
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 2 | Rita Xu | + 2704 word(s) | 2704 | 2021-08-05 10:08:57 | | | |
| 3 | Conner Chen | Meta information modification | 2704 | 2021-10-11 09:55:50 | | |
Video Upload Options
Urinary tract infections (UTIs) affect any part of the urinary tract and may spread through the urinary tract towards the urethra, bladder and even the kidneys and they are associated with an increase in morbidity and mortality. UTIs may be resolved spontaneously or treated with antibiotics. However, regular use of antibiotics is related to nephrotoxicity and produces changes in the intestinal flora, alter immunity and metabolism, and finally, gut bacteria become a reservoir of genes for resistance to antibiotics. Regarding this concern, a new group of molecules called drug conjugates have been proposed as an alternative or a complement to de use of just antibiotics such as a new adjuvant in UTIs therapy called Itxasol©.
1. Introduction
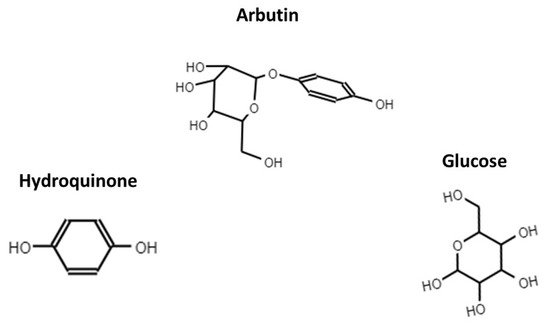
2. β-arbutin
| Pathogen | MIC (μg/mL) | Gram | Reference |
|---|---|---|---|
| Escherichia coli | 256 | Negative | [57] |
| Pseudomonas aeruginosa | 7.8 | Negative | [58] |
| Staphylococcus aureus | 15.6 | Positive | [58] |
| Staphylococcus aureus | 103 | Positive | [59] |
| Salmonella typhimurium | 512 | Negative | [57] |
| Bacillus cereus | 512 | Positive | [57] |
| Mycobacterium tuberculosis | 12.5 | Positive | [60] |
| Action | Main Findings/Use | Reference |
|---|---|---|
| Antibacterial action | Inhibits Pseudomonas aeruginosa growth at 128 mg/mL | [56] |
| Demonstration of antibacterial activity | [55] | |
| Arbutin destroys bacteria through wall cellular disruption (Gram + and Gram −) | [53] | |
| Reduction in bacterial load in prevention of UTI recurrence | [61] | |
| Clinical trial to reduce the use of antibiotics administering b-arbutin (results not yet published) | [62] | |
| Anti-inflammatory | Attenuated damage induced by lipopolysaccharide in rat | [67] |
| Anti-diabetic | Ameliorates hyperglycemia, hyperlipidemia and histopathological changes in pancreas, kidney and liver in a diabetes rat model | [68] |
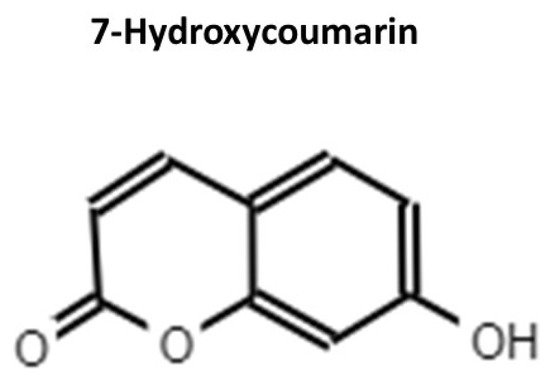
3. Umbelliferon (UMB)
| Action | Main Findings/Use | References |
|---|---|---|
| Antifungicidal/antibiotic | Antifungicidal activity | [74][82][83] |
| Decreases virulence and biofilm formation of E.coli O157:H7 | [84] | |
| Impedes biofilm formation of methicillin-resistant Staphylococcus epidermidis | [77] | |
| Destroys periodontal bacteria and inhibits biofilm formation | [76] | |
| Antibiofilm properties (Staphylococcus epidermidis) | [77] | |
| Antitumoral | Inhibits cell growth in lung carcinoma cell lines | [73] |
| Induces cell cycle arrest in G0/G1 in human cell carcinoma | [75] | |
| Anti-inflammatory | Reduction in inflammation in a model of brain damage in rats | [17] |
| Antihyperglycemic | Anti-diabetic effect in a diabetic mouse model induced with streptozotocin | [89] |
| Nephron protection | Reduction in the nephrotoxicity associated to cisplatin use | [85] |
