
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Asako Yamayoshi | + 1895 word(s) | 1895 | 2021-08-02 13:48:59 | | | |
| 2 | Frida Xu | -15 word(s) | 1880 | 2021-08-04 03:03:28 | | |
Video Upload Options
Nucleic acid drugs are not readily permeable through cell membranes and often exhibit poor blood serum stability, rapid renal clearance and poor endosomal escape/cytoplasmic escape. Therefore, they are commonly used in combination with drug delivery system (DDS) carriers. The drug carrier plays an important role in the process of drug delivery.
1. Introduction
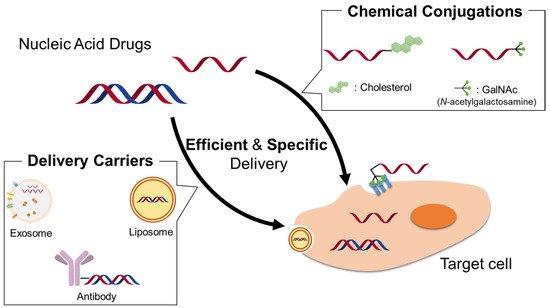
2. Nonviral Drug Delivery Systems for Nucleic Acid Drugs
3. Conjugation of Functional Molecules to Therapeutic Oligonucleotides
In recent decades, the derivatization of nucleic acid drugs has been studied extensively. The nucleic acid cargo can be covalently attached to functional carrier molecules or loaded into supramolecular delivery devices. Conjugations of uptake-enhancing or targeting ligands to oligonucleotides provide the advantage of generating a defined molecule that allows for traditional pharmaceutical quality assessment. Several molecules have been attached to therapeutic oligonucleotides to improve their delivery, biodistribution, and cellular uptake; some are detailed in this section.
GalNAc derivatives were first introduced to oligonucleotides by TsO’s research group in 1995 [23]. They developed GalNAc neoglycopeptide (ah-GalNAc)-conjugated oligodeoxynucleoside methylphosphonate (ah-GalNAc-oligo-MP) and successfully showed that the uptake of ah-GalNAc-oligo-MP by human hepatocellular carcinoma cells (Hep G2) is cell-type specific and can be completely inhibited by the addition of a 100-fold excess of free (ah-GalNAc) 3 in the culture medium, indicating the cell uptake of ah-GalNAc-oligo-MP was ligand dependent.
This specific and enhanced cellular uptake of GalNAc-conjugated oligonucleotides was also confirmed in vivo by several research groups [11][24]. Prakash et al. reported that antisense oligonucleotides conjugated to tri-antennary GalNAc improve the potency of therapeutic oligonucleotides about 10-fold in mice [24]. Now, there are various kinds of chemical modifications of GalNAc-conjugated, and from these reports, it has been shown that the GalNAc introduced into oligonucleotides does not necessarily have a tri-antennary structure, and, surprisingly, even mono-anntenary GalNAc-conjugation was also found effective [25][26]. In the future, we expect to uncover more detailed mechanisms of action of these monomeric GalNAc-conjugated oligonucleotides.
Folic acid (vitamin B9) binds with high affinity to the folate receptor protein to trigger cellular uptake via an endosomal pathway. The presence of the folate receptor on many cancer types has prompted the use of folate in targeted therapy [51]. Indeed, it has been used on liposomes or polyplexes to effectively deliver oligonucleotides to cancer cells that have the folate receptor [52][53]. Dohmen et al. were the first to develop folate-conjugated oligonucleotides, however, tethering folate to siRNA results in specific uptake but not silencing of reporter genes [54]. Folic acid– oligonucleotide conjugates are trapped in endosomes with insufficient endosomal escape to the cytosol for gene silencing. Later, Orellana’s group succeeded in eliciting the gene inhibitory effects of folic acid-conjugated oligonucleotides by connecting folic acid and oligonucleotides with a cleavable linker [55].
4. Conjugation of Functional Molecules to Therapeutic Oligonucleotides
5. Conclusions
nucleic acid drugs, such as ASOs and siRNAs, which promote the “disappearance” or “loss of function” of the target protein and act by new mechanisms that utilize the inherent characteristics of oligonucleotides. Although there are no nucleic acid drugs approved for cancer treatment yet, recent results of several clinical trials suggest that anti-cancer nucleic acid drugs will probably be approved in the near future. Furthermore, it is expected that nucleic acid drugs will be developed and practically used in a coordinated manner according to the characteristics of cancer types.
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30.
- Lameire, N. Nephrotoxicity of recent anti-cancer agents. Clin. Kidney J. 2014, 7, 11–22.
