 as shown in Figure 3a,b. HAsPBPE nanoparticles showed an accelerated CAPE release through irradiation (Figure 4). Then, HAsPBPE nanoparticles specifically decomposed and released CAPE when they were exposed to irradiation, i.e., drug release can be suppressed in the absence of irradiation and then irradiation of nanoparticles accelerated drug release. Even though nanoparticles in the in vitro cell culture showed a similar protective activity compared to CAPE, they were improved by the biological activity in the in vivo animal irradiation study, as shown in Figure 2 and Figure 5.
Figure 4. (a) The effect of drug contents on the drug release from HAsPBPE nanoparticles. (b) The effect of irradiation on the changes of the particle size of CAPE-incorportaed HAsPBPE nanoparticles.
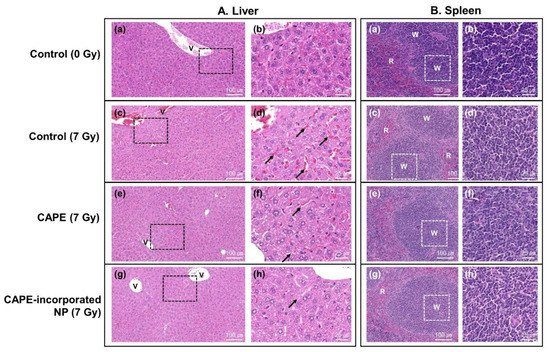
Figure 5. (A) H&E staining of mouse liver. Photomicrographs of liver sections stained with H&E demonstrated the protective effective of CAPE or CAPE-incorporated HAsPBPE nanoparticles when the whole body of the mouse was exposed to irradiation. (a,b) Control group (0 Gy); (c,d) control (7 Gy): The liver histology of the irradiation group showed a hepatocyte edema and sinusoidal dilation (d, black arrow); (e,f) CAPE (7 Gy); (g,h) CAPE-incorporated HAsPBPE nanoparticles group (7 Gy). (B) H&E staining of mouse spleen. Photomicrographs of spleen sections stained with H&E demonstrated the protective effective of CAPE with empty HAsPBPE nanoparticles or CAPE-incorporated HAsPBPE nanoparticles when the whole body of the mouse was exposed to irradiation. (a,b) Control group; (c,d) irradiation-only group; the spleen histology of the irradiation group showed a decrease in the lymphocytes in the white pulp; (e,f) CAPE + empty nanoparticles group; (g,h) CAPE-incorporated HAsPBPE nanoparticles group. Arrows, dilated sinusoid, exudate red blood cells (RBC); v, hepatic vein; W, white pulp; R, red pulp.