| UMB attenuates renal toxicity induced by gentamicin | [1][86] | |
| Enhances renal function in diabetic mouse model | [87] | |
| Antifibrotic | Ameliorates the liver fibrosis signs induced by carbon tetrachloride (CCl4) in rats | [88] |
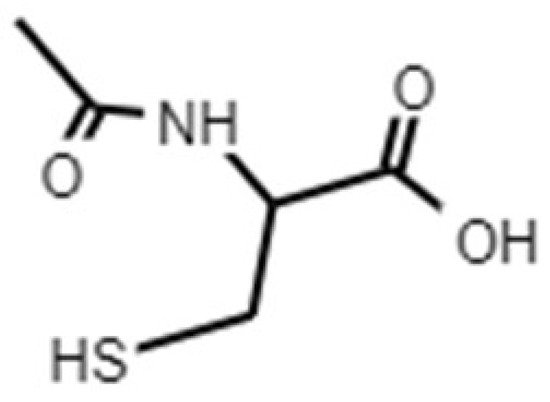
4. N-Acetyl L-Cysteine (NAC)
| Organism | NAC Concentration | Reference |
|---|---|---|
| C. albicans C. parapsilosis C. guilliermondii C. tropicalis C. glabrata |
10–50 mg/mL | [96] |
| Pseudomonas aeruginosa | 0.15–0.23 mg/mL | [98] |
| P. aeruginosa | 3–10 mg/mL | [99] |
| P. aeruginosa | 32 mg/mL | [100] |
| Candida albicans | 25 mg/mL | [101] |
| Staphylococcus aureus | 0.5 mg/mL | [102] |
| Stenotrophomonas maltophilia | 16–32 mg/mL | [103] |
| Acinetobacter baumannii | 16–128 mg/mL | [104] |
| Candida tropicalis | 1000 mg/mL | [105] |
| Actinomyces naeslundii, Lactobacillus salivarius, Streptococcus mutans, Enterococcus faecalis | 25–100 mg/mL | [106] |
| Pseudomonas aeruginosa | 10 mg/mL | [107] |
| Staphylococcus pseudintermedius Pseudomonas aeruginosa Corynebacterium spp. and β-hemolytic Streptococcus spp. | 0.115–80 mg/mL | [108] |
| Actinomyces naeslundii, Lactobacillus salivarius, Streptococcus mutans, Enterococcus faecalis | 0.78–1.56 mg/mL | [109] |
References
- Garud, M.S.; Kulkarni, Y.A. Attenuation of renal damage in type I diabetic rats by umbelliferone—A coumarin derivative. Pharmacol. Rep. 2017, 69, 1263–1269.
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284.
- Gandaglia, G.; Varda, B.; Sood, A.; Pucheril, D.; Konijeti, R.; Sammon, J.D.; Sukumar, S.; Menon, M.; Sun, M.; Chang, S.L.; et al. Short-term perioperative outcomes of patients treated with radical cystectomy for bladder cancer included in the National Surgical Quality Improvement Program (NSQIP) database. J. Can. Urol. Assoc. 2014, 8, E681–E687.
- Foxman, B. The epidemiology of urinary tract infection. Nat. Rev. Urol. 2010, 7, 653–660.
- Czajkowski, K.; Broś-Konopielko, M.; Teliga-Czajkowska, J. Urinary tract infection in women. Prz. Menopauzalny 2021, 20, 40–47.
- Geerlings, S.E. Clinical Presentations and Epidemiology of Urinary Tract Infections. Microbiol. Spectr. 2016, 4, 4–5.
- Albert, X.; Huertas, I.; Pereiro, I.; Sanfélix, J.; Gosalbes, V.; Perrotta, C. Antibiotics for preventing recurrent urinary tract infection in non-pregnant women. Cochrane Database Syst. Rev. 2004, 2004, CD001209.
- Scholes, D. Risk factors for recurrent urinary tract infection in young women. J. Infect. Dis. 2000, 182, 1177–1182.
- Mathiyalagen, P.; Peramasamy, B.; Vasudevan, K.; Basu, M.; Cherian, J.; Sundar, B. A descriptive cross-sectional study on menstrual hygiene and perceived reproductive morbidity among adolescent girls in a union territory, India. J. Fam. Med. Prim. Care 2017, 6, 360.
- Lindh, I.; Othman, J.; Hansson, M.; Ekelund, A.C.; Svanberg, T.; Strandell, A. New types of diaphragms and cervical caps versus older types of diaphragms and different gels for contraception: A systematic review. BMJ Sex. Reprod. Health 2020, 47, e12.