- Suter, T.M.; Ewer, M.S. Cancer drugs and the heart: Importance and management Eur. Heart J. 2013, 34, 1102–1111.
- Levin, A.A. Treating disease at the RNA level with oligonucleotides. N. Engl. J. Med. 2019, 380, 57–70.
- Wang, F.; Zuroske, T.; Watts, J.K. RNA therapeutics on the rise. Nat. Rev. Drug Discov. 2020, 19, 441–442.
- Craig, K.; Abrams, M.; Amiji, M. Recent preclinical and clinical advances in oligonucleotide conjugates. Expert Opin. Drug Deliv. 2018, 15, 629–640.
- Quemener, A.M.; Bachelot, L.; Forestier, A.; Donnou-Fournet, E.; Gilot, D.; Galibert, M.D. The powerful world of antisense oligonucleotides: From bench to bedside. Wiley Interdiscip. Rev. RNA 2020, 11, e1594.
- Mukalel, A.J.; Riley, R.S.; Zhang, R.; Mitchell, M.J. Nanoparticles for nucleic acid delivery: Applications in cancer immunotherapy. Cancer Lett. 2019, 458, 102–112.
- Fumoto, S.; Yamamoto, T.; Okami, K.; Maemura, Y.; Terada, C.; Yamayoshi, A.; Nishida, K. Understanding In Vivo Fate of Nucleic Acid and Gene Medicines for the Rational Design of Drugs. Pharmaceutics 2021, 13, 159.
- Judge, A.D.; Robbins, M.; Tavakoli, I.; Levi, J.; Hu, L.; Fronda, A.; Ambegia, E.; McClintock, K.; MacLachlan, I. Confirming the RNAi-mediated mechanism of action of siRNA-based cancer therapeutics in mice. J. Clin. Investig. 2009, 119, 661–673.
- Nair, J.K.; Willoughby, J.L.; Chan, A.; Charisse, K.; Alam, M.R.; Wang, Q.; Hoekstra, M.; Kandasamy, P.; Kel’in, A.V.; Milstein, S.; et al. Multivalent N-acetylgalactosamine-conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing. J. Am. Chem. Soc. 2014, 136, 16958–16961.
- Khvorova, A.; Watts, J.K. The chemical evolution of oligonucleotide therapies of clinical utility. Nat. Biotechnol. 2017, 35, 238–248.
- Garrelfs, S.F.; Frishberg, Y.; Hulton, S.A.; Koren, M.J.; O’Riordan, W.D.; Cochat, P.; Deschênes, G.; Shasha-Lavsky, H.; Saland, J.M.; Van’t Hoff, W.G.; et al. Lumasiran, an RNAi Therapeutic for Primary Hyperoxaluria Type 1. N. Engl. J. Med. 2021, 384, 1216–1226.
- Santel, A.; Aleku, M.; Keil, O.; Endruschat, J.; Esche, V.; Durieux, B.; Löffler, K.; Fechtner, M.; Röhl, T.; Fisch, G.; et al. RNA interference in the mouse vascular endothelium by systemic administration of siRNA-lipoplexes for cancer therapy. Gene Ther. 2006, 13, 1360–1370.
- Schultheis, B.; Strumberg, D.; Kuhlmann, J.; Wolf, M.; Link, K.; Seufferlein, T.; Kaufmann, J.; Feist, M.; Gebhardt, F.; Khan, M.; et al. Safety, Efficacy and Pharcacokinetics of Targeted Therapy with The Liposomal RNA Interference Therapeutic Atu027 Combined with Gemcitabine in Patients with Pancreatic Adenocarcinoma. A Randomized Phase Ib/IIa Study. Cancers 2020, 12, 3130.
- Atu027 Plus Gemcitabine in Advanced or Metastatic Pancreatic Cancer (Atu027-I-02) (Atu027-I-02). ClinicalTrials.gov, Identifier: NCT01808638 ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT01808638 (accessed on 7 July 2021).
- Golan, T.; Khvalevsky, E.Z.; Hubert, A.; Gabai, R.M.; Hen, N.; Segal, A.; Domb, A.; Harari, G.; David, E.B.; Raskin, S.; et al. RNAi therapy targeting KRAS in combination with chemotherapy for locally advanced pancreatic cancer patients. Oncotarget 2015, 6, 24560–24570.
- Shemi, A.; Khvalevsky, E.Z.; Gabai, R.M.; Gabai, R.M.; Hen, N.; Segal, A.; Domb, A.; Harari, G.; David, E.B.; Raskin, S.; et al. Multistep, effective drug distribution within solid tumors. Oncotarget 2015, 6, 39564–39577.