- Behzadi, P.; Behzadi, E.; Pawlak-Adamska, E.A. Urinary tract infections (UTIs) or genital tract infections (GTIs)? It’s the diagnostics that count. GMS Hyg. Infect. Control 2019, 14, Doc14.
- Jung, C.; Brubaker, L. The etiology and management of recurrent urinary tract infections in postmenopausal women. Climacteric 2019, 22, 242–249.
- Hooton, T.M.; Scholes, D.; Hughes, J.P.; Winter, C.; Roberts, P.L.; Stapleton, A.E.; Stergachis, A.; Stamm, W.E. A Prospective Study of Risk Factors for Symptomatic Urinary Tract Infection in Young Women. N. Engl. J. Med. 1996, 335, 468–474.
- Etienne, M.; Galperine, T.; Caron, F. Urinary Tract Infections in Older Men. N. Engl. J. Med. 2016, 374, 2191–2192.
- Lenis, A.T.; Lec, P.M.; Chamie, K. Bladder cancer a review. JAMA J. Am. Med. Assoc. 2020, 324, 1980–1991.
- Foxman, B. Urinary tract infection syndromes. Occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect. Dis. Clin. N. Am. 2014, 28, 1–13.
- Germoush, M.O.; Othman, S.I.; Al-Qaraawi, M.A.; Al-Harbi, H.M.; Hussein, O.E.; Al-Basher, G.; Alotaibi, M.F.; Elgebaly, H.A.; Sandhu, M.A.; Allam, A.A.; et al. Umbelliferone prevents oxidative stress, inflammation and hematological alterations, and modulates glutamate-nitric oxide-cGMP signaling in hyperammonemic rats. Biomed. Pharmacother. 2018, 102, 392–402.
- Nicolle, L.E.; Harding, G.K.M.; Preiksaitis, J.; Ronald, A.R. The association of urinary tract infection with sexual intercourse. J. Infect. Dis. 1982, 146, 579–583.
- Detweiler, K.; Mayers, D.; Fletcher, S.G. Bacteruria and Urinary Tract Infections in the Elderly. Urol. Clin. N. Am. 2015, 42, 561–568.
- Anger, J.T.; Saigal, C.S.; Wang, M.M.; Yano, E.M. Urologic Disease Burden in the United States: Veteran Users of Department of Veterans Affairs Healthcare. Urology 2008, 72, 37–41.
- Linsenmeyer, T.A. Catheter-associated urinary tract infections in persons with neurogenic bladders. J. Spinal Cord Med. 2018, 41, 132–141.
- Ghoreifi, A.; Van Horn, C.M.; Xu, W.; Cai, J.; Miranda, G.; Bhanvadia, S.; Schuckman, A.K.; Daneshmand, S.; Djaladat, H. Urinary tract infections following radical cystectomy with enhanced recovery protocol: A prospective study. Urol. Oncol. Semin. Orig. Investig. 2020, 38, 75.e9–75.e14.
- Clark, P.E.; Agarwal, N.; Biagioli, M.C.; Eisenberger, M.A.; Greenberg, R.E.; Herr, H.W.; Inman, B.A.; Kuban, D.A.; Kuzel, T.M.; Lele, S.M.; et al. Bladder cancer: Clinical practice guidelines in oncology. JNCCN J. Natl. Compr. Cancer Netw. 2013, 11, 446–475.
- Shigemura, K.; Tanaka, K.; Matsumoto, M.; Nakano, Y.; Shirakawa, T.; Miyata, M.; Yamashita, M.; Arakawa, S.; Fujisawa, M. Post-operative infection and prophylactic antibiotic administration after radical cystectomy with orthotopic neobladderurinary diversion. J. Infect. Chemother. 2012, 18, 479–484.
- Griebling, T.L. Urologic diseases in America project: Trends in resource use for urinary tract infections in women. J. Urol. 2005, 173, 1281–1287.
- Ventola, C.L. The antibiotic resistance crisis: Causes and threats. Pharm. Ther. J. 2015, 40, 277–283.
- Kakoullis, L.; Papachristodoulou, E.; Chra, P.; Panos, G. Mechanisms of antibiotic resistance in important gram-positive and gram-negative pathogens and novel antibiotic solutions. Antibiotics 2021, 10, 415.
- Morales-Alvarez, M.C. Nephrotoxicity of Antimicrobials and Antibiotics. Adv. Chronic Kidney Dis. 2020, 27, 31–37.
- Barber, A.E.; Norton, J.P.; Spivak, A.M.; Mulvey, M.A. Urinary tract infections: Current and emerging management strategies. Clin. Infect. Dis. 2013, 57, 719–724.
- Waller, T.A.; Pantin, S.A.L.; Yenior, A.L.; Pujalte, G.G.A. Urinary Tract Infection Antibiotic Resistance in the United States. Prim. Care Clin. Off. Pract. 2018, 45, 455–466.
- Foxman, B.; Gillespie, B.; Koopman, J.; Zhang, L.; Palin, K.; Tallman, P.; Marsh, J.V.; Spear, S.; Sobel, J.D.; Marty, M.J.; et al. Risk factors for second urinary tract infection among college women. Am. J. Epidemiol. 2000, 151, 1194–1205.
- Salo, J.; Uhari, M.; Helminen, M.; Korppi, M.; Nieminen, T.; Pokka, T.; Kontiokari, T. Cranberry juice for the prevention of recurrences of urinary tract infections in children: A randomized placebo-controlled trial. Clin. Infect. Dis. 2012, 54, 340–346.
- Gupta, K.; Hooton, T.M.; Naber, K.G.; Wullt, B.; Colgan, R.; Miller, L.G.; Moran, G.J.; Nicolle, L.E.; Raz, R.; Schaeffer, A.J.; et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin. Infect. Dis. 2011, 52, e103–e120.
- Pietrucha-Dilanchian, P.; Hooton, T.M. Diagnosis, Treatment, and Prevention of Urinary Tract Infection. Microbiol. Spectr. 2016, 4.
- Blunston, M.A.; Yonovitz, A.; Woodahl, E.L.; Smolensky, M.H. Gentamicin-induced ototoxicity and nephrotoxicity vary with circadian time of treatment and entail separate mechanisms. Chronobiol. Int. 2015, 32, 1223–1232.
- Pitout, J.D.D. Extraintestinal pathogenic Escherichia coli: A combination of virulence with antibiotic resistance. Front. Microbiol. 2012, 3, 9.
- Asadi Karam, M.R.; Habibi, M.; Bouzari, S. Urinary tract infection: Pathogenicity, antibiotic resistance and development of effective vaccines against Uropathogenic Escherichia coli. Mol. Immunol. 2019, 108, 56–67.
- Hopkins, A.M.; Kichenadasse, G.; Karapetis, C.S.; Rowland, A.; Sorich, M.J. Concomitant Antibiotic Use and Survival in Urothelial Carcinoma Treated with Atezolizumab. Eur. Urol. 2020, 78, 540–543.
- Zilberberg, M.D.; Shorr, A.F.; Micek, S.T.; Vazquez-Guillamet, C.; Kollef, M.H. Multi-drug resistance, inappropriate initial antibiotic therapy and mortality in Gram-negative severe sepsis and septic shock: A retrospective cohort study. Crit. Care 2014, 18, 596.
- Teillant, A.; Gandra, S.; Barter, D.; Morgan, D.J.; Laxminarayan, R. Potential burden of antibiotic resistance on surgery and cancer chemotherapy antibiotic prophylaxis in the USA: A literature review and modelling study. Lancet Infect. Dis. 2015, 15, 1429–1437.
- Rabin, N.; Zheng, Y.; Opoku-Temeng, C.; Du, Y.; Bonsu, E.; Sintim, H.O. Biofilm formation mechanisms and targets for developing antibiofilm agents. Future Med. Chem. 2015, 7, 493–512.
- Hall, C.W.; Mah, T.F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301.
- Goel, N.; Fatima, S.W.; Kumar, S.; Sinha, R.; Khare, S.K. Antimicrobial resistance in biofilms: Exploring marine actinobacteria as a potential source of antibiotics and biofilm inhibitors. Biotechnol. Rep. 2021, 30, e00613.
- Atriwal, T.; Azeem, K.; Husain, F.M.; Hussain, A.; Khan, M.N.; Alajmi, M.F.; Abid, M. Mechanistic Understanding of Candida albicans Biofilm Formation and Approaches for Its Inhibition. Front. Microbiol. 2021, 12, 932.
- Liu, L.; Shi, H.; Yu, H.; Yan, S.; Luan, S. The recent advances in surface antibacterial strategies for biomedical catheters. Biomater. Sci. 2020, 8, 4074–4087.
- Cal, P.M.S.D.; Matos, M.J.; Bernardes, G.J.L. Trends in therapeutic drug conjugates for bacterial diseases: A patent review. Expert Opin. Ther. Pat. 2017, 27, 179–189.
- Ibrahim, M.A.; Panda, S.S.; Birs, A.S.; Serrano, J.C.; Gonzalez, C.F.; Alamry, K.A.; Katritzky, A.R. Synthesis and antibacterial evaluation of amino acid-antibiotic conjugates. Bioorg. Med. Chem. Lett. 2014, 24, 1856–1861.