- A Phase 2 Study of siG12D LODER in Combination with Chemotherapy in Patients with Locally Advanced Pancreatic Cancer (PROTACT). ClinicalTrials.gov, Identifier: NCT01676259 ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT01676259 (accessed on 7 July 2021).
- Merkel, O.M.; Beyerle, A.; Librizzi, D.; Pfestroff, A.; Behr, T.M.; Sproat, B.; Barth, P.J.; Kissel, T. Nonviral siRNA delivery to the lung: Investigation of PEG-PEI polyplexes and their in vivo performance. Mol. Pharm. 2009, 6, 1246–1260.
- Hussain, M.; Shchepinov, M.; Sohail, M.; Benter, I.F.; Hollins, A.J.; Southern, E.M.; Akhtar, S. A novel anionic dendrimer for improved cellular delivery of antisense oligonucleotides. J. Control. Release 2004, 99, 139–155.
- Ko, Y.T.; Bhattacharya, R.; Bickel, U. Liposome encapsulated polyethylenimine/ODN polyplexes for brain targeting. J. Control. Release 2009, 133, 230–237.
- Hangeland, J.J.; Levis, J.T.; Lee, Y.C.; Ts’O, P.O. Cell-type specific and ligand specific enhancement of cellular uptake of oligodeoxynucleoside methylphosphonates covalently linked with a neoglycopeptide, YEE(ah-GalNAc)3. Bioconjug. Chem. 1995, 6, 695–701.
- Prakash, T.P.; Graham, M.J.; Yu, J.; Carty, R.; Low, A.; Chappell, A.; Schmidt, K.; Zhao, C.; Aghajan, M.; Murray, H.F.; et al. Targeted delivery of antisense oligonucleotides to hepatocytes using triantennary N-acetyl galactosamine improves potency 10-fold in mice. Nucleic Acids Res. 2014, 42, 8796–8807.
- Rajeev, K.G.; Nair, J.K.; Jayaraman, M.; Charisse, K.; Taneja, N.; O’Shea, J.; Willoughby, J.L.; Yucius, K.; Nguyen, T.; Shulga-Morskaya, S.; et al. Hepatocyte-specific delivery of siRNAs conjugated to novel non-nucleosidic trivalent N-acetylgalactosamine elicits robust gene silencing in vivo. Chembiochem 2015, 16, 903–908.
- Yamamoto, T.; Sawamura, M.; Wada, F.; Harada-Shiba, M.; Obika, S. Serial incorporation of a monovalent GalNAc phosphoramidite unit into hepatocyte-targeting antisense oligonucleotides. Bioorg. Med. Chem. 2016, 24, 26–32.
- Yamayoshi, A.; Oyama, S.; Kishimoto, Y.; Konishi, R.; Yamamoto, T.; Kobori, A.; Harada, H.; Ashihara, E.; Sugiyama, H.; Murakami, A. Development of Antibody–Oligonucleotide Complexes for Targeting Exosomal MicroRNA. Pharmaceutics 2020, 12, 545.
- Balwani, M.; Sardh, E.; Ventura, P.; Peiró, P.A.; Rees, D.C.; Stölzel, U.; Bissell, M.; Bonkovsky, H.L.; Windyga, J.; Anderson, K.E.; et al. Phase 3 Trial of RNAi Therapeutic Givosiran for Acute Intermittent Porphyria. N. Engl. J. Med. 2020, 382, 2289–2301.
- Ray, K.K.; Wright, R.S.; Kallend, D.; Koenig, W.; Leiter, L.A.; Raal, F.J.; Bisch, J.A.; Richardson, T.; Jaros, M.; Wijngaard, P.L.J.; et al. Two Phase 3 Trials of Inclisiran in Patients with Elevated LDL Cholesterol. N. Engl. J. Med. 2020, 382, 1507–1519.
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J.A. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345.
- Kooijmans, S.A.A.; Stremersch, S.; Braeckmans, K.; de Smedt, S.C.; Hendrix, A.; Wood, M.J.A.; Schiffelers, R.M.; Raemdonck, K.; Vader, P. Electroporation-induced siRNA precipitation obscures the efficiency of siRNA loading into extracellular vesicles. J. Control. Release 2013, 172, 229–238.