- Lima, L.M.; da Silva, B.N.M.; Barbosa, G.; Barreiro, E.J. β-lactam antibiotics: An overview from a medicinal chemistry perspective. Eur. J. Med. Chem. 2020, 208, 112829.
- Piddock, L.J.V. The crisis of no new antibiotics-what is the way forward? Lancet Infect. Dis. 2012, 12, 249–253.
- Sengupta, S.; Chattopadhyay, M.K.; Grossart, H.P. The multifaceted roles of antibiotics and antibiotic resistance in nature. Front. Microbiol. 2013, 4, 47.
- Vincent, J.F.V.; Bogatyreva, O.A.; Bogatyrev, N.R.; Bowyer, A.; Pahl, A.K. Biomimetics: Its practice and theory. J. R. Soc. Interface 2006, 3, 471–482.
- Sticher, O.; Soldati, F.; Lehmann, D. High-performance liquid chromatographic separation and quantitative determination of arbutin, methylarbutin, hydroquinone and hydroquinone-monomethylether in Arctostaphylos, Bergenia, Calluna and Vaccinium species (author’s transl). Planta Med. 1979, 35, 253–261.
- Ma, C.; He, N.; Zhao, Y.; Xia, D.; Wei, J.; Kang, W. Antimicrobial Mechanism of Hydroquinone. Appl. Biochem. Biotechnol. 2019, 189, 1291–1303.
- Schindler, G.; Patzak, U.; Brinkhaus, B.; Von Nieciecki, A.; Wittig, J.; Krähmer, N.; Glöckl, I.; Veit, M. Urinary excretion and metabolism of arbutin after oral administration of Arctostaphylos uvae ursi extract as film-coated tablets and aqueous solution in healthy humans. J. Clin. Pharmacol. 2002, 42, 920–927.
- Żbikowska, B.; Franiczek, R.; Sowa, A.; Połukord, G.; Krzyzanowska, B.; Sroka, Z. Antimicrobial and Antiradical Activity of Extracts Obtained from Leaves of Five Species of the Genus Bergenia: Identification of Antimicrobial Compounds. Microb. Drug Resist. 2017, 23, 771–780.
- Ng, T.B.; Ling, J.M.L.; Wang, Z.T.; Cai, J.N.; Xu, G.J. Examination of coumarins, flavonoids and polysaccharopeptide for antibacterial activity. Gen. Pharmacol. 1996, 27, 1237–1240.
- Kim, M.H.; Jo, S.H.; Ha, K.S.; Song, J.H.; Jang, H.D.; Kwon, Y.I. Antimicrobial activities of 1,4-benzoquinones and wheat germ extract. J. Microbiol. Biotechnol. 2010, 20, 1204–1209.
- Jeyanthi, V.; Velusamy, P.; Kumar, G.V.; Kiruba, K. Effect of naturally isolated hydroquinone in disturbing the cell membrane integrity of Pseudomonas aeruginosa MTCC 741 and Staphylococcus aureus MTCC 740. Heliyon 2021, 7, e07021.
- Rúa, J.; Fernández-Álvarez, L.; De Castro, C.; Del Valle, P.; De Arriaga, D.; García-Armesto, M.R. Antibacterial activity against foodborne Staphylococcus aureus and antioxidant capacity of various pure phenolic compounds. Foodborne Pathog. Dis. 2011, 8, 149–157.
- Jyoti, A.; Nam, K.-W.; Jang, W.S.; Kim, Y.-H.; Kim, S.-K.; Lee, B.-E.; Song, H.-Y. Antimycobacterial activity of methanolic plant extract of Artemisia capillaris containing ursolic acid and hydroquinone against Mycobacterium tuberculosis. J. Infect. Chemother. 2016, 22, 200–208.
- Genovese, C.; Davinelli, S.; Mangano, K.; Tempera, G.; Nicolosi, D.; Corsello, S.; Vergalito, F.; Tartaglia, E.; Scapagnini, G.; Di Marco, R. Effects of a new combination of plant extracts plus d-mannose for the management of uncomplicated recurrent urinary tract infections. J. Chemother. 2018, 30, 107–114.
- Afshar, K.; Fleischmann, N.; Schmiemann, G.; Bleidorn, J.; Hummers-Pradier, E.; Friede, T.; Wegscheider, K.; Moore, M.; Gágyor, I. Reducing antibiotic use for uncomplicated urinary tract infection in general practice by treatment with uva-ursi (REGATTA)—A double-blind, randomized, controlled comparative effectiveness trial. BMC Complement. Altern. Med. 2018, 18, 203.
- Adesunloye, B.A. Acute renal failure due to the herbal remedy CKLS. Am. J. Med. 2003, 115, 506–507.
- Nowak, A.K.; Shilkin, K.B.; Jeffrey, G.P. Darkroom hepatitis after exposure to hydroquinone. Lancet 1995, 345, 1187.
- Jurica, K.; Benković, V.; Sikirić, S.; Brčić Karačonji, I.; Kopjar, N. The effects of strawberry tree (Arbutus unedo L.) water leaf extract and arbutin upon kidney function and primary DNA damage in renal cells of rats. Nat. Prod. Res. 2020, 34, 2354–2357.
- Jurica, K.; Brčić Karačonji, I.; Mikolić, A.; Milojković-Opsenica, D.; Benković, V.; Kopjar, N. In vitro safety assessment of the strawberry tree (Arbutus unedo L.) water leaf extract and arbutin in human peripheral blood lymphocytes. Cytotechnology 2018, 70, 1261–1278.
- Zhang, B.; Zeng, M.; Li, B.; Kan, Y.; Wang, S.; Cao, B.; Huang, Y.; Zheng, X.; Feng, W. Arbutin attenuates LPS-induced acute kidney injury by inhibiting inflammation and apoptosis via the PI3K/Akt/Nrf2 pathway. Phytomedicine 2021, 82, 153466.
- Madić, V.; Petrović, A.; Jušković, M.; Jugović, D.; Djordjević, L.; Stojanović, G.; Vasiljević, P. Polyherbal mixture ameliorates hyperglycemia, hyperlipidemia and histopathological changes of pancreas, kidney and liver in a rat model of type 1 diabetes. J. Ethnopharmacol. 2021, 265, 113210.
- Hoult, J.R.S.; Payá, M. Pharmacological and biochemical actions of simple coumarins: Natural products with therapeutic potential. Gen. Pharmacol. 1996, 27, 713–722.
- Kumar, V.; Anwar, F.; Verma, A.; Mujeeb, M. Therapeutic effect of umbelliferon-α-D-glucopyranosyl-(2I→1II)-α-D-glucopyranoside on adjuvant-induced arthritic rats. J. Food Sci. Technol. 2015, 52, 3402–3411.
- Kumar, V.; Ahmed, D.; Verma, A.; Anwar, F.; Ali, M.; Mujeeb, M. Umbelliferone β-D-galactopyranoside from Aegle marmelos (L.) corr. An ethnomedicinal plant with antidiabetic, antihyperlipidemic and antioxidative activity. BMC Complement. Altern. Med. 2013, 13, 273.
- Li, H.; Yao, Y.; Li, L. Coumarins as potential antidiabetic agents. J. Pharm. Pharmacol. 2017, 69, 1253–1264.
- Lopez-Gonzalez, J.S.; Prado-Garcia, H.; Aguilar-Cazares, D.; Molina-Guarneros, J.A.; Morales-Fuentes, J.; Mandoki, J.J. Apoptosis and cell cycle disturbances induced by coumarin and 7-hydroxycoumarin on human lung carcinoma cell lines. Lung Cancer 2004, 43, 275–283.
- Navarro-García, V.M.; Rojas, G.; Avilés, M.; Fuentes, M.; Zepeda, G. In vitro antifungal activity of coumarin extracted from Loeselia mexicana Brand. Mycoses 2011, 54, e569–e571.
- Vijayalakshmi, A.; Sindhu, G. Umbelliferone arrest cell cycle at G0/G1 phase and induces apoptosis in human oral carcinoma (KB) cells possibly via oxidative DNA damage. Biomed. Pharmacother. 2017, 92, 661–671.
- Amin, A.; Hanif, M.; Abbas, K.; Ramzan, M.; Rasheed, A.; Zaman, A.; Pieters, L. Studies on effects of umbelliferon derivatives against periodontal bacteria; antibiofilm, inhibition of quorum sensing and molecular docking analysis. Microb. Pathog. 2020, 144, 104184.
- Swetha, T.K.; Pooranachithra, M.; Subramenium, G.A.; Divya, V.; Balamurugan, K.; Pandian, S.K. Umbelliferone Impedes Biofilm Formation and Virulence of Methicillin-Resistant Staphylococcus epidermidis via Impairment of Initial Attachment and Intercellular Adhesion. Front. Cell. Infect. Microbiol. 2019, 9, 357.
- Cruz, L.F.; de Figueiredo, G.F.; Pedro, L.P.; Amorin, Y.M.; Andrade, J.T.; Passos, T.F.; Rodrigues, F.F.; Souza, I.L.A.; Gonçalves, T.P.R.; Lima, L.A.R.D.S.; et al. Umbelliferone (7-hydroxycoumarin): A non-toxic antidiarrheal and antiulcerogenic coumarin. Biomed. Pharmacother. 2020, 129, 110432.