- Didiot, M.C.; Hall, L.M.; Coles, A.H.; Haraszti, R.A.; Godinho, B.M.D.C.; Chase, K.; Sapp, E.; Ly, S.; Alterman, J.F.; Hassler, M.R. Exosome-mediated Delivery of Hydrophobically Modified siRNA for Huntingtin mRNA Silencing. Mol. Ther. 2016, 24, 1836–1847.
- Zhao, L.; Gu, C.; Gan, Y.; Shao, L.; Chen, H.; Zhu, H. Exosome-mediated siRNA delivery to suppress postoperative breast cancer metastasis. J. Control. Release 2020, 318, 1–15.
- Munagala, R.; Aqil, F.; Jeyabalan, J.; Kandimalla, R.; Wallena, M.; Tyagi, N.; Wilcher, S.; Yan, J.; Schultz, D.J.; Spencer, W.; et al. Exosome-mediated delivery of RNA and DNA for gene therapy. Cancer Lett. 2021, 505, 58–72.
- Kamerkar, S.; LeBleu, V.S.; Sugimoto, H.; Yang, S.; Ruivo, C.F.; Melo, S.A.; Lee, J.J.; Kalluri, R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 2017, 546, 498–503.
- iExosomes in Treating Participants with Metastatic Pancreas Cancer With KrasG12D Mutation. ClinicalTrials.gov, Identifier: NCT03608631 ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03608631 (accessed on 7 July 2021).
- Semple, S.C.; Klimuk, S.K.; Harasym, T.O.; Dos Santos, N.; Ansell, S.M.; Wong, K.F.; Maurer, N.; Stark, H.; Cullis, P.R.; Hope, M.J.; et al. Efficient encapsulation of antisense oligonucleotides in lipid vesicles using ionizable aminolipids: Formation of novel small multilamellar vesicle structures. Biochim. Biophys. Acta 2001, 1510, 152–166.
- Morrissey, D.V.; Lockridge, J.A.; Shaw, L.; Blanchard, K.; Jensen, K.; Breen, W.; Hartsough, K.; Machemer, L.; Radka, S.; Jadhav, V.; et al. Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat. Biotechnol. 2005, 23, 1002–1007.
- Judge, A.D.; Bola, G.; Lee, A.C.; MacLachlan, I. Design of noninflammatory synthetic siRNA mediating potent gene silencing in vivo. Mol. Ther. 2006, 13, 494–505.
- A Study of NBF-006 in Non-Small Cell Lung, Pancreatic, or Colorectal Cancer. ClinicalTrials.gov, Identifier: NCT03819387 ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03819387 (accessed on 7 July 2021).
- Sahin, U.; Oehm, P.; Derhovanessian, E.; Jabulowsky, R.A.; Vormehr, M.; Gold, M.; Maurus, D.; Schwarck-Kokarakis, D.; Kuhn, A.N.; Omokoko, T.; et al. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature 2020, 585, 107–112.
- Trial With BNT111 and Cemiplimab in Combination or as Single Agents in Patients with Anti-PD1-refractory/Relapsed, Unresectable Stage III or IV Melanoma. ClinicalTrials.gov, Identifier: NCT04526899 ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04526899 (accessed on 7 July 2021).
- Song, E.; Zhu, P.; Lee, S.K.; Chowdhury, D.; Kussman, S.; Dykxhoorn, D.M.; Feng, Y.; Palliser, D.; Weiner, D.B.; Shankar, P.; et al. Antibody mediated in vivo delivery of small interfering RNAs via cell-surface receptors. Nat. Biotechnol. 2005, 23, 709–717.
- Ma, Y.; Kowolik, C.M.; Swiderski, P.M.; Kortylewski, M.; Yu, H.; Horne, D.A.; Jove, R.; Caballero, O.L.; Simpson, A.J.G.; Lee, F.-T.; et al. Humanized Lewis-Y specific antibody based delivery of STAT3 siRNA. ACS Chem. Biol. 2011, 6, 962–970.
- Xia, C.F.; Zhang, Y.; Zhang, Y.; Boado, R.J.; Pardridge, W.M. Intravenous siRNA of brain cancer with receptor targeting and avidin-biotin technology. Pharm. Res. 2007, 24, 2309–2316.
- Arnold, A.E.; Malek-Adamian, E.; Le, P.U.; Meng, A.; Martínez-Montero, S.; Petrecca, K.; Damha, M.J.; Shoichet, M.S. Antibody-Antisense Oligonucleotide Conjugate Downregulates a Key Gene in Glioblastoma Stem Cells. Mol. Ther. Nucleic Acids 2018, 11, 518–527.