- Monte, J.; Abreu, A.C.; Borges, A.; Simões, L.C.; Simões, M. Antimicrobial Activity of Selected Phytochemicals against Escherichia coli and Staphylococcus aureus and Their Biofilms. Pathogens 2014, 3, 473–498.
- Hu, X.-L.; Xu, Z.; Liu, M.-L.; Feng, L.-S.; Zhang, G.-D. Recent Developments of Coumarin Hybrids as Anti-fungal Agents. Curr. Top. Med. Chem. 2017, 17, 3219–3231.
- Kumar, R.; Saha, A.; Saha, D. A new antifungal coumarin from Clausena excavata. Fitoterapia 2012, 83, 230–233.
- Jia, C.; Zhang, J.; Yu, L.; Wang, C.; Yang, Y.; Rong, X.; Xu, K.; Chu, M. Antifungal activity of coumarin against Candida albicans is related to apoptosis. Front. Cell. Infect. Microbiol. 2019, 9, 445.
- Xu, K.; Wang, J.L.; Chu, M.P.; Jia, C. Activity of coumarin against Candida albicans biofilms. J. Mycol. Med. 2019, 29, 28–34.
- Lee, J.H.; Kim, Y.G.; Cho, H.S.; Ryu, S.Y.; Cho, M.H.; Lee, J. Coumarins reduce biofilm formation and the virulence of Escherichia coli O157:H7. Phytomedicine 2014, 21, 1037–1042.
- McSweeney, K.R.; Gadanec, L.K.; Qaradakhi, T.; Ali, B.A.; Zulli, A.; Apostolopoulos, V. Mechanisms of cisplatin-induced acute kidney injury: Pathological mechanisms, pharmacological interventions, and genetic mitigations. Cancers 2021, 13, 1572.
- Hassanein, E.H.M.; Ali, F.E.M.; Kozman, M.R.; Abd El-Ghafar, O.A.M. Umbelliferone attenuates gentamicin-induced renal toxicity by suppression of TLR-4/NF-κB-p65/NLRP-3 and JAK1/STAT-3 signaling pathways. Environ. Sci. Pollut. Res. 2021, 28, 11558–11571.
- Wang, H.Q.; Wang, S.S.; Chiufai, K.; Wang, Q.; Cheng, X.L. Umbelliferone ameliorates renal function in diabetic nephropathy rats through regulating inflammation and TLR/NF-κB pathway. Chin. J. Nat. Med. 2019, 17, 346–354.
- Mahmoud, A.M.; Hozayen, W.G.; Hasan, I.H.; Shaban, E.; Bin-Jumah, M. Umbelliferone Ameliorates CCl4-Induced Liver Fibrosis in Rats by Upregulating PPARγ and Attenuating Oxidative Stress, Inflammation, and TGF-β1/Smad3 Signaling. Inflammation 2019, 42, 1103–1116.
- Kumar, V.; Ahmed, D.; Anwar, F.; Ali, M.; Mujeeb, M. Enhanced glycemic control, pancreas protective, antioxidant and hepatoprotective effects by umbelliferon-α-D-glucopyranosyl-(2I→1II)-α-Dglucopyranoside in streptozotocin induced diabetic rats. SpringerPlus 2013, 2, 1–20.
- Aldini, G.; Altomare, A.; Baron, G.; Vistoli, G.; Carini, M.; Borsani, L.; Sergio, F. N-Acetylcysteine as an antioxidant and disulphide breaking agent: The reasons why. Free Radic. Res. 2018, 52, 751–762.
- Tardiolo, G.; Bramanti, P.; Mazzon, E. Overview on the effects of N-acetylcysteine in neurodegenerative diseases. Molecules 2018, 23, 3305.
- Samuni, Y.; Goldstein, S.; Dean, O.M.; Berk, M. The chemistry and biological activities of N-acetylcysteine. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 4117–4129.
- Blasi, F.; Page, C.; Rossolini, G.M.; Pallecchi, L.; Matera, M.G.; Rogliani, P.; Cazzola, M. The effect of N-acetylcysteine on biofilms: Implications for the treatment of respiratory tract infections. Respir. Med. 2016, 117, 190–197.
- Dinicola, S.; De Grazia, S.; Carlomagno, C.; Pintucci, J.P. N-acetylcysteine as powerful molecule to destroy bacterial biofilms. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2942–2948.
- Li, X.; Kim, J.; Wu, J.; Ahamed, A.I.; Wang, Y.; Martins-Green, M. N-Acetyl-cysteine and Mechanisms Involved in Resolution of Chronic Wound Biofilm. J. Diabetes Res. 2020, 2020, 9589507.
- Kelly, A.M.; Dwamena, B.; Cronin, P.; Bernstein, S.J.; Carlos, R.C. Meta-analysis: Effectiveness of drugs for preventing contrast-induced nephropathy. Ann. Intern. Med. 2008, 148, 284–294.
- Aydin, A.; Sunay, M.M.; Karakan, T.; Özcan, S.; Hasçiçek, A.M.; Yardimci, İ.; Surer, H.; Korkmaz, M.; Hücümenoğlu, S.; Huri, E. The examination of the nephroprotective effect of montelukast sodium and N-acetylcysteine ın renal ıschemia with dimercaptosuccinic acid imaging in a placebo-controlled rat model. Acta Cir. Bras. 2020, 35, 1–9.
- Guerini, M.; Grisoli, P.; Pane, C.; Perugini, P. Microstructured lipid carriers (MLC) based on N-acetylcysteine and chitosan preventing Pseudomonas Aeruginosa biofilm. Int. J. Mol. Sci. 2021, 22, 891.
- Kundukad, B.; Udayakumar, G.; Grela, E.; Kaur, D.; Rice, S.A.; Kjelleberg, S.; Doyle, P.S. Weak acids as an alternative anti-microbial therapy. Biofilm 2020, 2, 100019.
- Lababidi, N.; Montefusco-Pereira, C.V.; de Souza Carvalho-Wodarz, C.; Lehr, C.M.; Schneider, M. Spray-dried multidrug particles for pulmonary co-delivery of antibiotics with N-acetylcysteine and curcumin-loaded PLGA-nanoparticles. Eur. J. Pharm. Biopharm. 2020, 157, 200–210.
- Nunes, T.S.B.S.; Rosa, L.M.; Vega-Chacón, Y.; de Oliveira Mima, E.G. Fungistatic action of N-acetylcysteine on Candida albicans biofilms and its interaction with antifungal agents. Microorganisms 2020, 8, 980.
- Pijls, B.G.; Sanders, I.M.J.G.; Kuijper, E.J.; Nelissen, R.G.H.H. Synergy between induction heating, antibiotics, and N-acetylcysteine eradicates Staphylococcus aureus from biofilm. Int. J. Hyperth. 2020, 37, 130–136.
- Pollini, S.; Di Pilato, V.; Landini, G.; Di Maggi, T.; Cannatelli, A.; Sottotetti, S.; Cariani, L.; Aliberti, S.; Blasi, F.; Sergio, F.; et al. In vitro activity of N-acetylcysteine against Stenotrophomonas maltophilia and Burkholderia cepacia complex grown in planktonic phase and biofilm. PLoS ONE 2018, 13, e0203941.
- Feng, J.; Liu, B.; Xu, J.; Wang, Q.; Huang, L.; Ou, W.; Gu, J.; Wu, J.; Li, S.; Zhuo, C.; et al. In vitro effects of N-acetylcysteine alone and combined with tigecycline on planktonic cells and biofilms of Acinetobacter baumannii. J. Thorac. Dis. 2018, 10, 212–218.
- Fernández-Rivero, M.E.; Del Pozo, J.L.; Valentín, A.; de Diego, A.M.; Pemán, J.; Cantón, E. Activity of amphotericin B and anidulafungin combined with rifampicin, clarithromycin, ethylenediaminetetraacetic acid, N-acetylcysteine, and farnesol against Candida tropicalis biofilms. J. Fungi 2017, 3, 16.
- Choi, Y.S.; Kim, C.; Moon, J.H.; Lee, J.Y. Removal and killing of multispecies endodontic biofilms by N-acetylcysteine. Braz. J. Microbiol. 2018, 49, 184–188.
- Kundukad, B.; Schussman, M.; Yang, K.; Seviour, T.; Yang, L.; Rice, S.A.; Kjelleberg, S.; Doyle, P.S. Mechanistic action of weak acid drugs on biofilms. Sci. Rep. 2017, 7, 4783.
- May, E.R.; Conklin, K.A.; Bemis, D.A. Antibacterial effect of N-acetylcysteine on common canine otitis externa isolates. Vet. Dermatol. 2016, 27, 188-e47.
- Moon, J.H.; Choi, Y.S.; Lee, H.W.; Heo, J.S.; Chang, S.W.; Lee, J.Y. Antibacterial effects of N-acetylcysteine against endodontic pathogens. J. Microbiol. 2016, 54, 322–329.