- Sugo, T.; Terada, M.; Oikawa, T.; Miyata, K.; Nishimura, S.; Kenjo, E.; Ogasawara-Shimizu, M.; Makita, Y.; Imaichi, S.; Murata, S.; et al. Development of antibody-siRNA conjugate targeted to cardiac and skeletal muscles. J. Control. Release 2016, 237, 1–13.
- Fishman, J.B.; Rubin, J.B.; Handrahan, J.V.; Connor, J.R.; Fine, R.E. Receptor-mediated transcytosis of transferrin across the blood-brain barrier. J. Neurosci. Res. 1987, 18, 299–304.
- Junghans, M.; Kreuter, J.; Zimmer, A. Antisense delivery using protamine-oligonucleotide particles. Nucleic Acids Res. 2000, 28, E45.
- Dinauer, N.; Lochmann, D.; Demirhan, I.; Bouazzaoui, A.; Zimmer, A.; Chandrac, A.; JörgKreuter; Briesena, H. Intracellular tracking of protamine/antisense oligonucleotide nanoparticles and their inhibitory effect on HIV-1 transactivation. J. Control. Release 2004, 96, 497–507.
- Low, P.S.; Henne, W.A.; Doornereerd, D.D. Discovery and Development of Folic-Acid-Based Receptor Targeting for Imaging and Therapy of Cancer and Inflammatory Diseases. Acc. Chem. Res. 2008, 41, 120–129.
- Zhou, W.; Yuan, X.; Wilson, A.; Yang, L.; Mokotoff, M.; Pitt, B.; Li, S. Efficient intracellular delivery of oligonucleotides formulated in folate receptor-targeted lipid vesicles. Bioconjug. Chem. 2002, 13, 1220–1225.
- Wang, M.; Hu, H.; Sun, Y.; Qiua, L.; Zhang, J.; Guan, G.; Zhao, X.; Qiao, M.; Cheng, L.; Cheng, L.; et al. A pH-sensitive gene delivery system based on folic acid-PEG-chitosan—PAMAM-plasmid DNA complexes for cancer cell targeting. Biomaterials 2013, 34, 10120–10132.
- Dohmen, C.; Fröhlich, T.; Lächelt, U.; Röhl, I.; Vornlocher, H.-P.; Hadwiger, P.; Wagner, E. Defined Folate-PEG-siRNA Conjugates for Receptor-specific Gene Silencing. Mol. Ther. Nucleic Acids 2012, 1, e7.
- Orellana, O.; Tenneti, S.; Rangasamy, L.; Lyle, T.; Low, P.S.; Kasinski, A.L. FolamiRs: Ligand-targeted, vehicle-free delivery of microRNAs for the treatment of cancer. Sci. Transl. Med. 2017, 9, eaam9327.
- Juliano, R.L.; Ming, X.; Nakagawa, O. Cellular uptake and intracellular trafficking of antisense and siRNA oligonucleotides. Bioconjug. Chem. 2012, 23, 147–157.
- Wolfrum, C.; Shi, S.; Jayaprakash, K.N.; Jayaraman, M.; Wang, G.; Pandey, R.K.; Rajeev, K.G.; Nakayama, T.; Charrise, K.; Ndungo, E.M.; et al. Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nat. Biotechnol. 2007, 25, 1149–1157.
- Wong, S.C.; Klein, J.J.; Hamilton, H.L.; Chu, Q.; Frey, C.L.; Truvetskoy, V.S.; Hegge, J.; Wakefield, D.; Rozema, D.B.; Lewis, D.L. Co-injection of a targeted, reversibly masked endosomolytic polymer dramatically improves the efficacy of cholesterol-conjugated small interfering RNAs in vivo. Nucleic Acid Ther. 2012, 22, 380–390.
- Frankel, A.D.; Pabo, C.O. Cellular uptake of the tat protein from human immunodeficiency virus. Cell 1988, 55, 1189–1193.
- Derossi, D.; Joliot, A.H.; Chassaing, G.; Prochiantz, A. The third helix of the Antennapedia homeodomain translocates through biological membranes. J. Biol. Chem. 1994, 269, 10444–10450.
- Pooga, M.; Hällbrink, M.; Zorko, M.; Langel, U. Cell penetration by transportan. FASEB J. 1998, 12, 67–77.
- Futaki, S.; Suzuki, T.; Ohashi, W.; Yagami, T.; Tanaka, S.; Ueda, K.; Sugiura, Y. Arginine-rich peptides. An abundant source of membrane-permeable peptides having potential as carriers for intracellular protein delivery. J. Biol. Chem. 2001, 276, 5836–5840.




